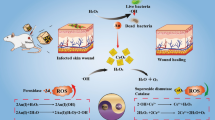
Abstract
The development of advanced sustainable biomedical materials with superior biosafety and bioactivity for clinical applications is highly desirable. In the present investigation, biomass-based nanoparticles (NPs) were assembled through the Mannich reaction between the plant polyphenols and the broad-spectrum antibiotic tigecycline (TG). The fabricated NPs with uniform size demonstrated excellent oxidative balance effects, pH-responsive release properties and antibacterial performances. Furthermore, the intracellular and in vivo studies confirmed that the NPs are capable of reducing oxidative damage to cells and significantly repairing tissue injury in mice with peritonitis. This work presents an effective method and idea for constructing biomass-based materials for the treatment of infection-induced diseases.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Peritonitis is usually triggered by bacterial infection of the peritoneum, which could further damage healthy tissues such as the heart muscle, lung parenchyma and gastrointestinal tract, ultimately leading to multi-organ dysfunction and even threatening life [1, 2]. Despite several broad-spectrum antibiotics that have been developed for bacteria clearance in clinic, there still exists a huge challenge to achieve great therapeutic effects due to chaotic levels of reactive oxygen species (ROS) induced by the infection [3, 4].
The simultaneous uses of antibiotics and small molecular antioxidants became the most direct strategy in the current study, but the rapid metabolism, poor bioavailability and high toxicity extremely limited the further therapeutic effects [5]. In recent years, nanotechnology has emerged as an excellent strategy for better applications [6,7,8]. For instance, polymers and lipids have been designed to deliver antibiotics and antioxidants for the therapy of peritonitis. [9,10,11] Despite their many advantages and achievements, the wide applications of these materials are limited by the resource constraints, complex synthesis and high cost. In contrast, biomass is a renewable organic material from plants and animals which is abundant, fast-growing and low-cost. In addition, the development of advanced biomass-based biomedical materials for clinical applications is highly desirable owing to their high biocompatibility and unique bioactivities in the human body, which make them uniquely attractive for biomaterial construction [12]. Polyphenols, as classical biomass, are a class of secondary metabolites widely present in all plants and have received increasing attention in the biomedical field due to their unique bioactivities such as anti-inflammatory, antimicrobial and antioxidant activities [13,14,15,16]. A large number of recent studies have focused on the scavenging capacity of excess free radicals in polyphenol-based materials, aiming to exploit their potential to balance complex oxidative stress microenvironments [13, 17, 18]. Moreover, the polyphenolic components also possess outstanding physicochemical properties such as covalent and noncovalent interactions, which make polyphenol used as excellent building blocks for the construction of nanomaterials [19,20,21]. Therefore, it is a potential strategy to improve the therapeutic effects of antibiotics against the complex oxidative stress microenvironment in peritonitis with the assistance of polyphenolic platforms.
Herein, tigecycline (TG) which has been clinically used to relieve complex peritoneal infections and epigallocatechin gallate (EGCG) with excellent antioxidative properties as well as reactive activities were employed to construct functional nanoparticles (NPs) with both antimicrobial and antioxidative effects via Mannich reaction and subsequent assembly. The as-prepared NPs responsively release TG for killing bacteria under the peritonitis microenvironment, and EGCG blocks could scavenge the excess ROS accumulated by bacterial infection. The in vivo results further confirmed the improved therapeutic effects of NPs in peritonitis treatment, such as reduced tissue damage and delayed disease progression. It is anticipated that this material construction strategy could provide new ideas for the treatment of intractable diseases with bacterial infection and subsequent oxidative stress microenvironment.
2 Material and methods
2.1 Material
EGCG (> 98.0%, Nanjing, Dasf Biotechnology Co., Ltd.), tigecycline (> 96%, Shanghai, Aladdin Biochemical Technology Co., Ltd.), formaldehyde (37%, AR, Chengdu, Chron Chemicals Co., Ltd.), 1,1-diphenyl-2-picryl-hydrazyl (DPPH, 95%, Shanghai, Alfa Aesar Chemical Co., Ltd.). 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid ammonium salt (ABTS, 98.0%, TCI), H2O2 (30%, Chengdu, Jinshan Chemical Reagent Co., Ltd.). Tryptone, yeast extract, tryptic soy broth (TSB) and agar used for bacteria culture were obtained from Oxoid (Basingstoke, UK). Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) were obtained from ATCC (American Type Culture Collection, USA). Dulbecco's modified Eagle's medium (DMEM), and penicillin–streptomycin solution were obtained from Hyclone Laboratories (Logan, Utah, USA). JC-1 (95%). All chemicals were not further decontaminated before use.
2.2 Fabrication of NPs
In brief, 20 mg of EGCG was dissolved in water followed by the addition of 124 μL of formaldehyde solution (0.3%) with stirring. After 10 min, 10.4 mg of tigecycline (TG) was added into the solution. Finally, the solution became turbid after stirring for 12 h at room temperature, implying the formation of nanoparticles. Nanoparticles were collected by centrifugation (14 000 rpm, 5 min), and washed three times with water. Nanoparticles with different TG loadings could be obtained from the reaction system by varying the ratio of the raw materials (the specific parameters were listed in Additional file 1: Table S2). They were named NP-1, NP-2, NP-3 and NP-4 respectively.
Escalab MKII spectrometer was employed to conduct X-ray photoelectron spectroscopy (XPS) measurement. The narrow-scan XPS spectra of the N 1 s of the samples were deconvolved by XPSPEAK software (version 4.1).
2.3 In vitro antibiotic release
1.0 mL NPs solution (2 mg/mL) was transferred into the dialysis apparatus (molecule weight cut-off, 3.5 kDa), then immersed in 30 mL of phosphate-buffered saline (PBS) at pH 7.2, 6.5 and 5.5 in centrifuge tubes and placed in a shaker at 37 °C, 100 rpm. At specified intervals, 9.0 mL of the PBS solution was sampled and the absorbance at 351 nm was measured by UV–Vis spectrophotometry to obtain a cumulative TG release curve. At the same time, an equal amount of fresh medium was supplied to keep a constant solution volume. Three parallel experiments were performed on each sample.
2.4 In vitro antibacterial assay
Escherichia coli (ATCC 2922) and Staphylococcus aureus (ATCC 25923) were selected to evaluate the antibacterial activity of NP-1. Bacterial samples were suspended in LB medium and then 250 μL NP-1 was added at various concentrations. The final bacterial concentration was ∼105 CFU/mL. The optical density (OD) values of the suspension in each well were measured at 600 nm using a Thermo Fisher enzyme marker after 24 h incubation at 37 °C. In agar plate counting assays, four different concentrations (0–64 μg/mL) of NP-1 solutions were incubated with bacterial cultures (~ 105 CFU/mL) at 37 °C for 24 h, then 200 μL of the mixture was applied to LB plates under moderate dilution in PBS, cultivated for a further 24 h to count the colonies.
Moreover, calcein AM and propidium iodide (calcein AM/PI) were employed to perform the staining results for 15 min, followed by observation of live and dead bacteria by an inverted fluorescence microscope. Then, a suspension containing bacteria of 107 CFU/mL concentration was admitted to 6-well plates coated with sterile slides and allowed to incubate under 37 °C for 12 h. The medium was then removed and NP-1 at a concentration of 64 μg/mL was carefully incorporated into the wells. After a further 12 h incubation, the treated bacteria were fixed in the dark with 2.5% glutaraldehyde and then sequentially washed and dehydrated with 30%, 50%, 70%, 90% and 100% ethanol gradients. At last, the morphological changes of the bacteria were observed by scanning electron microscopy (SEM, Phenom Pro microscopy).
2.5 Cell culture and cytocompatibility assay
RAW264.7 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM), and the cells were taken for experiments when entering the logarithmic growth phase. First, the effect of NP-1 on cell viability was inspected. Cells were inoculated in 96-well plates at a density of 5000 cells/well. After 24 h of cell culture, the solution was replaced with fresh medium containing NP-1, equal levels of free EGCG or TG. Different samples were co-incubated with cells for 24 h before assessing cell viability using the Alamar-Blue assay. To acquire images of live-dead staining, cells after different treatments were stained with calcein AM/PI for 15 min and photographed with a fluorescent inverted microscope. Then the protective effect of NP-1 against cellular oxidative stress was tested. Cells were inoculated in 6-well plates (5 × 105 cells per well), pretreated with various concentrations of NP-1 for 12 h, and then exposed to 600 μM hydrogen peroxide for 3 h. Subsequently, the relative cell viability was measured using the same method.
2.6 Intracellular ROS scavenging
The ROS was monitored using a 2,7-dichlorofluorescein diacetate (DCFH-DA) fluorescent probe. RAW264.7 cells were treated with NP-1 and hydrogen peroxide in 6-well plates, and the cells were stained with DCFH-DA (10 μM) in serum-free medium for 30 min shielded from light. Fluorescent microscope images were collected after washing the cells 3 times with PBS. To get quantitative results, the collected cells were resuspended in 500 µL PBS for flow cytometry analysis. Moreover, Malondialdehyde (MDA) levels were analyzed for each group of cells according to the kit instructions.
2.7 In vivo biocompatibility assessment
Healthy KM mice (female, 2–8 weeks, ~ 20 g) were randomly divided into two groups (n = 6) to assess the in vivo biocompatibility of NP-1. Mice without bacterial infection were taken peritoneal injections of NP-1 (64 μg/mL, 200 μL) and sterile saline respectively. 24 h later, the mice were sacrificed and blood samples were gathered for biochemical analysis. The levels of ALT, AST, CREA and UREA were evaluated to ascertain the function of the liver and kidney. The heart, liver, spleen, lung and kidney tissues of the mice were harvested and fixed in paraformaldehyde, then stained with hematoxylin and eosin (H&E) for histological analysis.
2.8 Peritonitis infection and treatment in mice
Peritonitis infection models were established by intraperitoneal injection of E. coli ATCC 25922 (OD600 = 0.3, 200 μL) into female KM mice (2–8 weeks old, ~ 20 g). Upon infection for 1 h, mice were given equal amounts of saline, NP-1 suspension (64 μg/mL), or TG solution (same antibiotic content as the NP-1 group), respectively. After 24 h of handling, the mice were sacrificed. Serum was obtained to determine blood biochemical parameters including ALT, AST, CREA, and UREA. The heart, liver, spleen, lung, and kidney of the mice were isolated, and a portion of these organs was utilized for further Gram and H&E staining, while the other portion was homogenized for CFU counting and MDA quantification. Dihydroethidium (DHE) staining was also carried out on the liver and kidneys to detect ROS levels and immunohistochemical staining was undertaken to assess the levels of inflammatory factors (TNF-α, IL-6).
2.9 Statistical analysis
Statistical analysis involved the two-sided Student's t-test for two groups and one-way ANOVA for multiple groups. P < 0.05 was considered statistically significant.
3 Results and discussion
3.1 Fabrication and characterization
In this study, naturally occurring EGCG with multiple phenolic hydroxyl groups and TG with the amino group were condensed via Mannich reaction under formaldehyde activation, and then assembled into NPs in an aqueous solution (Fig. 1a and Additional file 1: Fig. S1). The resulting NPs with different relative TG/EGCG ratios were named NP-i (i = 1–4) where the amount of TG increased slightly with the initial reaction ratios (Additional file 1: Tables S1 and S2). The uniformly dispersed spherical morphology of NPs was visualized with scanning electron microscope (SEM) and transmission electron microscope (TEM) images (Fig. 1b and Additional file 1: Fig. S2-3), and the average sizes of NPs were counted as approximately 300 nm. Besides, the electron energy loss spectrum (EELS) mapping reflected the uniform distribution of C, N and O elements in the NP-1, indicating the homogeneous participation of EGCG and TG. Furthermore, the NPs presented a negative surface charge in zeta potential assay due to the reservation of phenolic groups, which benefits the further therapeutic effects [22]. Interestingly, the zeta potential increased with the increase of TG components which might be attributed to the increase of tertiary and secondary amines from TG which were prone to protonation. Thus far, the homogeneous and stable NPs from EGCG and TG were successfully fabricated, following which their structures and properties will be detailly discussed.
Synthesis and characterization of NPs. a Illustration of NPs formation processes and roles in the treatment of peritonitis. b SEM image of NP-1. c Elemental mapping images of NP-1. (Scale bar: 100 nm) d UV–Vis absorption spectra and e FTIR spectra of EGCG, TG and NPs. f 1H NMR spectra of EGCG, TG and NP-1. g ESI–MS spectrum of NP-1 and the possible structures assigned to specific peaks. N 1 s XPS spectra of h TG and i NP-1 (The same color represented the same bonds.)
The UV–Vis spectra were first utilized to evaluate the structures of NPs. The characteristic absorption peaks were assigned to EGCG and TG, which demonstrated the successful coupling of both functional building blocks (Fig. 1d). The broader absorption range of NPs could be attributed to the expanded conjugation system following the oligomerization of EGCG [23,24,25]. Moreover, compared with monomers as a result of the involvement of the secondary amide of TG together with the aryl hydrogen of EGCG in Mannich reaction, the in-plane bending vibrational peaks of EGCG aromatic-CH at 953, 852 cm−1 and the amide II and amide III bands of TG secondary amide at 1520, 1286 cm−1 from Fourier-transform infrared spectroscopy (FTIR) [26, 27] significantly weakened (Fig. 1e). The merging and shifting of the EGCG hydroxyl stretching vibrational peaks at 3360 and 3480 cm−1 were assigned to the formation of hydrogen bonds in this synthetic system [28]. Additionally, the appearance of new signals at 3.99, 4.80, 5.16 and 5.30 ppm in the 1H nuclear magnetic resonance (NMR) spectra (Fig. 1f) suggested the formation of methylene bridges (Ar-CH2-Ar), methylene ether bridges (CH2-O-CH2), benzyl amide bonds (Ar-CH2-CON) and methoxy amide bonds (CON-CH2-O) attesting to the occurrence of phenolic condensation and Mannich condensation [29]. To further clarify the reaction mechanism, cation-mode electrospray ionization mass spectrometry (ESI–MS) was adopted to identify the possible intermediate structures during NPs formation. The peaks at 286.90, 343.65, 382.40 and 586.30 fully confirmed the occurrence of the Mannich reaction and the phenolic condensation between EGCG and TG (Fig. 1g, Additional file 1: Fig. S1 and S4). Moreover, as shown in the X-ray photoelectron spectroscopy (XPS) spectrum, the signal peaks at 399.2, 398.6 and 398.0 eV in the N 1 s spectrum of TG could be assigned to amide, tertiary amine and secondary amine separately, [30, 31] and the percentage of tertiary amines increased with decreasing amide contents which also supported the Mannich condensation process (Fig. 1h and Fig. 1i). In conclusion, NPs were fabricated by integrating these antimicrobial and antioxidative building blocks via Mannich reaction, which has great potential for further application.
3.2 Release behaviors and antibacterial properties of NPs
Uncontrolled bacterial contamination at the abdominal site could cause rapid bacteraemia and sepsis [3], and the removal of intra-abdominal microorganisms plays a vital role in the therapy of peritonitis. Before the evaluation of NPs’s antibacterial properties, the responsive release behaviors from NPs were first studied at different pH values. Taking NP-1 as an example, the NPs structure gradually disintegrated after 120 h incubation in acid phosphate buffer solution (PBS, pH = 5.5) (Additional file 1: Fig. S5), which simulated the microenvironment of bacteria and inflammation [10, 32, 33]. The cumulative TG release ratios were determined by UV–Vis spectra at 351 nm which corresponded to the characteristic peak of TG. As shown in Fig. 2a, the release of TG from NP-1 could reach 91.7% after 72 h incubation (pH = 5.5), which was much higher than that of the neutral environment (pH = 7.2). The other NPs (NP-2 ~ 4) also showed similar results, and these universal release behaviors of NPs could boost the accumulation of the antibiotic in the target position and reduce non-specific side effects [34]. Furthermore, NP-1 with the highest release efficiency was selected as a representative for antibacterial assays in vitro, Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) as the most commonly infected species of peritonitis were selected to evaluate the bacteria-killing abilities. [35] Clear zones of inhibition could be observed in the NP-1-coated filter group (Additional file 1: Fig. S6), which preliminarily performed excellent antibacterial activities. Quantitative results indicated that NP-1 could significantly inhibit the growth of bacteria at low concentration of 4 μg/mL (97% for S. aureus and 80% for E. coli) (Fig. 2e-f) and completely eradicated bacteria at the concentration of 32 μg/mL (Fig. 2g-h and Additional file 1: Fig. S7). The above results were also confirmed by calcein AM and propidium iodide (calcein AM/PI) double staining. [36] Bacteria accompanied by intense red fluorescence after NP-1 treatment (Fig. 2i) reflected the effective bacteria-killing for both S. aureus and E. coli. Moreover, roughly and severely deformed surface with the inwards sunken membrane and disrupted structural integrity of bacteria were observed with SEM images after NP-1 treatment, whereas the control group showed a clear and smooth border (Fig. 2j). All the results confirmed that NPs maintained the excellent antibacterial properties of TG and were effective in eliminating bacteria to avoid continuous infection towards the peritoneum [37, 38].
The antibacterial activity of NPs. a–d Cumulative rates of release of TG from NPs over 72 h, respectively. Optical density (OD) at 600 nm of e S. aureus and f E. coli bacterial suspensions treated with different concentrations of NP-1. Survival of g S. aureus and h E. coli after incubation with different concentrations of NP-1 for 24 h. i Live/dead stained images of S. aureus and E. coli treated with 64 μg/mL NP-1. Untreated bacteria were used as controls. j Typical SEM images of S. aureus and E. coli treated with/without 64 μg/mL NP-1
3.3 In vitro antioxidative evaluation
Apart from being able to annihilate pathogenic bacteria at the lesion sites, the abilities against subsequent oxidative stress microenvironments after peritonitis infection were further evaluated [39, 40]. The typical 2,2-diphenyl-1-trinitrophenylhydrazine (DPPH) and 2,2′-biazido-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) free radical scavenging assays were first performed to assess the antioxidative capacity in vitro [41, 42]. All NPs expressed excellent free radical scavenging behaviors, and NP-1 performed the best scavenging efficiency in both assays (Fig. 3a, b) due to the greater exposure of phenolic hydroxyl groups as demonstrated in the zeta potential results (Additional file 1: Table S1). Meanwhile, the cytocompatibility of NP-1 was tested with RAW264.7 cells for further in vivo application. As seen in Additional file 1: Fig. S8, NP-1 performed remarkably excellent cytocompatibility compared to naked EGCG and TG, which could promote cell proliferation at 32 μg/mL and keep around 80% cell viability at 256 μg/mL, and live/dead staining also confirmed great cytocompatibility of NP-1 (Additional file 1: Fig. S9). The intracellular protective effect of NPs towards oxidative stress was further evaluated with the stimulation of H2O2. There existed increasing survival rates of cells with oxidative stress after NP-1 co-incubation, and enhanced protective effects were detected with increasing NP-1 concentrations due to upregulated ROS scavenging abilities. [43] To precisely assess the intracellular ROS levels, a 2,7-dichlorofluorescein diacetate (DCFH-DA) fluorescent probe was applied via both fluorescence microscopy observation and flow cytometry [44,45,46], and significant reducing trends in mean fluorescence intensity could be observed after the co-incubation with NP-1 (Fig. 3d-e). The average fluorescence intensity could be diminished from 100.0% ± 3.7% to 21.1% ± 0.1% with 32 μg/mL NP-1(Fig. 3f), which showed the effective ROS scavenging effects. Interestingly, NP-1 performed the lower ROS levels compared with naked EGCG, illustrating that NP-1 could perpetuate the antioxidative capacity and enhance the stability of naked EGCG. Moreover, the protective effects of NP-1 towards oxidative stress could be further quantificationally evaluated by the contents of malondialdehyde (MDA) which could evaluate the oxidative stress levels as the side product of lipid peroxidation in living organisms [47]. After co-incubation of NP-1, the intracellular MDA content could back up to near normal level, and significant differences could be observed with positive control (Fig. 3h). The above results implied that NP-1 could balance the intracellular ROS level and thus lessen the severity of cellular lipid peroxidation, ultimately protecting the cells from H2O2-induced oxidative damage. The comprehensive in vitro experiments confirmed that the prepared NP-1 possessed obvious antibacterial and antioxidative abilities which provide huge potential towards peritonitis therapy in vivo.
The antioxidative activities of NPs in vitro. a DPPH and b ABTS free radical scavenging activities of NP-i (i = 1–4) with different incubation times (DPPH: 0.10 × 10−3 M, ABTS: 0.23 × 10.−3 M, NPs: 0.017 mg/mL). c Protective effects of NP-1 against hydrogen peroxide (600 μM, stimulated for 3 h) mediated oxidative stress. d Images of intracellular DCFH-DA staining. e Flow cytometry results of ROS production in RAW 264.7 cells. Fluorescence quantification of ROS from f staining images and g flow cytometry results. h MDA content changes of cells with treatment of different NP-1 concentrations. NC: negative control (without any treatment); PC: positive control (treated with H2O2 only); ns, no significance, *P < 0.05, **P < 0.01, ***P < 0.001
3.4 In vivo therapeutic efficacy of NP-1 on peritonitis
Encouraged by the powerful antibacterial and antioxidative capacity of NP-1 in vitro, the therapeutic effects of NP-1 in vivo against peritonitis were further investigated. Prior to a systematic evaluation in vivo, the biosafety of NP-1 was first tested. Hemolytic analysis revealed that the hemolysis rate of NP-1 was below the 5% threshold when NPs’ concentration was less than 100 μg/mL (Additional file 1: Fig. S10) [48], implicating their good biosafety for systemic injection. Blood serum analysis consisting of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (CREA) and urea (UREA) in vivo showed that NP-1 did not cause hepatotoxicity or nephrotoxicity, and no significant differences were observed between mice treated with NP-1 and control group (p > 0.05, Additional file 1: Fig. S11) [49, 50]. The above results were also confirmed with histopathological hematoxylin–eosin (H&E) stained histological images, and no distinguishable tissue injury could be observed in the major organs (heart, liver, spleen, lung and kidney) with NP-1 treatment (Additional file 1: Fig. S12). In summary, NP-1 was demonstrated favorable in vivo biocompatibility and showed excellent potential for the treatment of peritonitis in vivo.
Subsequently, to evaluate the in vivo therapeutic effects of NP-1, the peritonitis model was established by intraperitoneal injection of E. coli, then different treatments were carried out after 1 h. Administration of TG or NP-1 could eliminate bacterial infection in each organ by 1 order of magnitude (Fig. 4a-d), and there were no significant differences in the statistical results between both groups. Typical photographs of E. coli colonies along with Gram staining results also showed consistent trends (Additional file 1: Fig. S12-13). Furthermore, the abilities to scavenge excess in vivo ROS from the respiratory burst of immune cells undergoing bacterial invasion were evaluated in each group via the quantitative assays of MDA in tissues [51]. Interestingly, the NP-1 could efficiently downregulate the MDA levels, but TG treated group failed to remove excess ROS with the absence of antioxidative abilities (Fig. 4e-h). Moreover, dihydroethidium (DHE) staining of liver and kidney sections reflected weaker red fluorescence intensity in the NP-1 treated group, also confirming the down-regulating ROS levels (Fig. 4i) [52, 53]. Next, the effect of NP-1 on inflammatory levels was investigated by immunohistochemical staining of the liver and kidney. The E. coli-induced pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were suppressed through NP-1 treatment indicating the palliation of the inflammatory cascade (Fig. 4j), which could greatly balance the microenvironment in the process of peritonitis [54, 55].
The bacterial infection, oxidative stress and inflammatory levels with different treatments (n = 6). a–d Bacterial counts of bacteria in the liver, kidney, spleen and lung with saline, TG or NP-1 treatment, respectively: e–h MDA levels in each group. i DHE staining of liver and kidney in each group. j Immunohistochemical staining of the liver (IL-6) and kidney (TNF-α). NC: negative control (without any treatment); PC: positive control (treated with E. coli and saline); ns, no significance, *P < 0.05, **P < 0.01, ***P < 0.001
Furthermore, the physiological functions with NP-1 treatment were roundly evaluated, and the serum analyses were first performed. Similar CREA and UREA levels were observed in either TG or NP-1-treated mice compared with the NC group, displaying a positive recovery of renal excretory function (Fig. 5a, b). However, the AST and ALT levels in the TG group appeared the abnormal trends, which could be attributed to mitochondrial dysfunction and a certain toxicity to hepatocytes of TG (Fig. 5c-d) [56]. Interestingly, the AST and ALT levels could be downregulated to near normal levels with NP-1 treatment, and it might be attributed to the reduced unnecessary antibiotic exposure. Eventually, H&E staining was used to investigate the histological characteristics in major organs (Fig. 5e), and extensive histopathology was observed in PC group like loss of healthy structures in the liver lobule with poorly defined border and disturbed liver cord, [49] the necrosis of glomeruli, dilatation on renal tubule and deposition of intracellular debris in the lumen, [57] atrophy of the red medullary region for spleen [58], thickening of the alveolar wall accompanied by infiltration of inflammatory cells in the lung [59], and disruption of myocardial cell arrays interspersed with vacuolization. Whereas, all above-mentioned destroys were maximally suppressed with the treatment of NP-1, and the histological structures were closed to the tissues of the normal group. In conclusion, NP-1 could promote the histological structure remodeling and function recovery by regulating bacterial infection, oxidative stress and inflammatory levels.
The functional and histological effects of NP-1 towards peritonitis. a CREA, b UREA, c ALT and d AST levels after various treatments (n = 6). e Histopathological staining of liver, kidney, spleen and lung, respectively. Red circles indicated hemolysis or congestion; orange circles represented cellular debris deposition; boxes showed degenerative changes in the glomerulus; blue circles pointed to inflammatory cell infiltration; green circles marked fibrous vacuolation. (ns, no significance, *P < 0.05, **P < 0.01, ***P < 0.001.)
4 Conclusion
Biomass-derived nanoparticles with antimicrobial and antioxidative properties were successfully constructed by a simple and novel preparation method. The excellent therapeutic effects were achieved compared to those of the naked TG via minimizing bacterial infection, clearing excess ROS, alleviating inflammatory responses and potentiating tissue repair in mice with peritonitis. This study proposed an effective strategy to fabricate antibiotics-contained NPs for infection-induced diseases including peritonitis.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EGCG:
-
Epigallocatechin gallate
- TG:
-
Tigecycline
- NPs:
-
Nanoparticles
- ROS:
-
Reactive oxygen species
- PBS:
-
Phosphate-buffered saline
- S. aureus :
-
Staphylococcus aureus
- E.coli :
-
Escherichia coli
- DCFH-DA:
-
2,7-Dichlorofluorescein diacetate
- MDA:
-
Malondialdehyde
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- CREA:
-
Creatinine
- UREA:
-
Urea
References
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54.
Zheng D, Yu Y, Li M, Wang G, Chen R, Fan G-C, Martin C, Xiong S, Peng T. Inhibition of microRNA 195 prevents apoptosis and multiple-organ injury in mouse models of sepsis. J Infect Dis. 2016;213(10):1661–70.
Ross JT, Matthay MA, Harris HW. Secondary peritonitis: principles of diagnosis and intervention. BMJ. 2018;361: k1407.
Zhang J, Fu Y, Yang P, Liu X, Li Y, Gu Z. ROS scavenging biopolymers for anti-inflammatory diseases: classification and formulation. Adv Mater Interfaces. 2020;7(16):2000632.
Yang Y, Ding Y, Fan B, Wang Y, Mao Z, Wang W, Wu J. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J Control Release. 2020;321:463–74.
Li Y, Jiang Y, Zheng Z, Du N, Guan S, Guo W, Tang X, Cui J, Zhang L, Liu K. Co-delivery of precisely prescribed multi-prodrug combination by an engineered nanocarrier enables efficient individualized cancer chemotherapy. Adv Mater. 2022;34(12):2110490.
Yang X, Jiang X, Yang H, Bian L, Chang C, Zhang L. Biocompatible cellulose-based supramolecular nanoparticles driven by host–guest interactions for drug delivery. Carbohydr Polym. 2020;237: 116114.
Zou Y, Wang Y, Xu S, Liu Y, Yin J, Lovejoy DB, Zheng M, Liang XJ, Park JB, Efremov YM. Brain Co-delivery of temozolomide and cisplatin for combinatorial glioblastoma chemotherapy. Adv Mater. 2022;34(33):2203958.
Costa-Fernandez S, Matos JK, Scheunemann GS, Salata GC, Chorilli M, Watanabe I-S, de Araujo GL, Santos MF, Ishida K, Lopes LB. Nanostructured lipid carriers containing chitosan or sodium alginate for co-encapsulation of antioxidants and an antimicrobial agent for potential application in wound healing. Int J Biol Macromol. 2021;183:668–80.
Zhang CY, Gao J, Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30(43):1803618.
Wang T, Li Y, Cornel EJ, Li C, Du J. Combined antioxidant–antibiotic treatment for effectively healing infected diabetic wounds based on polymer vesicles. ACS Nano. 2021;15(5):9027–38.
Liu J, Hu Y, Li L, Wang C, Wang J, Li Y, Chen D, Ding X, Shen C, Xu F. Biomass-derived multilayer–Structured microparticles for accelerated hemostasis and bone repair. Adv Science. 2020;7(22):2002243.
Wang T, Zhao J, Yang Z, Xiong L, Li L, Gu Z, Li Y. Polyphenolic sunscreens for photoprotection. Green Chem. 2022;24(9):3605–22.
Gao X, Xu Z, Liu G, Wu J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021;119:57–74.
Fu Y, Wan R, Yang L, Xiong L, Hu J, Tang J, He H, Gu Z, Li L, Li Y. Propolis inspired sunscreens for efficient UV-protection and skin barrier maintenance. Nano Res. 2022;15(9):8237–46.
Zhu F, Zhang J, Zhong J, Wang T, Li Y, Gu Z. Natural polyphenol-based nanoparticles for the treatment of iron-overload disease. J Control Release. 2023;356:84–92.
Li X, Wang X, Liu Q, Yan J, Pan D, Wang L, Xu Y, Wang F, Liu Y, Li X. ROS-responsive boronate–Stabilized polyphenol–poloxamer 188 assembled dexamethasone nanodrug for macrophage repolarization in osteoarthritis treatment. Adv Healthcare Mater. 2021;10(20):2100883.
Cao H, Yang L, Tian R, Wu H, Gu Z, Li Y. Versatile polyphenolic platforms in regulating cell biology. Chem Soc Rev. 2022;51:4175–98.
Kim H, Yuk SA, Dieterly AM, Kwon S, Park J, Meng F, Gadalla HH, Cadena MJ, Lyle LT, Yeo Y. Nanosac, a noncationic and soft polyphenol nanocapsule, enables systemic delivery of siRNA to solid tumors. ACS Nano. 2021;15(3):4576–93.
Chen Z, Farag MA, Zhong Z, Zhang C, Yang Y, Wang S, Wang Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv Drug Delivery Rev. 2021;176: 113870.
Li H, Zhang J, Yang L, Cao H, Yang Z, Yang P, Zhang W, Li Y, Chen X, Gu Z. Synergistic antimicrobial and antibiofilm nanoparticles assembled from naturally occurring building blocks. Adv Funct Mater. 2023;33(21):2212193.
Wang T, Fan Q, Hong J, Chen Z, Zhou X, Zhang J, Dai Y, Jiang H, Gu Z, Cheng Y. Therapeutic nanoparticles from grape seed for modulating oxidative stress. Small. 2021;17(45):2102485.
Fu Y, Yang L, Zhang J, Hu J, Duan G, Liu X, Li Y, Gu Z. Polydopamine antibacterial materials. Mater Horiz. 2021;8(6):1618–33.
Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014;114(9):5057–115.
Tao K, Fan Z, Sun L, Makam P, Tian Z, Ruegsegger M, Shaham-Niv S, Hansford D, Aizen R, Pan Z. Quantum confined peptide assemblies with tunable visible to near-infrared spectral range. Nat Commun. 2018;9(1):1–11.
Yi Z, Chen G, Chen X, Sun Z, Ma X, Su W, Deng Z, Ma L, Ran Y, Tong Q. Modular assembly of versatile nanoparticles with epigallocatechin gallate. ACS Sustain Chem Eng. 2020;8(26):9833–45.
Su W, Ran Y, Ma L, Ma X, Yi Z, Chen G, Chen X, Deng Z, Tong Q, Li X. Micro-/nanomechanics dependence of biomimetic matrices upon collagen-based fibrillar aggregation and arrangement. Biomacromol. 2020;21(9):3547–60.
Chen Y, Wang J, Rao Z, Hu J, Wang Q, Sun Y, Lei X, Zhao J, Zeng K, Xu Z. Study on the stability and oral bioavailability of curcumin loaded (-)-epigallocatechin-3-gallate/poly (N-vinylpyrrolidone) nanoparticles based on hydrogen bonding-driven self-assembly. Food Chem. 2022;378: 132091.
Chen X, Yi Z, Chen G, Ma X, Su W, Deng Z, Ma L, Tong Q, Ran Y, Li X. Carrier-enhanced photodynamic cancer therapy of self-assembled green tea polyphenol-based nanoformulations. ACS Sustain Chem Eng. 2020;8(43):16372–84.
Feng J, Sun Y, Song P, Lei W, Wu Q, Liu L, Yu Y, Wang H. Fire-resistant, strong, and green polymer nanocomposites based on poly (lactic acid) and core–shell nanofibrous flame retardants. ACS Sustain Chem Eng. 2017;5(9):7894–904.
Jansen R, Van Bekkum H. XPS of nitrogen-containing functional groups on activated carbon. Carbon. 1995;33(8):1021–7.
Certo M, Tsai C-H, Pucino V, Ho P-C, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21(3):151–61.
Ye M, Zhao Y, Wang Y, Yodsanit N, Xie R, Gong S. pH-responsive polymer–drug conjugate: An effective strategy to combat the antimicrobial resistance. Adv Funct Mater. 2020;30(39):2002655.
Song Z, Wu Y, Cao Q, Wang H, Wang X, Han H. pH-responsive, light-triggered on-demand antibiotic release from functional metal–organic framework for bacterial infection combination therapy. Adv Funct Mater. 2018;28(23):1800011.
Grotelüschen R, Heidelmann LM, Lütgehetmann M, Melling N, Reeh M, Ghadban T, Dupree A, Izbicki JR, Bachmann KA. Antibiotic sensitivity in correlation to the origin of secondary peritonitis: a single center analysis. Sci Rep. 2020;10(1):1–9.
Ma K, Dong P, Liang M, Yu S, Chen Y, Wang F. Facile assembly of multifunctional antibacterial nanoplatform leveraging synergistic sensitization between silver nanostructure and vancomycin. ACS Appl Mater Interfaces. 2020;12(6):6955–65.
Yang L, Wang C, Li L, Zhu F, Ren X, Huang Q, Cheng Y, Li Y. Bioinspired integration of naturally occurring molecules towards universal and smart antibacterial coatings. Adv Funct Mater. 2022;32(4):2108749.
Li H, Zhang J, Xue H, Li L, Liu X, Yang L, Gu Z, Cheng Y, Li Y, Huang Q. An injectable all-small-molecule dynamic metallogel for suppressing sepsis. Mater Horiz. 2023;10(5):1789–94.
Chen X, Zhu X, Gong Y, Yuan G, Cen J, Lie Q, Hou Y, Ye G, Liu S, Liu J. Porous selenium nanozymes targeted scavenging ROS synchronize therapy local inflammation and sepsis injury. Appl Mater Today. 2021;22:100929.
Ma B, Xu H, Zhuang W, Wang Y, Li G, Wang Y. Reactive oxygen species responsive theranostic nanoplatform for two-photon aggregation-induced emission imaging and therapy of acute and chronic inflammation. ACS Nano. 2020;14(5):5862–73.
Long L, Hu C, Liu W, Wu C, Lu L, Yang L, Wang Y. Injectable multifunctional hyaluronic acid/methylcellulose hydrogels for chronic wounds repairing. Carbohydr Polym. 2022;289:119456.
Mo X, Xiang H, Wei L, Xia L, Chen X, Chen Y, Zhang B. Nature-inspired allomelanin nanomedicine alleviates cardiac ischemia/reperfusion injury via scavenging free radicals and ameliorating myocardial microenvironment. Nano Today. 2022;46:101589.
Su H, Liu D-D, Zhao M, Hu W-L, Xue S-S, Cao Q, Le X-Y, Ji L-N, Mao Z-W. Dual-enzyme characteristics of polyvinylpyrrolidone-capped iridium nanoparticles and their cellular protective effect against H2O2-induced oxidative damage. ACS Appl Mater Interfaces. 2015;7(15):8233–42.
Bao X, Zhao J, Sun J, Hu M, Yang X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano. 2018;12(9):8882–92.
Ji X, Ge L, Liu C, Tang Z, Xiao Y, Chen W, Lei Z, Gao W, Blake S, De D. Capturing functional two-dimensional nanosheets from sandwich-structure vermiculite for cancer theranostics. Nat Commun. 2021;12(1):1–17.
Sun Y, Sun X, Li X, Li W, Li C, Zhou Y, Wang L, Dong B. A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization. Biomaterials. 2021;268:120614.
Ge C, Fang G, Shen X, Chong Y, Wamer WG, Gao X, Chai Z, Chen C, Yin J-J. Facet energy versus enzyme-like activities: the unexpected protection of palladium nanocrystals against oxidative damage. ACS Nano. 2016;10(11):10436–45.
Liu T, Xiao B, Xiang F, Tan J, Chen Z, Zhang X, Wu C, Mao Z, Luo G, Chen X. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11(1):1–16.
Lai Z, Jian Q, Li G, Shao C, Zhu Y, Yuan X, Chen H, Shan A. Self-assembling peptide dendron nanoparticles with high stability and a multimodal antimicrobial mechanism of action. ACS Nano. 2021;15(10):15824–40.
Wu S, Huang Y, Yan J, Li Y, Wang J, Yang YY, Yuan P, Ding X. Bacterial outer membrane-coated mesoporous silica nanoparticles for targeted delivery of antibiotic rifampicin against Gram-negative bacterial infection in vivo. Adv Funct Mater. 2021;31(35):2103442.
Lei Y, Wang K, Deng L, Chen Y, Nice EC, Huang C. Redox regulation of inflammation: old elements, a new story. Med Res Rev. 2015;35(2):306–40.
Liao X, Song X, Li J, Li L, Fan X, Qin Q, Zhong C, Yang P, Zhan J, Cai Y. An injectable co-assembled hydrogel blocks reactive oxygen species and inflammation cycle resisting myocardial ischemia-reperfusion injury. Acta Biomater. 2022;149:82–95.
Zhao X, Wang LY, Li JM, Peng LM, Tang CY, Zha XJ, Ke K, Yang MB, Su BH, Yang W. Redox-mediated artificial non-enzymatic antioxidant MXene nanoplatforms for acute kidney injury alleviation. Adv Sci. 2021;8(18):2101498.
Xi J, An L, Huang Y, Jiang J, Wang Y, Wei G, Xu Z, Fan L, Gao L. Ultrasmall FeS2 nanoparticles-decorated carbon spheres with laser-mediated ferrous ion release for antibacterial therapy. Small. 2021;17(13):2005473.
Tu C, Lu H, Zhou T, Zhang W, Deng L, Cao W, Yang Z, Wang Z, Wu X, Ding J. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286: 121597.
Yu Z, Zhao Y, Jin J, Zhu J, Yu L, Han G. Prevalence and risk factors of tigecycline-induced liver injury: A multicenter retrospective study. Int J Infect Dis. 2022;120:59–64.
Zhou G, Zhao W, Zhang C, Gao X, Cheng Q, Gao F. Manganese carbonyl-loaded hollow mesoporous silica nanoparticles coated with neutrophil membranes for acute kidney injury therapy. ACS Appl Nano Mater. 2022;5(3):4130–45.
Wei W, Cheng W, Dai W, Lu F, Cheng Y, Jiang T, Ren Z, Xie Y, Xu J, Zhao Q. A nanodrug coated with membrane from brain microvascular endothelial cells protects against experimental cerebral malaria. Nano Lett. 2021;22(1):211–9.
Zhao D, Li D, Cheng X, Zou Z, Chen X, He C. Mucoadhesive, antibacterial, and reductive nanogels as a mucolytic agent for efficient nebulized therapy to combat allergic asthma. ACS Nano. 2022;16(7):11161–73.
Funding
This work was supported by the project of National Key R&D Program of China (2022YFB3804500).
Author information
Authors and Affiliations
Contributions
JHD: writing-original draft, data extraction and analysis. TYW: investigation, editing of the manuscript. HTL, JHZ, HJZ and WJL participated in the analysis of the data. XCC and QF: supervision, project administration, funding acquisition. XRY, YWL and ZPG: reviewing, editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal studies were approved by the Animal Ethics Committee of West China Hospital, Sichuan University (WCHSIRB-D-2017-263).
Competing interests
The authors of this manuscript have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Fig. S1. Proposed reaction pathways for the preparation of NPs from EGCG and TG. Fig. S2. The SEM images of NP-i (i = 2–4), respectively. Fig. S3. TEM images of NP-1 corresponding to the element mapping shown in Figure 1c. Fig. S4. The possible intermediate structures in the reaction process corresponding to the peaks shown in Figure 1g. Fig. S5. SEM image of NP-1 after being soaked in phosphate-buffered saline (PBS) buffer at pH=5.5 for 120 hours. Fig. S6. Representative inhibition zone images of S. aureus and E. coil treated with/without NP-1. Fig. S7. Photographs of S. aureus and E. coli bacterial colonies growing on agar plates after treatment with NP-1 of different concentrations. Fig. S8. Viabilities of RAW264.7 cells treated with varying concentrations of EGCG, TG or NP-1 for 24 h. Fig. S9. AM/PI staining of RAW264.7 cells treated with NP-1 of different concentrations. Fig. S10. Hemolysis ratios of NP-1 with different concentrations (n=3). Fig. S11. Biochemical analysis of serum from mice treated by intraperitoneal injection of NP-1. Fig. S12. Histological analysis of kidney and liver tissue from mice treated with saline or NP-1. Fig. S13. Digital photographs of bacterial colony-forming units obtained from tissues in each group. Fig. S14. Representative images of Gram staining in each group of tissues. The blue circles are E. coli. Fig. S15. DAPI staining of each group for liver and kidney tissues. Table S1. Statistical parameters of prepared NP-i (i=1~4) NPs. Table S2. Specific parameters for the synthesis of NP-i (i=1~4), respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, J., Wang, T., Li, H. et al. Polyphenol-based antibacterial and antioxidative nanoparticles for improved peritonitis therapy. Collagen & Leather 5, 34 (2023). https://doi.org/10.1186/s42825-023-00141-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-023-00141-7