Abstract
Purpose
We aimed to evaluate the influence of technological advances on ablation outcomes in patients with persistent atrial fibrillation (AF) (PeAF). Radiofrequency ablation for patients with AF has advanced, including contact force (CF)-sensing catheters and the ablation index (AI).
Methods
Between 2009 and 2018, we analyzed 173 patients with PeAF who underwent catheter ablation. We categorized them into three groups: AF ablation without CF and AI information (no-CF group, n = 63), with CF without AI (CF-only group, n = 49), and with optimal AI-guided ablation (AI group, n = 61). Early (within 3 months, ER) and late (from 3 months to 1 year, LR) AF recurrence after ablation was assessed. Procedure-related complications were also evaluated.
Results
The baseline characteristics were similar among the 3 groups, excluding the baseline antiarrhythmic drug history. Additional substrate modification after pulmonary vein isolation was significantly low in frequency in the AI group (71.4%, no-CF; 69.4%, CF-only; 41.0%, AI, p = 0.001). The AI group had a shorter mean procedure-related time than the other groups. Both ER and LR of PeAF showed a trend of reduction with technological advances. With a short experience (less than 1 year), the CF-only group showed more ER and LR than that shown by the AI group. However, with a long experience (more than 1 year), ER and LR occurred similarly in the two groups. Procedure-related complications improved with technological advances.
Conclusion
As ablation technology advanced, favorable clinical outcomes with short procedural times were observed. However, prospective, large multicenter studies are needed to verify these results.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia, and the global incidence and prevalence of AF have increased continuously over the past 20 years [1]. The prevalence rates of paroxysmal AF (PAF) and persistent AF (PeAF) have been reported to be similar [2], and the progression rate from PAF to PeAF has been reported to be approximately 36% [3]. PeAF is associated with a higher risk of thromboembolic events and worse survival outcomes than those associated with PAF [4].
Catheter ablation is used effectively and safely for AF rhythm control worldwide [5]. Catheter ablation shows better rhythm control than that shown by antiarrhythmic drugs in patients with symptomatic AF [6,7,8]. Recent guideline recommends AF catheter ablation for pulmonary vein (PV) isolation (PVI) as a class I indication to control rhythm and improve symptoms related to recurrent AF in patients who experience failure of antiarrhythmic drug treatment [9]. Regarding the technique of AF ablation, complete PVI is the cornerstone of all AF catheter ablation procedures; however, the benefits of additional substrate modification beyond PVI are not well established [9].
During AF catheter ablation, adequate electrode–tissue contact is important for complete PVI [10]. Before introducing a contact force (CF)-sensing catheter, indirect signs of tissue contact such as tactile feedback, fluoroscopy images, electrogram diminution, and impedance changes are used as surrogate markers of tissue contact [11]. Catheter ablation using CF-sensing catheters reduces the procedure time and arrhythmia recurrence compared to those associated with catheter ablation using non-CF-sensing catheters in patients with PeAF [12]. Recently, the ablation index (AI) has been developed as a marker of ablation lesion quality, integrated time and power, and CF [13]. When optimal AI-guided ablation is compared to CF-guided ablation, AI-guided ablation improves the acute and long-term outcomes of PVI [14,15,16]. Optimal AI-guided ablation shows a high rate of clinical success and improved durability of PVI for PeAF [17]. However, whether these technological advances, including CF-sensing technology and AI-guided ablation, improve the efficacy and safety outcomes of catheter ablation for PeAF remains unclear. Therefore, in this study, we aimed to evaluate the influence of technological advances on ablation outcomes in patients with PeAF.
Methods
Study population
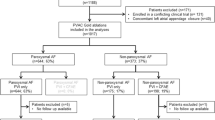
This was a single-center, retrospective study. Adult patients with AF who underwent radiofrequency (RF) catheter ablation between 2009 and 2018 at our institution were enrolled. Finally, 165 patients with PeAF were selected for the analysis. The study protocol was authorized by the Institutional Review Board of Seoul National University Hospital (H21011581191).
Covariates
Baseline clinical information was collected at the time of the first ablation for PeAF based on a retrospective review of electronic medical records. The patients’ age, sex, and comorbidities were also collected. Hypertension was defined based on a previous diagnosis of hypertension, current anti-hypertensive medications, or systolic/diastolic blood pressure higher than 140/90 mmHg. Diabetes mellitus was defined based on a previous diagnosis of diabetes mellitus, current anti-diabetic medications, or fasting blood glucose levels > 126 mg/dL in repeated tests. Dyslipidemia was defined based on a previous diagnosis of dyslipidemia, current anti-dyslipidemia medications, or low-density lipoprotein cholesterol levels > 130 mg/dL. Coronary artery disease was defined on the basis of a previous diagnosis of coronary artery disease or significant stenosis of the coronary arteries on invasive coronary angiography or coronary computed tomography angiography. Heart failure was defined based on a previous diagnosis of heart failure, symptoms of heart failure, or left ventricular dysfunction on echocardiography. Stroke/systemic embolism was defined based on previous diagnoses. The CHA2DS2-VASc score was calculated based on each patient’s medical history. The body mass index was calculated as body weight divided by height in square meters (kg/m2). Echocardiography measurements were taken from the study performed within 1 month of the ablation date. Information on baseline antiarrhythmic drugs, including propafenone, flecainide, pilsicainide, amiodarone, dronedarone, and sotalol, was also obtained.
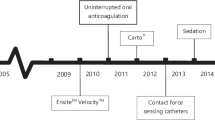
Technological advances in catheter ablation
The study patients were categorized into 3 groups based on technological advances in catheter ablation as follows: ablation without CF and AI information (no-CF group), ablation with CF without AI information (CF-only group), and AI-guided ablation (AI group). Since both CF and the AI are recently developed technologies, these strategies may require a learning curve for optimal effects. In this respect, the CF-only and AI groups were divided again based on the operator’s experience (less than 1 year and 1 year after exposure to the new use of each technology) to assess the impact of proficiency in the use of these new technologies.
Ablation protocol: pulmonary vein antrum isolation and additional linear or focal ablation
The protocol for PVI and additional substrate modification in our hospital has been described previously [14, 18, 19]. In brief, under deep conscious sedation, we performed single or double transseptal puncture(s) and introduced SL1 non-steerable long sheath(s) into the left atrium (LA). Three-dimensional (3D) electroanatomical geometry mapping of the LA and PV was performed using the CARTO 3 system (Biosense Webster, Inc., Diamond Bar, CA, USA) and/or merged with a reconstructed computed tomography image of the LA and PVs. For PVI, a circumferential lesion set for each PV was created using point-by-point RF ablation. The RF power ranged from 25 to 35 W at the anterior wall and 20 to 30 W at the posterior wall. In the CF-only group, the CARTO Visitag module (Biosense Webster, Inc., Diamond Bar, CA, USA) was used to display real-time ablation parameters including CF, ablation time, power, and impedance at each ablation location. During CF-guided ablation, the operators targeted the CF from 5 to 20 g [18]. In both the no-CF and CF-only groups, the ablation duration per point was 20–40 s to achieve local electrogram signal abolition by > 80% and determined at the operators’ discretion. In the AI group, the ablation power and CF target were similar to those in the no-CF and CF-only groups, but the CARTO Visitag module and target AI values were ≥ 450 for the anterior wall and ≥ 350 for the posterior wall (named OPTIMUM protocol) [14]. In all three groups, the maximal interlesion diameter between neighboring lesions was 4 mm. In addition, for the ablation of inferior/posterior regions of the left inferior PV, 25 W of RF energy was applied for 15 s to prevent the occurrence of esophageal injury. If PVI was not achieved after first-pass ablation or early reconnection was confirmed, additional touch-up ablation was delivered until isolation was achieved. Additional linear ablation or ablation for a non-PV trigger was performed, if needed at the physicians’ discretion. If AF persisted after PVI and additional ablation, internal or external direct current cardioversion was performed.
Efficacy and safety assessment
Twelve-lead electrocardiography was performed during follow-up visits 1, 3, 6, 9, and 12 months after the index ablation procedure, and 24-h Holter monitoring was performed at the 3- and 12-month follow-up visits. The clinical recurrence of AF for efficacy assessment was defined as documented atrial tachycardia or AF persisting for longer than 30 s on 12-lead ECG and 24-h Holter monitoring. Early recurrence (ER) of AF was defined as AF recurrence within 3 months after the index ablation procedure. Late recurrence (LR) of AF was defined as AF recurrence 1 year after ablation. For efficacy assessment, ER and LR of AF were evaluated. Cardiac tamponade was evaluated for safety assessments.
Statistical analysis
Continuous variables are expressed as means ± standard deviations, and categorical variables are expressed as n (%). Student’s t test was used to compare continuous variables, and Pearson’s Chi-square test or Fisher’s exact test was used to compare categorical variables between the presence and absence of events. One-way analysis of variance was conducted to evaluate differences among the groups with different ablation strategies. Cox proportional hazard analysis was performed to determine independent associations between ablation technologies and AF recurrence. The confounding factors included age, sex, body mass index, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, congestive heart failure, stroke, systemic embolism, antiarrhythmic drugs, and AF duration. Kaplan–Meier survival curves were generated using a log-rank test to show differences in freedom from recurrence among the different ablation strategies. P values < 0.05 were considered statistically significant. All statistical analyses were conducted using SPSS version 25 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
The baseline characteristics of the study patients are described in Table 1. A total of 173 patients with PeAF were divided into three groups according to technological advances in catheter ablation: 36.4% (n = 63) in the no-CF group, 28.3% (n = 49) in the CF-only group, and 35.3% (n = 61) in the AI group. The mean age was 57.8 years, and 77.5% were males. There were no significant differences in the prevalence of comorbidities among the three groups. The mean duration of PeAF after the first diagnosis was 4.7 ± 5.1 years, and the mean CHA2DS2-VASc score was 1.7 ± 1.3. There were no significant differences in the duration of PeAF and CHA2DS2-VASc scores among the three groups. The prevalence of patients who did not take any antiarrhythmic drugs before ablation was 6.9%, and those of patients who took class Ic and class III drugs were 47.4% and 45.7%, respectively. Patients in the no-CF group used more class III antiarrhythmic drugs than those in the other groups (p = 0.018). The mean left ventricular ejection fraction was 57.7 ± 5.4%, and the LA anteroposterior diameter was 45.9 ± 6.5 mm. Among the three groups, echocardiography measurements showed no significant differences.
Procedural data
The procedural data of each group are described in Table 2. Only 9 patients in the no-CF group underwent ablation without anesthesia. All other patients underwent ablation with deep sedation. Over half of the AI group (59.0%) had PVI alone, with or without cavotricuspid isthmus (CTI) ablation. More than two-thirds of the no-CF and CF-only groups (71.4% and 69.4%, respectively) underwent additional substrate modifications including those involving the LA roof, inferior, anteromedial and anterolateral wall ablation, mitral isthmus, CTI, superior vena cava, appendage, coronary sinus, ligament of Marshall, complex fractionated atrial electrograms, and ganglionated plexi ablation. With advances in ablation technology, the total procedure time, ablation time, and fluoroscopy time were significantly reduced (no-CF group 340 ± 86 min, 98 ± 29 min, 83 ± 50 min; CF-only group 244 ± 51 min, 91 ± 29 min, 26 ± 8 min; AI group 188 ± 40 min, 72 ± 20 min, 20 ± 10 min; p < 0.001, respectively). When we looked into the total procedure time in a subgroup who received PVI only (± CTI ablation) without additional substrate modification, the results were consistent with the main results, including the total study population.
Procedural outcomes: efficacy and safety
The prevalence of ER and LR of AF in each group is shown in Fig. 1. ER in the no-CF and AI groups showed a significant difference (55.6% vs. 32.8%, p = 0.011). In addition, there were statistically non-significant trends of reduction in both ER and LR with technological advances. Figure 2 presents a comparison of the cumulative probability of freedom from recurrence among the three groups. In the Kaplan–Meier curves for ER, the CF-only and AI groups showed a significantly lower recurrence of atrial tachyarrhythmia than that in the no-CF group, whereas the CF-only and AI groups did not show significant differences. Regarding LR, there was a similar trend as that in ER, but the log-rank test among the three groups showed no significant differences. Cox proportional hazard analysis for ablation technologies and AF recurrence is shown in Table 3. In univariate analysis, the CF-only and AI groups showed a significantly lower risk of ER and numerical trend of lower risk of LR. In multivariable analysis after adjustment for potential confounders including age, sex, body mass index, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, congestive heart failure, stroke, systemic embolism, antiarrhythmic drugs, AF duration, and ablation strategy, the significance of ER in the AI group disappeared, but the numerical trend of a lower risk of LR in the AI group strengthened in the AI group. Regarding safety assessments, cardiac tamponade occurred only in the no-CF group (n = 6, 10.5%).
The CF-only and AI groups were divided based on 1 year of operator experience (Fig. 3). The CF-only group showed a numerically higher rate of ER and LR than that in the AI group when the operator's experience was less than 1 year. In contrast, the rates of ER and LR were similar between the CF-only and AI groups when the operator's experience was more than 1 year.
Discussion
This study was performed to evaluate how technological advances influence ablation outcomes in patients with PeAF. The major findings of this study were as follows: (1) the clinical outcomes of ER and LR may have improved as technology advanced, despite a PVI-only strategy; (2) the procedural time shortened and procedure-related cardiac tamponade decreased with technological advances; and (3) although the clinical outcome of AF ablation only with CF was influenced by the operator’s exposure time after new technology introduction, optimal AI-guided AF ablation was less affected by the operator’s exposure time.
The process of appropriate ablation lesion formation is affected by multiple factors, including electrode temperature, power, and duration of energy delivery [20]. It is well known from a few decades prior that optimal tissue contact of the ablation catheter is a key factor for optimal lesion formation [21]. Adequate contact is correlated with increased lesion width, volume, and depth [22, 23]. Many indirect signs, including tactile feedback, fluoroscopy images, electrogram diminution, and impedance changes, were used until the CF-sensing catheter was introduced.
The site at which ablation is performed and extent of ablation are also important factors that may influence the clinical outcomes of AF ablation. The previous guideline suggested considering more extensive ablation than conventional PVI for PeAF [24]. However, the STAR-AF II trial revealed no advantage of additional substrate modification (ablation of complex fractionated electrograms and linear ablation) over conventional PVI for PeAF [25]. This result was reproduced in the randomized Alster-Lost-AF trial [26]. Other studies that compared PVI with additional posterior wall isolation or linear ablation showed consistent results [27,28,29]. Recent guidelines underline the importance of durable PVI and suggest that PVI should be considered as first-line therapy, even for PeAF [9]. The data from a landmark randomized clinical trial [30] and a subsequently reported meta-analysis [31] showed that the addition of complex fractionated atrial electrograms and left atrial linear ablation offers no significant improvement in arrhythmia-free survival, in comparison with PVI alone, in patients with persistent AF. Based on this evidence, the benefit of additional ablation beyond PVI in patients with persistent AF is not well established and has been recommended as class IIb in the current guidelines [9, 32]. We assumed that these results affected physicians’ decisions; as a result, the proportion of patients receiving additional ablation beyond PVI decreased from non-CF, CF to AI groups.
The SMART-AF trial is the first study to demonstrate the safety and effectiveness of an irrigated CF-sensing catheter for the treatment of paroxysmal AF [33]. In this trial, 12-month freedom from symptomatic AF recurrence was significantly higher in patients whose CF working range remained within the selected range (5 to 40 g) more than 80% of the time during ablation than that in those whose working range remained within the selected range less than 80% of the time. Similar results were obtained for PeAF in other studies, including the recent PRECEPT trial [34]. In particular, the TOCCASTAR trial demonstrated the importance of optimal CF with clinical outcomes [35]. In this trial, the optimal CF group showed a significantly higher success rate than that of the non-optimal CF group. This result emphasizes the importance of optimal CF for durable PVI, which reduces the recurrence of AF. CF-sensing catheters also result in improved safety outcomes. In our study, procedure-related cardiac tamponade was not reported after the introduction of the CF-sensing catheter. A previous study revealed better outcomes with increased experience with the CF-sensing technology [36]. Our result showed a similar trend in the CF-only group, although this was not statistically significant.
The force–time integral (FTI) does not incorporate power; thus, a novel lesion quality marker named the AI, which utilizes CF, time, and power in a weighted formula, was developed. When blinded to the AI, the FTI does not secure the optimal AI range on its own, and vice versa [16]. This novel marker independently predicted reconnection of the PVI segment, and the requirement of a higher AI value was demonstrated in some critical segments to prevent reconnection [13]. The OPTIMUM trial has shown improvement in acute outcomes with optimal AI-guided PVI [14]. In the PRAISE trial with optimal AI-guided AF ablation, late PV reconnection was significantly lower than in other studies that did not use the AI [17]. Meta-analysis of observational studies has shown lower PV reconnection with an AI-guided procedure compared to that with a CF-only guided procedure [37]. In our study, these results may be associated with a longer learning curve for the optimal outcome of AF ablation with CF only than with the AI. We may expect consistent outcomes of the treatment of AF with optimal AI-guided ablation.
As mentioned, data have been reported that the introduction of AI and AI-targeted ablation in PVI improves outcome and reduces procedure time compared to CF alone. In this study, it was found that these benefits appear differently depending on the operators’ experience (Fig. 3). We would like to interpret that, for less experienced operators, these technologic advance can shorten the learning curve and help standardize the procedure.
Limitations
This study had some limitations. First, this was a retrospective, single-center study. Second, the number of subjects enrolled in this study was small. Further evaluation in a prospective and larger multicenter study is needed to verify the results of this study. Third, most of the results only exhibited numerical trends. Fourth, the class of baseline antiarrhythmic drugs was different among the groups. The number of patients who used class III drugs was twice that of those who used class Ic drugs in the no-CF group. In contrast, class Ic drugs were used by more patients in the CF-only and AI groups. The most notable limitation was that different ablation technologies were applied with technological advances over time, not simultaneously. Although operators must learn new technology initially, they developed proficiency at the time of introduction of the new technology because the main stem of the AF ablation technique was not changed. In our study, the fact that a shorter experienced operator was only involved in the AI group, not in the other two groups, may have diluted the increased proficiency with time flows. Fifth, there is a possibility that LA substrate has not much progressed in patients receiving PVI only, who were included larger proportion in AI group than other groups. Finally, other technological advances such as a multi-electrode catheters, the 3D merge system, or the CARTO sound system, were not considered in this study. These technologies may have influenced our results.
Conclusion
In conclusion, more tolerable procedural times and favorable clinical outcomes are expected with advances in ablation technology, although prospective multicenter studies are needed to verify these results.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- PAF:
-
Paroxysmal atrial fibrillation
- PeAF:
-
Persistent atrial fibrillation
- PVI:
-
Pulmonary vein isolation
- CF:
-
Contact force
- AI:
-
Ablation index
- LA:
-
Left atrium
- 3D:
-
Three-dimensional
- PV:
-
Pulmonary vein
- RF:
-
Radiofrequency
- ER:
-
Early recurrence
- LR:
-
Late recurrence
- CTI:
-
Cavotricuspid isthmus
- FTI:
-
Force–time integral
References
Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2020. https://doi.org/10.1177/174749301989787.
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20.
Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian P, Green MS, Humphries KH, Klein GJ, Sheldon R, Talajic M, Kerr CR. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm. 2017;14:801–7.
Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, Becker RC, Singer DE, Halperin JL, Hacke W, Nessel CC, Berkowitz SD, Mahaffey KW, Fox KA, Califf RM, Piccini JP, Committee R-AS and Investigators. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:288–96.
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8.
Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clementy J, Haissaguerre M. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–505.
Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli R, Raviele A, Themistoclakis S, Rossillo A, Bonso A, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–40.
Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA, ThermoCool AFTI. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–40.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL and Group ESCSD. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498
Avitall B, Mughal K, Hare J, Helms R, Krum D. The effects of electrode-tissue contact on radiofrequency lesion generation. Pacing Clin Electrophysiol. 1997;20:2899–910.
Rolf S, Hindricks G, Sommer P, Richter S, Arya A, Bollmann A, Kosiuk J, Koutalas E. Electroanatomical mapping of atrial fibrillation: review of the current techniques and advances. J Atr Fibrillation. 2014;7:1140.
Hussein AA, Barakat AF, Saliba WI, Tarakji KG, Bassiouny M, Baranowski B, Tchou P, Bhargava M, Dresing T, Callahan T, Cantillon D, Kanj M, Lindsay BD, Wazni OM. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol. 2017;28:483–8.
Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed Y, Bonnett LJ, Waktare JEP, Todd DM, Hall MCS, Snowdon RL, Modi S, Gupta D. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace. 2017;19:775–83.
Lee SR, Choi EK, Lee EJ, Choe WS, Cha MJ, Oh S. Efficacy of the optimal ablation index-targeted strategy for pulmonary vein isolation in patients with atrial fibrillation: the OPTIMUM study results. J Interv Card Electrophysiol. 2019;55:171–81.
Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Y, Tavernier R, Duytschaever M. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ’CLOSE’-protocol. Europace. 2018;20:f419–27.
Munkler P, Kroger S, Liosis S, Abdin A, Lyan E, Eitel C, Eitel I, Meyer C, Willems S, Heeger CH, Tilz RR. Ablation index for catheter ablation of atrial fibrillation- clinical applicability and comparison with force-time integral. Circ J. 2018;82:2722–7.
Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni A, Shaw M, Todd D, Hall M, Modi S, Natale A, Dello Russo A, Snowdon R, Gupta D. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE study results. Circ Arrhythm Electrophysiol. 2018;11: e006576.
So-Ryoung L, Hyoung-Seob P, Eue-Keun C, Soonil K, Youngjin C, Il-Young O, Seil O, Seongwook H. Contact force-guided ablation reduced poor contact segments and improved acute reconnection in patients with atrial fibrillation. J Atr Fibrillation. 2020;12:2185.
Lee SR, Park HS, Choi EK, Lee E and Oh S. Acute and long-term efficacy of ablation index-guided higher power shorter duration ablation in patients with atrial fibrillation: A prospective registry. J Arrythm. 2021.
Ariyarathna N, Kumar S, Thomas SP, Stevenson WG, Michaud GF. Role of contact force sensing in catheter ablation of cardiac arrhythmias: evolution or history repeating itself? JACC Clin Electrophysiol. 2018;4:707–23.
Haines DE. Determinants of lesion size during radiofrequency catheter ablation: the role of electrode-tissue contact pressure and duration of energy delivery. J Cardiovasc Electrophysiol. 1991;2:509–15.
Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, Ikeda A, Pitha JV, Sharma T, Lazzara R, Jackman WM. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354–62.
Wong MC, Edwards G, Spence SJ, Kalman JM, Kumar S, Joseph SA, Morton JB. Characterization of catheter-tissue contact force during epicardial radiofrequency ablation in an ovine model. Circ Arrhythm Electrophysiol. 2013;6:1222–8.
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr., Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D, Heart Rhythm Society Task Force on C and Surgical Ablation of Atrial F. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696 e21.
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P, Investigators SAI. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22.
Fink T, Schluter M, Heeger CH, Lemes C, Maurer T, Reissmann B, Riedl J, Rottner L, Santoro F, Schmidt B, Wohlmuth P, Mathew S, Sohns C, Ouyang F, Metzner A and Kuck KH. Stand-Alone Pulmonary Vein Isolation Versus Pulmonary Vein Isolation With Additional Substrate Modification as Index Ablation Procedures in Patients With Persistent and Long-Standing Persistent Atrial Fibrillation: The Randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation). Circ Arrhythm Electrophysiol. 2017;10.
Sutter JS, Lokhnygina Y, Daubert JP, Bahnson T, Jackson K, Koontz JI, Sun AY, Hegland DD, Thomas KL, Jackson L, Lewis R, Granger C, Piccini JP, Atwater BD. Safety and efficacy outcomes of left atrial posterior wall isolation compared to pulmonary vein isolation and pulmonary vein isolation with linear ablation for the treatment of persistent atrial fibrillation. Am Heart J. 2020;220:89–96.
Lee JM, Shim J, Park J, Yu HT, Kim TH, Park JK, Uhm JS, Kim JB, Joung B, Lee MH, Kim YH, Pak HN, Investigators P-A. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin Electrophysiol. 2019;5:1253–61.
Jin MN, Lim B, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, Hwang C, Pak HN. Long-term outcome of additional superior vena cava to septal linear ablation in catheter ablation of atrial fibrillation. J Am Heart Assoc. 2019;8: e013985.
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P. STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812–22.
Scott PA, Silberbauer J, Murgatroyd FD. The impact of adjunctive complex fractionated atrial electrogram ablation and linear lesions on outcomes in persistent atrial fibrillation: a meta-analysis. Europace. 2016;18(3):359–67.
Yu HT, Jeong DS, Pak HN, Park HS, Kim JY, Kim J, Lee JM, Kim KH, Yoon NS, Roh SY, Oh YS, Cho YJ, Shim J. Korean guidelines for catheter ablation of atrial fibrillation: Part II. Int J Arrhythm. 2018;19(3):235–84.
Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, Kantipudi C, Mansour MC, Melby DP, Packer DL, Nakagawa H, Zhang B, Stagg RB, Boo LM, Marchlinski FE. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol. 2014;64:647–56.
Mansour M, Calkins H, Osorio J, Pollak SJ, Melby D, Marchlinski FE, Athill CA, Delaughter C, Patel AM, Gentlesk PJ, DeVille B, Macle L, Ellenbogen KA, Dukkipati SR, Reddy VY, Natale A. Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol. 2020;6:958–69.
Reddy VY, Dukkipati SR, Neuzil P, Natale A, Albenque JP, Kautzner J, Shah D, Michaud G, Wharton M, Harari D, Mahapatra S, Lambert H, Mansour M. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the tacticath contact force ablation catheter study for atrial fibrillation (TOCCASTAR) Study. Circulation. 2015;132:907–15.
Barbhaiya CR, Knotts RJ, Bockstall K, Bernstein S, Park D, Holmes D, Aizer A, Chinitz LA. Contact-force radiofrequency ablation of non-paroxysmal atrial fibrillation: improved outcomes with increased experience. J Interv Card Electrophysiol. 2020;58:69–75.
Pranata R, Vania R, Huang I. Ablation-index guided versus conventional contact-force guided ablation in pulmonary vein isolation - Systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2019;19:155–60.
Acknowledgements
None.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korean Government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (Project Number: 202013B14) and by the Korea National Research Foundation funded by the Ministry of Education, Science and Technology (Grant 2020R1F1A106740).
Author information
Authors and Affiliations
Contributions
Dr. SRL has concepted and designed the study, WKJ performed the analysis, and all authors contributed to the analysis, interpretation of the data, and drafting of the manuscript. All authors have reviewed and approved the submission of the paper to the journal.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was authorized by the Institutional Review Board of Seoul National University Hospital (H21011581191). This is a retrospective study and informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeon, W.K., Lee, SR., Choi, EK. et al. Clinical outcomes in patients with persistent atrial fibrillation after technologic advances including contact force-guided and ablation index-guided ablation. Int J Arrhythm 23, 13 (2022). https://doi.org/10.1186/s42444-022-00064-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-022-00064-0