Abstract
Background
Sandhoff disease (SD) i s an autosomal recessive lysosomal disease with clinical manifestations such as epilepsy, psychomotor retardation and developmental delay. However, infantile SD with onset of infantile epilepsy spasm syndrome (IESS) is extremely rare.
Case presentation
The case presented here was a 22-month-old boy, who presented with IESS and psychomotor retardation/regression at 6 months of age. The patient showed progressive aggravation of seizures and excessive startle responses. The whole exome sequencing data, which initially revealed negative results, were reanalyzed and indicated a homozygous mutation at the c.1613 + 4del splice site of the HEXB gene. The activities of β-hexosaminidase A and total hexosaminidase were significantly decreased. The fundus examination showed cherry red spots at the macula.
Conclusions
IESS can be an epileptic phenotype of infantile SD. Clinical phenotypes should be adequately collected in genetic testing. In the case of negative sequencing results, gene variant reanalysis can be performed when the patients show clinically suspicious indications.
Similar content being viewed by others
Background
Infantile Sandhoff disease (SD; OMIM 268800) usually develops within the first 6 months of life, mostly with psychomotor retardation and regression as the first symptom. The characteristic clinical symptoms include seizures, excessive startle responses, hypotonia, cherry red spots on the fundus of the eye, and optic atrophy. Usually, the disease has a poor prognosis among children, with death occurring at two to three years of age [1]. The HEXB gene is the only gene known to cause SD [2]. It is located on chromosome 5q13.3 and contains 14 exons distributed in approximately 40 kb of DNA [1], encoding a β-subunit containing 556 amino acids. Mutations of the HEXB gene can cause deficiencies of β-hexosaminidase A (HexA) and B (HexB) enzymes, leading to abnormal degradation of GM2 ganglioside and resulting in disease [1].
Infantile epilepsy spasm syndrome (IESS) is a special developmental epileptic encephalopathy in infancy, which is characterized by clusters of epileptic spasms. Most IESS patients show quite abnormal interictal EEG features with hypsarrhythmia or multifocal discharges [3]. Convulsive seizures are often accompanied by slow development, arrest or regression.
Thus far, there have been no reports of infantile SD with onset of IESS. Here, we report a case of infantile SD with IESS as the predominant epileptic phenotype. This case was finally diagnosed with SD by reanalysis of the genetic raw data due to the remarkable clinical phenotype.
Case presentation
Clinical course
The case was a 22-month-old boy who was born at 38+1 weeks with a birth weight of 2.7 kg. He was born to healthy nonconsanguineous parents as a second child, with no significant family history of disease. At 6 months old, the child still could not sit steadily by himself, and rehabilitation training did not have any effect. At 11 months old, he could not roll over, sit alone, or grasp objects, and had poor eye-tracking and sound-tracking ability. The Peabody Motor Development Scale test showed that the child had approximately 4-month delay of motor development. After a respiratory infection at 13 months of age, the child showed obvious regression of psychomotor development and hypotonia. At the age of 16 months, he had seizures that manifested as nodding, lifting of both upper extremities, and clusters of epileptic spasms (Fig. 1a, b). At the age of 18 months, he had a tonic seizure with generalized rigidity and extension of both upper limbs, with head tilting back, eyes rolling upward, and loss of consciousness. The seizure was self-resolving and lasted for approximately 5 s. The frequency was a single attack every 1–2 weeks. Upon sound stimulation, the patient showed a startle response with postural changes. Video-EEG showed paroxysmal delta activities during wakefulness with multifocal epileptiform discharges significant in bilateral frontal regions, and a high degree of arrhythmia during sleep (Fig. 1c–f). Cranial MRI showed abnormal signals in the bilateral basal ganglia and periventricular white matter and a thin corpus callosum. The patient was diagnosed with infantile epileptic spasm syndrome. Topiramate, prednisolone acetate, clobazam and vigabatrin were added successively, but all showed poor efficacy.
EEG recordings of the case. a The background EEG at the age of 16 months. b EEG recordings at the attack period of at the age of 16 months. c The background EEG at the age of 22 months. d The EEG recordings during a tonic seizure at the age of 22 months. e Atypical high arrhythmia during sleep at the age of 22 months. f The EEG recordings during a sound-evoked startle response in the child, manifested as eye squeezing, nervousness, and shoulder lifting
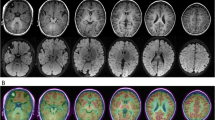
The patient was admitted to our hospital at the age of 22 months. Physical examination indicated that he was flaccid, lethargic and severely malnourished. The patient had a head circumference of 48.2 cm. The liver could be touched at 1 cm below the costal margin of the midline of the right clavicle, and the spleen was not palpable. The body weight was below the 3rd percentile of other boys of the same age. No autonomous movement or sound chasing was observed. His bilateral pupils were equal in size, round and sensitive to light reflection. He showed muscle hypotonia with a grade I strength. His breathing rate was 50 times/min, and thick breath sounds were present in both lungs with laryngeal stridor and coarse wet rales; phlegm in the throat was obvious after eating. Sudden sound stimulation could induce tonic seizures. Video-EEG showed slow waves in the bilateral frontal, parietal, occipital, and temporal lobes, mixed with multifocal sharp waves and spikes, slightly significant in bilateral frontal and temporal regions. Cranial MRI showed multiple abnormal brain signals and sulcus fissure widening (Fig. 2a–d). Prednisone acetate and aminoglutethimide were gradually discontinued, and sodium valproate and levetiracetam were added for anti-seizure treatment, but the efficacy was poor.
Cranial MRI of the case at age 22 months. a, b T2 weighted images; c, d T1 weighted images. Multiple symmetrical patches of slightly longer T1 and slightly longer T2 signals were seen in the white matter of the bilateral cerebral hemispheres and bilateral basal ganglia nuclei. Myelination of white matter is delayed. The corpus callosum is slender. The ventricular system, sulcus, fissure and pool are widened. e Fundus film of the case. The borders of the optic discs of both eyes were clear and pale, and the vascular pathways were acceptable, with cherry red spots visible in the macula. (white arrows)
Reanalysis of raw data of genetic sequencing for genetic variation
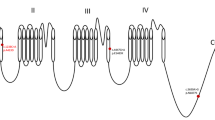
The whole exome sequencing (WES), copy number variant and mitochondrial DNA test data of all three members of the family obtained from the hospital of previous visits all showed negative results. Bioinformatics analysis was repeated on the raw data obtained, and after quality control, the clean reads were mapped to the UCSC hg19 human reference genome using the Sentieon BWA software. The parameter BWA of the Sentieon software was used to remove the duplicated reads and correct the base so that the mapped reads could be used for detecting variations. SNP and InDel variants were detected by the parameter driver. Then, the data were transformed into the VCF format. Variants were further annotated by the ANNOVAR software and associated with multiple databases, such as 1000 Genomes, ESP6500, dbSNP, EXAC, based on the inheritance patterns, variant types, population frequencies, and lists of genes associated with the main phenotypic characteristics of patient variants. Variant pathogenicity was predicted with the REVEL, SIFT, PolyPhen-2, MutationTaster and GERP + + softwares. Possible disease-associated variants were confirmed by Sanger sequencing. The reanalysis of the original data revealed that the child had a homozygous mutation at the c.1613 + 4del splicing site of the HEXB gene (Fig. 3a), and both parents had heterozygous mutations.
Complementary examinations, treatment and prognosis
Enzymatic activity assessment indicated that the activity of HexA was 16.6 nmol/g per minute (54.5–140.3) and that of the total hexosaminidase was 2.7 nmol/mg per hour (75.4–158.6), both significantly decreased. Ophthalmological examination of the fundus film showed a cherry red spot in the central fovea of each eye (Fig. 2e).
Combined with the clinical manifestations, enzymatic activity results and other auxiliary examinations, the patient was finally diagnosed with infantile SD (Fig. 3b). After oral administration of levocarnitine, vigabatrin, sodium valproate, levetiracetam and clobazam for 2 months, his seizure frequency was slightly reduced, swallowing improved, and weight increased.
Discussion
SD has been widely reported as a metabolic genetic disorder in the Jewish population in the United States s, but only a few cases have been reported in the Chinese population [4]. We search the Pubmed, MEDLINE and other databases and summarize the clinical manifestations of infantile SD with seizures reported in recent 20 years. The main forms of seizure in infantile SD are generalized tonic–clonic and myoclonus seizures (Table 1). Other common manifestations are exaggerated startle responses to noise, psychomotor developmental delay/regression and presence of macular cherry red spot. This boy experienced tonic, tonic–clonic and myoclonic seizures successively, which is consistent with the literature. However, IESS was the initial epilepsy phenotype, which had never been reported in the literature. This case expands the seizure phenotype of infantile SD.
The Human Phenotype Ontology (HPO) is a comprehensive resource for systematically defining and integrating human phenotypes and contributes to prediction of pathogenic genes. Studies have demonstrated the importance of deep phenotyping in improving the diagnostic power of clinical exome sequencing [15]. The phenotype-centric and phenotype-driven selection of genes can avoid missed diagnoses [16]. In this case, mental retardation and poor myelination were initially sent as HPO for testing, but the results were negative, which means no pathogenic gene consistent with clinical manifestations was found. Reanalysis of the original data after adding epilepsy, excessive startle response, and hypotonia to the HPO revealed a genetic variant that was consistent with the clinical phenotype. We speculate that the inadequate combination of the data with the clinical manifestations may have resulted in inaccurate phenotype entry, or that practitioners may have only focused on the positions of canonical “ ± 1–2” during analyzing, ignoring the possible impact of “ + 4” on splicing, resulting in the failure to detect any variant during the initial genetic testing of the case. The homozygous mutation of the c.1613 + 4del splice site of the HEXB gene in this child has not been previously reported. Our result extends the genetic spectrum of infantile SD.
Conclusions
IESS can be the epileptic phenotype of infantile SD. Genetic testing and enzymatic activity testing are helpful in obtaining a definitive diagnosis. Genetic testing results should be comprehensively analyzed in combination with clinical evaluations. Genetic factors cannot be ruled out for difficult-to-interpret clinical manifestations in the nervous system. If necessary, the original genetic data can be reanalyzed to provide supportive evidence for disease diagnosis.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
References
Tim-Aroon T, Wichajarn K, Katanyuwong K, Tanpaiboon P, Vatanavicharn N, Sakpichaisakul K, et al. Infantile onset Sandhoff disease: clinical manifestation and a novel common mutation in Thai patients. BMC Pediatr. 2021;21(1):22.
Stenson PD, Mort M, Ball EV, Evans K, Hayden M, Heywood S, et al. The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet. 2017;136(6):665–77.
Zuberi SM, Wirrell E, Yozawitz E, Wilmshurst JM, Specchio N, Riney K, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1349–97.
Liu M, Huang D, Wang H, Zhao L, Wang Q, Chen X. Clinical and Molecular Characteristics of Two Chinese Children with Infantile Sandhoff Disease and Review of the Literature. J Mol Neurosci. 2020;70(4):481–7.
Gowda VK, Amoghimath R, Srinivasan VM, Bhat M. Sandhoff Disease without Hepatosplenomegaly Due to Hexosaminidase B Gene Mutation. J Pediatr Neurosci. 2017;12(1):78–9.
Maulik K, Kumar S, Singh P, Saini AG. Microcephaly in infantile Sandhoff's disease. BMJ Case Rep. 2017;2017:bcr2017220912.
Muralidharan CG, Tomar RPS. Infantile Sandhoff Disease: Unusual presentation. Med J Armed Forces India. 2016;72(Suppl 1):S91–3.
Zhang W, Zeng H, Huang Y, Xie T, Zheng J, Zhao X, et al. Clinical, biochemical and molecular analysis of five Chinese patients with Sandhoff disease. Metab Brain Dis. 2016;31(4):861–7.
Jain A, Kohli A, Sachan D. Infantile Sandhoff’s disease with peripheral neuropathy. Pediatr Neurol. 2010;42(6):459–61.
Saouab R, Mahi M, Abilkacem R, Boumdin H, Chaouir S, Agader O, et al. A case report of Sandhoff disease. Clin Neuroradiol. 2011;21(2):83–5.
Tavasoli AR, Parvaneh N, Ashrafi MR, Rezaei Z, Zschocke J, Rostami P. Clinical presentation and outcome in infantile Sandhoff disease: a case series of 25 patients from Iranian neurometabolic bioregistry with five novel mutations. Orphanet J Rare Dis. 2018;13(1):130.
Sahyouni JK, Odeh LBM, Mulla F, Junaid S, Kar SS. Al Boot Almarri NMJ Infantile Sandhoff disease with ventricular septal defect a case report. J Med Case Rep. 2022;16(1):317.
Beker-Acay M, Elmas M, Koken R, Unlu E, Bukulmez A. Infantile Type Sandhoff Disease with Striking Brain MRI Findings and a Novel Mutation. Pol J Radiol. 2016;81:86–9.
Ozaal S, Jayasena S, Jayakody S, Schröder S, Jayawardana A, Jasinge E. Clinical Presentation and Genetic Heterogeneity Including Two Novel Variants in Sri Lankan Patients With Infantile Sandhoff Disease. Child Neurol Open. 2022;9:2329048X221139495.
Robinson PN. Deep phenotyping for precision medicine. Hum Mutat. 2012;33(5):777–80.
Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27(9):1389–97.
Acknowledgements
We are sincerely grateful to this patient and his parents, and all of our colleagues at the department of pediatric, the First Medical Center of Chinese PLA General Hospital.
Funding
This research was funded by the Capital’s Funds for Health Improvement and Research (No. 2022–1-5081).
Author information
Authors and Affiliations
Contributions
Jing Wang and Liping Zou analysed the case, draft and revised the manuscript. Qi Zhang and Qian Lu collected the data of the case, draft and revised the manuscript. Qiuhong Wang and Shuo Dun provide advice. All authors had read and approved the final manuscript prior to submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the the Institutional Review Board of Chinese PLA General Hospital(The ethics number: 2023–143). Written informed consent to participate in this study was provided by the participants’ legal guardian.
Competing interests
Author Liping Zou is the member of the Editorial Board for Acta Epileptologica, who was not involved in the journal’s review of, or decisions related to this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Q., Zou, L., Lu, Q. et al. Genetic variant reanalysis reveals a case of Sandhoff disease with onset of infantile epileptic spasm syndrome. Acta Epileptologica 6, 6 (2024). https://doi.org/10.1186/s42494-024-00149-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42494-024-00149-4