Abstract
Background
Grewia flava infusions are consumed to assist with kidney problems and stomach ailments. However, there are no scientific data on the phytochemical profile or biological properties to validate its folklore use. Thus, the study aimed to assess the phytochemical profile, antioxidant, and antimicrobial activities of Grewia flava twig extracts.
Methodology
The antioxidant activities of the extracts were assayed using 1,1-diphenyl-2-picrylhydrazyl radical scavenging, reducing power, metal chelation, and total phenolic and flavonoid content assays. The agar well diffusion and microdilution methods were used for crude extracts and fractions (from 80% methanol extract) for antimicrobial screening against P. aeruginosa, S. aureus, E. coli, B. subtilis, A. niger, and R. oryzae.
Results
The 80% methanol twig extract (250.00 ± 2 GAE/g) exhibited a high concentration of phenolic content, followed by the distilled water extract (192.00 ± 2 mg GAE/g) and the hexane extract (43.10 ± 0.2 mg GAE/g). Fraction 14 of the methanol twig extract exhibited MIC values of 0.21–0.31 mg/mL against all test microorganisms. The root and twig extracts exhibited significant antioxidant and antimicrobial activities, which were attributed to the extracts of bioactive phytochemical compounds such as alkaloids, flavonoids, saponins, steroids, glycosides, anthraquinones, and tannins that were detected in the extracts. Also, the root and twig non-polar extracts were subjected to gas chromatography–mass spectrometry analysis, which identified several bioactive compounds like betulin, β-amyrin, palmitic acid, lupenone, and phytol, highlighting the potential of the plant species as a botanical drug.
Conclusions
The study supports the traditional use of plant roots and twigs for treating various ailments, indicating their medicinal value. The twigs can be used in place of the roots to guarantee Grewia flava harvesting that is sustainable. However, a comparison of the quantities of the active compound in the twigs and roots using LC–MS is crucial.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Throughout history, medicinal plants have been utilized worldwide for treating various illnesses, particularly in rural communities of developing countries, where these plants were available and they were considered to be more effective and at a lower cost when compared to synthetic drugs (Motamedi et al. 2010; Motlhanka and Nthoiwa 2013; Sinha et al 2015). Medicinal plant extracts have been shown to have bioactive phytochemicals such as tannins, terpenoids, alkaloids, anthraquinones, flavonoids, and saponins, which exhibit ethnopharmacological properties (Dhawan and Gupta 2017; Kaur et al. 2024). Herbal infusions and decoctions are rich in phenolic compounds, which have shown potent antioxidant properties. In the human body, antioxidants are known to work against both free radicals and reactive oxygen species. Synthetic antioxidants are commonly used to prevent and treat chronic diseases, but their toxicity has led to a search for safer natural alternatives (Chiavaroli et al. 2011; Ahmed et al. 2019; Stobiecka et al. 2022).
Grewia flava, which is referred to as a raisin tree or brandy bush in English and commonly known as moretlwa, moseme, and ntewa in Tswana. It is a shrub in the Malvaceae family. It is widespread in the drier bush land and deciduous woodlands in the northern, central, and eastern parts of Botswana, as well as in South Africa, Zimbabwe, Namibia, and Eswatini (Mashungwa et al. 2019). Typically growing up to 2–3 m tall, Grewia flava has grayish-brown young branchlets that turn dark purplish to black as they age. It produces edible reddish-brown globular fruits (Lamola et al. 2017). According to folklore medicine, the plant's twigs are used to prepare herbal tea that is believed to help with kidney problems, while a mixture of stem bark, roots, and milk is thought to aid stomach problems caused by bacterial infections (Mashungwa et al. 2019). Dried berries are consumed in their natural form or can be used to make porridge and can also be fermented to make a local Tswana wine called khadi, which is believed to have antioxidant properties (Motlhanka and Nthoiwa 2013; Motlhanka et al. 2018, 2020). Previous studies have focused mainly on the ethnobotanical properties of the roots, barks, leaves, and berries of Grewia flava. In contrast, this study profiled the phytochemical properties of the plant by determining, the antioxidant, and antimicrobial activities of the twigs, which have been minimally researched. The use of Grewia flava twigs over roots would be more sustainable and is, therefore, recommended. This study determined the chemical profile, in vitro antioxidant, and antimicrobial activities of Grewia flava twigs.
Methods
Sample collection and preparation
G. flava twigs and roots were collected in October 2020 from Mmashoro village in the Central district of Botswana. Coordinates: 21°48′15.9′′S 26°27′22.4′′E, Botswana. The plant (Fig. 1) was identified at the University of Botswana herbarium by Dr. Mbaki Muzila, voucher number MZ002_2022. The twigs and roots were washed and left to air dry for two weeks. Plant samples were placed in zip-lock bags after being powdered and stored at room temperature in a locker.
Extraction and fractionation
The dried samples were pulverized (model; pulverisette 5 FRITSCH) for 30 min. Powdered twig and root samples of G. flava were macerated in n-hexane, acetone, distilled water (DW), and 80% methanol. In brief, 100 g of G. flava ground samples was extracted repeatedly with 500 mL of n-hexane (Hex), acetone (Ace), distilled water, and 80% methanol in water (80% MeOH). The Whatman No. 1 filter paper was used to filter the 24-h extracts, followed by solvent removal using a rotary evaporator. The extracts were stored in the fridge at 4 °C. The twig methanol extract was fractionated using a Merck silica gel, 60–80-mesh column chromatography. The column was subjected to different solvent systems of increasing polarity, starting with mixtures of n-hexane: ethyl acetate (EtOAc) (4:0, 4:1, 2:1, 4:3, 1:1), followed by mixtures of EtOAc: MeOH. (4:0, 4:1, 2:1, 4:3, 1:1). The column fractions Rf were monitored by thin-layer chromatography (TLC) (Silica gel 60, UV254). Similar fractions based on TLC analysis were pooled together, resulting in 24 column fractions.
Antioxidant activity assays
Total phenolic content (TPC)
The total phenolic content of the crude extracts was evaluated using the Folin-Ciocalteu assay as previously described (Lfitat et al. 2020; Maigoda et al. 2022). A UV–Vis spectrophotometer was used to measure absorbance at 725 nm for the aliquots. Aqueous methanol (80%) was used as a blank. All the experiments were repeated three times.
Total flavonoid content (TFC)
The aluminum chloride colorimetry method (Atere et al. 2018; Hmamou et al. 2022) was used to evaluate the total flavonoid content (TFC) of the crude extracts. Quercetin was used as the standard. TFC was expressed as mg quercetin equivalents per gram of the crude extract (mg QUE/g of the crude extract).
DPPH radical scavenging assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of extracts was measured following a reported protocol (Hmamou et al. 2022). Ascorbic acid was used as a standard. The blank was methanol, and all measurements were repeated three times.
Ferric reducing antioxidant power assay
Ferric ion reducing capacity was measured using reported methods and presented as mg ascorbic acid equivalents per gram of the extract dry matter (mg of AAE/g) (Kanmaz et al. 2020).
Metal chelation ability
Metal chelation ability was determined following a reported method (Saliu and Olabiyi 2017). Ethylenediaminetetraacetic acid (EDTA) was used as a standard chelator, and the experiment was done in triplicate.
Antimicrobial activity
Six different strains were selected for antibacterial and antifungal assays. The strains were Gram-positive (Bacillus subtilis and Staphylococcus aureus), Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacterial strains, and fungal strains (Aspergillus niger and Rhizopus oryzae). The strains were obtained from the microbiology laboratory at the Department of Biological Sciences and Biotechnology at Botswana International University of Science and Technology (BIUST). Mueller–Hinton broth was used to subculture the bacteria while the fungal strains were cultured in potato dextrose broth. Nutrient agar at 4 °C was used as bacterial strain media during experimentation.
Agar well diffusion method
The Kirby–Bauer agar diffusion method was adopted (Barberis et al. 2020). Briefly, 50 mL of Mueller–Hinton agar was poured into petri dishes and allowed to set. A McFarland standardized inoculum (1.0 × 108 CFU/mL) of each strain (100 µL) was introduced onto the surface of the set agar plate using a sterilized glass cell spreader. Using a 1000-µL pipette, tip wells (8 mm in diameter) were made in the agar plate. The test sample (100 µL of 10 mg/L extract) in dimethyl sulfoxide was introduced into the well and allowed to diffuse. The plates were then incubated at 37 °C for 24 h for bacteria and at 30 °C for 48 h for fungi. The negative control was 10% dimethyl sulfoxide. The antibacterial test positive control was chloramphenicol. After incubation, the zone of inhibition diameter (in millimeters) was measured and represented microbial growth inhibition (Rakholiya and Chanda 2014). The experiment was repeated thrice, and the mean values were obtained ± SEM (standard error of the mean values).
Determination of minimum inhibitory concentration (MIC)
To determine the MIC, the microbroth dilution method was used (Kowalska-Krochmal and Dudek-Wicher 2021; Kudumela et al. 2019). The extracts were selected based on their activity against the test strains in the Kirby–Bauer diffusion assay (Barberis et al. 2020). The serial dilution of the selected extracts ranged from 2 to 0.0313 mg/mL. The microbial strains were inoculated using the colony suspension method (Kowalska-Krochmal and Dudek-Wicher 2021) to obtain a suspension with a log phase absorbance of 0.4 at 600 nm. The final concentration was adjusted to 5 × 105 CFU/mL. To each well, 50 µL of Mueller–Hinton broth, 25 µL of test samples, and 25 µL of the test organism suspension were added. The negative control used was 10% dimethyl sulfoxide, while the positive control employed chloramphenicol for the antibacterial assay. Bacterial plates were incubated for 24 h at 37 °C while fungal plates were incubated for 48 h at 30 °C. The microplate reader (MultiSkan FC, ThermoSci) measured the wells absorbance at 600 nm. The test samples with the lowest concentration that inhibited at least 80% of microbial growth were taken as the sample MIC compared to the growth control (Kowalska-Krochmal and Dudek-Wicher 2021; Zamakshshari et al. 2021).
Preliminary phytochemical screening
Previously documented procedures were used to assess for the presence of tannins, saponins, flavonoids, steroids, glycosides, anthraquinones, and alkaloids in the 80% methanol root and twig extracts of Grewia flava (Maigoda et al. 2022; Kebal et al. 2022).
GC–MS analysis
GC–MS analysis was performed on the n-hexane non-polar extracts using an HP-5 MS capillary column (Hewlett-Packard, CA, USA) (30 m × 320 µm × 0.25), 0.25 mm thickness, in an Agilent 7890B GC system coupled to an Agilent 5977A mass detector. The helium carrier gas constant flow rate was set at 1 mL/min. The oven temperature initial temperature at 100 °C was held for 2 min. It was raised at a rate of 10 °C/min isothermally for 30 min. A sample of 0.3 mg/mL in dichloromethane was manually injected at 250 °C, at a volume of 1.0 µL in the splitless mode. Mass spectra were obtained by EI at an electron energy of 70 eV (Ahuchaogu et al. 2018; Phiri et al. 2021).
Phytochemical constituents were identified by matching their mass spectra with those found in the National Institute Standard and Technology (NIST 2012) database. Peak areas were used to determine the relative percentage composition of each compound (Akwu et al. 2019).
Statistical analysis
Statistical analysis for determining significant differences in active compound quantities was carried out based on the one-way analysis of variance (ANOVA), followed by the Tukey test. Values were regarded as statistically significant at P < 0.05.
Results
Extractions
Grewia flava roots and twigs were extracted using various solvents, and it was found that distilled water produced the highest extractible material from both plant parts (29.88% and 15.72% for the roots and twigs, respectively), whereas acetone resulted in the lowest yield in both scenarios (Table 1). When choosing an extraction method, extractive yield should be considered because low yield is a drawback in natural product research. Non-polar compounds were extracted at a higher yield from the twigs (6.97%) than from the root (0.38%) in hexane. The percentage yield of the extracts showed that water was the best extraction solvent for both twigs and roots, followed by 80% methanol, hexane, and acetone.
Phytochemical analysis
Phytochemical analysis was carried out on G. flava 80% methanol twig and root extracts. The screening exhibited the presence of alkaloids, flavonoids, saponins, steroids, glycosides, anthraquinones, and tannins in both extracts.
Total phenolic content and flavonoid content
The total phenolic content (TPC) and total flavonoid content (TFC) are presented in Table 2. The 80% methanol and distilled water extracts recorded high concentrations of both TFC and TPC. For the twig extracts, methanol extract (250.00 ± 2 GAE/g) exhibited a high concentration of phenolic compounds, followed by distilled water extract (192.00 ± 2 mg GAE/g), with the hexane extract (43.10 ± 0.2 mg GAE/g) exhibiting the lowest TPC value, and for the root extracts, a similar trend was observed except for the aqueous extract, which showed a high TPC compared to methanol extract. The same trend was also observed for the flavonoid estimation, where 80% of methanol extracts exhibited the highest flavonoid content (Table 1).
Antioxidant activities
The study examined the ability of G. flava extracts to scavenge free DPPH radicals. The results showed that all the extracts had a dose-dependent radical scavenging ability, with 80% methanol extracts showing the strongest ability under each plant part, with IC50 values of 14.50 ± 0.7 and 98 ± 7 µg/mL for the twig and root extract, respectively (Table 2).
The n-hexane and acetone twig extracts showed better activity, despite having relatively low total phenolic content (Tables 1 and 2), which suggests that the antiradical activity was not exclusive to phenolic compounds.
The study employed the FRAP assay to evaluate the extracts' capability to donate electrons and convert ferric iron to ferrous iron. The FRAP results are presented in Table 2. The outcomes indicated that the 80% methanol extracts had a stronger electron-donating ability, with the twig extract exhibiting a reducing power of 745.00 ± 1 mg AAE/g, while the root extract displayed a reducing ability of 637.00 ± 3 mg AAE/g. This trend was expected as the extract’s ability to reduce ions depends on the availability of phytochemicals that perform their antioxidant function by donating hydrogen or electrons, thus neutralizing free radicals. The observed pattern in electron-donating ability was similar to the trend in DPPH scavenging ability, indicating a comparable mechanism for both.
The chelating ability of plant extracts and the reference standard tested is shown in Table 2. Antioxidants from the plant extract compete with O-phenanthroline to form complexes with Fe2+ ions. O-phenanthroline forms red-colored complexes with Fe2+, which can be quantified spectrophotometrically. The assay showed that all extracts and EDTA had a dose-dependent response, with increased concentration leading to increased chelation ability. The aqueous methanol extracts showed strong chelation ability, with IC50 values of 98.00 ± 7 and 110.00 ± 24 µg/mL for the twig and root extracts, respectively. However, EDTA had a stronger chelation ability. Twig extracts exhibited higher chelation ability than root extracts in all scenarios except for the aqueous extract.
The DPPH radical scavenging abilities of the 80% methanol twig extract column fractions are displayed in Table 3. Fraction 14, ethyl acetate (EtOAc, 100%), demonstrated the most effectiveness in DPPH radical quenching, (IC50 of 10.60 ± 0.3 µg/mL), which was better than the parent extract. Fractions 1, 23, and 24 also showed significant radical quenching capacity (IC50 of 15.00 ± 0.6 to 18.00 ± 2 µg/mL) in comparison with the reference standard (23.00 ± 1 µg/mL). All the fractions, except fraction 9, displayed a concentration-dependent ability to scavenge DPPH radicals.
Biological activities of the extracts and column fractions
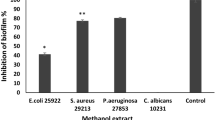
The MIC was determined for samples with zones of inhibition of ≥ 15 mm only. Samples with zones of inhibition < 15 mm were considered intermediate. The antimicrobial activity of methanol extracts of the twigs and roots of G. flava showed that both crude extracts were active against all organisms tested (Tables 4 and 5). The column fractions exhibited different degrees of activity against the microorganisms tested, with fractions 14 and 24 being the most effective.
GC–MS analysis
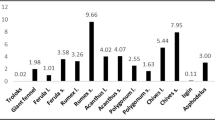
The study analyzed the n-hexane twig and root extract of G. flava using GC–MS, and several bioactive compounds such as lupeol, hexadecenoic acid, β-sitosterol, α-amyrin, betulin, and phytol were identified (Tables 6 and 7). The root extract contained 23 compounds, while the twig extract contained 28 compounds, with fatty acids being the major constituents in both the twig and root extracts, followed by triterpenes and fatty acid esters.
The predominant phytochemical constituents in the root extract were lupeol (23.58%), n-hexadecanoic acid (14.58%), oleic acid (10.14%), hexadecanoic acid, methyl ester (9.75%), and (Z)-9-O-octadecenamide (7.87%). The twig extract major compounds were 9,12-octadecadienoic acid (25.85%), octacosane (17.98%), n-hexadecanoic acid (17.34%), hentriacontane (7.88%), and hexadecanoic acid methyl ester (5.15%). Lupeol and γ-sitosterol were present in both plant extracts, while phytol was only identified in the twig extract.
Discussion
The differences in extract yields in various studies were attributed to factors such as the specific plant parts under study, the season of sampling, the geographic location of the plant, and the extraction techniques employed (Senhaji et al. 2020). The occurrence of various phytochemicals explains the application of the plant against different ailments, as they assist the body in combating illnesses and microbial invasion through their antioxidant and antimicrobial activities (Adebiyi et al. 2017; Aryal et al. 2019; Kaur et al. 2021). Common phenolic compounds are phenolic acids, hydroxycinnamic acid derivatives, anthocyanins, and flavonoids, which have reported antioxidant, anti-inflammatory, antibacterial, and anti-carcinogenic effects (Adebiyi et al. 2017).
Grewia species have been found to contain appreciable amounts of various phytochemicals, with flavonoids being the major group (Akwu et al. 2019; Kumar et al. 2022). The identified phytochemical composition was similar to that of other plants within the genus, such as G. Tenax (Kumar et al. 2022), G. tiliifolia (Kuruvilla and Anilkumar 2020; Dharmasoth et al. 2022), and G. asiatica (Sinha et al. 2015; Kaur et al. 2024). The study confirms earlier findings on the occurrence of tannins, saponins, flavonoids, anthraquinones, reducing sugars, and alkaloids in G. flava extracts (Gololo et al. 2016).
Many of the bioactive compounds identified by GC–MS have been reported to show various antibacterial, antifungal, and anti-inflammatory properties, which may further support the plant's traditional use (Agidew 2022; Kumar et al. 2022). Chlorpyrifos, a widely used insecticide, was unexpectedly identified in the twig extract, and contamination was suspected due to sampling in a farming area. Sampling from different areas is recommended to confirm the availability of this compound in the extract.
The GC–MS analysis also showed the presence of terpenoids and phenolics such as lupeol and hexadecanoic acid in n-hexane twig extract, which showed high antioxidant activity. Previous studies on other Grewia species isolated various flavonoids, alkaloids, lignans, sterols, and terpenoids (Kumar et al. 2022). The biological activities of plant extracts have been linked to phenolic compounds and flavonoids (Akwu et al. 2019; Kumar et al. 2022) that constitute the plant extracts. The identification of phenolic compounds in all extracts of Grewia flava suggests that the plant has the potential for medicinal use in treating various conditions, which is consistent with its traditional use in folklore. The chelation ability was also attributed to the extracts' phenolics and flavonoids, which have been reported to form complexes with iron (II) ions (Sudan et al. 2014). The total phenolic content was higher than the total flavonoid content in the twigs and root extracts. Phenolic compounds, which are inclusive of flavonoids, are known for their antioxidant activities, which depend on the number and position of hydroxyl groups. These hydroxylated phenolic compounds can deactivate free radicals by donating an electron (Ahmed et al. 2015) as well as hydrogen (Maigoda et al. 2022). This ability of phenol compounds to reduce oxidative stress and modulate biological processes has shown their potential in the management of non-communicable diseases (Díaz et al. 2023; Borsoi et al. 2023).
Crude extracts MIC values ranged from 0.11 to 0.96 mg/mL, and this was attributed to the presence of flavonoids and phenolic compounds (Table 1). MIC values of column fractions ranged from 0.02 to 1.70 mg/mL, and extracts with MIC values less than 0.1 mg/mL were considered to exhibit good antibacterial activity (Malada et al. 2023). Gram-negative bacteria were not resistant to plant extracts and fractions 14 and 24. Resistance is usually due to their complex and multi-layered cell wall, which acts as a barrier to many environmental substances, including synthetic and natural antibiotics, as previously reported (Rakholiya and Chanda 2014).
The study suggests that G. flava has the potential to be a source of antimicrobial agents with the ability to cross this barrier (Rakholiya and Chanda 2014). The activity of a plant extract exhibiting < 0.1 mg/mL MIC values is considered to be of pharmacological interest (Malada et al. 2023). Therefore, fractions 14 and 24 of the 80% methanol twig extract (MIC = 0.02–0.09 mg/mL) are worthy of further study to probe its use against microbial infections. Most of the plant extracts and fractions investigated exhibited moderate to good activities against the test organisms, thus validating the plant use in traditional medicine.
Conclusions
This study reports on the antioxidant and antimicrobial activity of crude extracts and column fractions of G. flava. The extracts showed moderate activities attributed to their phytochemical composition. Some column fractions exhibited better activities than the crude extracts, which merits further exploration of G. flava twig extract to isolate and identify bioactive compounds. The GC–MS analysis of the non-polar extracts showed the presence of important bioactive compounds such as 9,12-octadecadienoic acid (Z, Z) (5.84 and 27.19%), lupeol (23.58 and 6.82%), γ-sitosterol (0.9% and 5.53%), and n-hexadecanoic acid (14.58 and 16.99%) in twigs and root non-polar extracts, respectively. The twig extracts biological activities were comparable to those of the root extract; therefore, their use in herbal preparation can be adopted for G. flava sustainable harvesting. The observed G. grewia extracts biological activities and identified bioactive compounds support its use against microbial infections and folklore use. The study was limited in time and instrumentation to cover isolations and identify active compounds in the extract. We, therefore, recommend the use of NMR and LC–MS for the profiling and isolation of the twig extract components and pharmacological studies.
Availability of data and materials
Not applicable.
Abbreviations
- GAE:
-
Gallic acid equivalents
- Hex:
-
n-Hexane
- Ace:
-
Acetone
- 80% MeOH:
-
80% Methanol
- TLC:
-
Thin-layer chromatography
- TPC:
-
Total phenolic content
- UV-Vis:
-
Ultraviolet–visible
- TFC:
-
Total flavonoid content
- QUE:
-
Quercetin equivalents
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl radical
- FRAP:
-
Ferric reducing antioxidant power
- AAE:
-
Ascorbic acid equivalent
- EDTA:
-
Ethylenediaminetetraacetic acid
- MIC:
-
Minimum inhibitory concentration
- GC-MS:
-
Gas chromatography–mass spectrometry
- LC-MS:
-
Liquid chromatography–mass spectrometry
- NMR:
-
Nuclear magnetic resonance
References
Adebiyi OE, Olayemi FO, Ning-hua T, Guang-zhi Z (2017) In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ J Basic Appl Sci 6:10–14. https://doi.org/10.1016/j.bjbas.2016.12.003
Agidew MG (2022) Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull Natl Res Cent 46:87. https://doi.org/10.1186/s42269-022-00770-8
Ahmed D, Khan MM, Saeed R (2015) Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic, and aqueous extracts from Adiantum caudatum leaves. Antioxidants 4:394–409. https://doi.org/10.3390/antiox4020394
Ahmed SR, Romi IJ, Ahmed J et al (2019) Phytochemical profiling and antioxidant potentiality of medicinal plants along with their antibacterial efficacy. J Adv Biotechnol Exp Ther 2:140–145. https://doi.org/10.5455/jabet.2019.d37
Ahuchaogu AA, Ogbuehi GI, Obike AI et al (2018) GC-MS analysis of bioactive compounds from whole plant chloroform extract of Ageratum conyzoides. Inter J Med Plants Nat Prod 4:13–24. https://doi.org/10.20431/2454-7999.0402003
Akwu NA, Naidoo Y, Singh M et al (2019) Phytochemical screening, in vitro evaluation of the antimicrobial, antioxidant and cytotoxicity potentials of Grewia lasiocarpa E. Mey Ex S Afr J Bot 123:180–192. https://doi.org/10.1016/j.sajb.2019.03.004
Aryal S, Baniya MK, Danekhu K et al (2019) Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 8:1–12. https://doi.org/10.3390/plants8040096
Atere TG, Akinloye OA et al (2018) In vitro antioxidant capacity and free radical scavenging evaluation of standardized extract of Costus afer leaf. Food Sci Hum Wellness 7:266–272. https://doi.org/10.1016/j.fshw.2018.09.004
Barberis A, Deiana M, Spissu Y et al (2020) Antioxidant, antimicrobial, and other biological properties of Pompia juice. Molecules 25:3186. https://doi.org/10.3390/molecules25143186
Borsoi FT, Neri-Numa IA, de Oliveira WQ et al (2023) Dietary polyphenols and their relationship to the modulation of non-communicable chronic diseases and epigenetic mechanisms: a mini-review. Food Chem Mol Sci 6:100155. https://doi.org/10.1016/j.fochms.2022.100155
Chiavaroli V, Giannini C, De Marco S et al (2011) Unbalanced oxidant-antioxidant status and its effects in pediatric diseases. Redox Rex 16:101–107. https://doi.org/10.1179/174329211X13049558293551
Dharmasoth RD, Ganga B, Nageswara S, Basavaiah K (2022) Investigation of antibacterial activity of methanolic extract and isolation of sterol and triterpenoids from Grewia tiliaefolia VAHL LEAF. Int J Pharm Pharm Sci 14:34–43. https://doi.org/10.22159/ijpps.2022v14i4.44105
Dhawan D, Gupta J (2017) Comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. Int J Biol Chem 11:17–22. https://doi.org/10.3923/ijbc.2017.17.22
Díaz AY, Cotorruelo MSF, Battino M (2023) The role of dietary polyphenols in the control of chronic non-communicable diseases. Food Safety and Health 1:13–21. https://doi.org/10.1002/fsh3.12013
Gololo SS, Mogale MA, Shai LJ et al (2016) Phytochemical, antioxidant and antibacterial screening of the leaves of Barleria dinteri (Oberm), Grewia flava (DC) and Jatropha lagarinthoides (Sond). Res J Med Plant 8:56–60
Hmamou A, Eloutassi N, Alshawwa SZ et al (2022) Total phenolic content and antioxidant and antimicrobial activities of Papaver rhoeas l. organ extracts growing in Taounate region. Morocco. Molecules 27:584. https://doi.org/10.3390/molecules27030854
Kanmaz N, Uzer A, Hizal J, Apak R (2020) Determination of total antioxidant capacity of Cynara Scolymus L. (Globe artichoke) by using novel nanoparticle-based ferricyanide/Prussian blue assay. Talanta 216:1–7. https://doi.org/10.1016/j.talanta.2020.120960
Kaur D, Shri R, Kamboj A (2021) Bioactivity-directed isolation, characterization, and quantification of an anxiolytic flavonoid from Brassica oleracea L. J Food Biochem 45:1–9. https://doi.org/10.1111/jfbc.13608
Kaur S, Shams R, Dash KK et al (2024) Phytochemical and pharmacological characteristics of phalsa (Grewia asiatica L.): a comprehensive review. Heliyon 10:e25046. https://doi.org/10.1016/j.heliyon.2024.e25046
Kebal L, Pokajewicz K, Djebli N et al (2022) HPLC-DAD profile of phenolic compounds and In vitro antioxidant activity of Ficus carica L. fruits from two Algerian varieties. Biomed Pharmacother 155:113738. https://doi.org/10.1016/j.biopha.2022.113738
Kowalska-Krochmal B, Dudek-Wicher R (2021) The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens 10:165. https://doi.org/10.3390/pathogens10020165
Kudumela RG, Mazimba O, Masoko P (2019) Isolation and characterisation of sesquiterpene lactones from Schkuhria pinnata and their antibacterial and anti-inflammatory activities. S Afr J Bot 126:340–344. https://doi.org/10.1016/j.sajb.2019.04.002
Kumar S, Singh B, Bajpai V (2022) Traditional uses, phytochemistry, quality control and biological activities of genus Grewia phytomedicine plus traditional uses, phytochemistry, quality control and biological activities of genus Grewia. Phytomedicine plus 2:1–34. https://doi.org/10.1016/j.phyplu.2022.100290
Kuruvilla J, Anilkumar M (2020) Pharmacognostic and phytochemical evaluation of the bark of Grewia tiliifolia Vahl. Pharmacogn J 12:967–976. https://doi.org/10.5530/pj.2020.12.137
Lamola SM, Dzoyem JP, Botha F, Van Wyk C (2017) Anti-bacterial, free radical scavenging activity and cytotoxicity of acetone extracts of Grewia flava. Afr Health Sci 17:790–796. https://doi.org/10.4314/ahs.v17i3.22
Lfitat A, Zejli H, Bousselham A et al (2020) Comparative evaluation of Argania spinosa and Olea europaea leaf phenolic compounds and their antioxidant activity. Botanica 26:76–87. https://doi.org/10.2478/botlit-2020-0007
Maigoda T, Judiono J, Purkon DB et al (2022) Evaluation of Peronema canescens leaves extract: Fourier transform infrared analysis, total phenolic and flavonoid content, antioxidant capacity, and radical scavenger activity. Maced J Med Sci 10:117–124. https://doi.org/10.3889/oamjms.2022.8221
Malada MP, Mazimba O, Mogashoa MM, Masoko P (2023) Antimicrobial activity of Mystroxylon aethiopicum leave extracts and isolated 3-O-acetyloleanolic acid. Indian J Nat Prod Resour 14:464–469. https://doi.org/10.56042/ijnpr.v14i3.5520
Mashungwa NG, Mogotsi KK, Amosso C (2019) Grewia flava. In: Ulian T, Flores C, Lira R, Mamatsharaga A, Mogotsi KK, Muthoka P, Ngwako S, Nyamongo DO, Omondi W, Sanogo AK, Sanogo S, Mattana E (eds) Wild Plants for a Sustainable Future: 110 multipurpose species. Royal Botanic Gardens, Kew, pp 32–34
Motamedi H, Darabpour E, Gholipour M, Seyyed-Nejad SM (2010) In vitro assay for the anti-brucella activity of medicinal plants against cetracycline-resistant Brucella melitensis. J Zhejiang Uni Sci B 11:506–511. https://doi.org/10.1631/jzus.B0900365
Motlhanka DMT, Ngwako S (2009) Notes on Chetopoti: a traditional Botswana fermented alcoholic beverage from watermelons. Botswana Soc Rep 41:133–135. https://doi.org/10.2307/23237935
Motlhanka DMT, Nthoiwa GP (2013) Ethnobotanical survey of medicinal plants of Tswapong north, in eastern Botswana: a case of plants from Mosweu and Seolwane villages. European J Med Plants 3:10–24. https://doi.org/10.9734/EJMP/2013/1871
Motlhanka K, Zhou N, Lebani K (2018) Microbial and chemical diversity of traditional non-cereal based alcoholic beverages of Sub-Saharan Africa. Beverages 4:1–25. https://doi.org/10.3390/beverages4020036
Motlhanka K, Lebani K, Boekhout T, Zhou N (2020) Fermentative microbes of khadi, a traditional alcoholic beverage of Botswana. Fermentation 6:1–22. https://doi.org/10.3390/fermentation6020051
Phiri N, Serame EL, Pheko T (2021) Extraction, chemical characterization, and antioxidant analysis data of essential oil from Schinus molle medicinal plant cultivated in Botswana. Am J Essent Oil Nat Prod 9:01–09. https://doi.org/10.22271/23219114.2021.v9.i4a.236
Rakholiya KD, Chanda S (2014) Comparative study of hydroalcoholic extracts of Momordica charantia L. against foodborne pathogens. Indian J Pharm Sci 76:148–156. https://doi.org/10.4103/0250-474X.131529
Saliu JA, Olabiyi AA (2017) Aqueous extract of Securidaca longipendunculata oliv. and Olax subscropioidea inhibits key enzymes (acetylcholinesterase and butyrylcholinesterase) linked with Alzheimer’s disease in vitro. Pharm Biol 55:252–257. https://doi.org/10.1080/13880209.2016.1258426
Senhaji S, Lamchouri F, Toufik H (2020) Phytochemical content, antibacterial and antioxidant potential of endemic plant Anabasis aretioïdes Coss. & Moq. (Chenopodiaceae). BioMed Res Int 6:1–16. https://doi.org/10.1155/2020/6152932
Sinha J, Purwar S, Chuhan SK, Rai G (2015) Nutritional and medicinal potential of Grewia subinaequalis DC (syn. G. asiatica) (Phalsa). J Med Plant Res 9:594–612. https://doi.org/10.5897/JMPR2015.5724
Stobiecka M, Krol J, Brodziak A (2022) Antioxidant activity of milk and dairy products. Animals 12:245. https://doi.org/10.3390/ani12030245
Sudan R, Bhagat M, Gupta S, Singh J, Koul A (2014) Iron (FeII) chelation, ferric reducing antioxidant power, and immune-modulating potential of Arisaema jacquemontii (Himalayan cobra lily). BioMed Res Int 8:179865. https://doi.org/10.1155/2014/179865
Zamakshshari N, Ahmed IA, Nasharuddin MNA, Mohd HN, Mustafa MR, Othman R, Noordin MI (2021) Effect of extraction procedure on the yield and biological activities of hydroxychavicol from Piper betle L. leaves. J App Res Med Aromat Plant 24:1–10. https://doi.org/10.1016/j.jarmap.2021.100320
Acknowledgements
The authors would like to thank Botswana University of Science and Technology for the MSc studentship and initiation grant that made the project possible. The author also thanked the reviewers for their comments, which improved the manuscript.
Funding
This work was funded by the initiation grant for GC (S00325) from the Botswana International University of Science and Technology.
Author information
Authors and Affiliations
Contributions
All Authors have read and approved the manuscript. GC conducted data collection, analysis, interpretation, and manuscript writing. OM developed the study concept, data analysis, and interpretation, and manuscript writing supervision. KM and DL conducted the biological studies and data analysis. MM conducted plant collections, identifications, data analysis, and manuscript editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coin, G., Lekutlane, D., Masisi, K. et al. Grewia flava twig extracts: phytochemical, antioxidant, and antimicrobial evaluations. Bull Natl Res Cent 48, 75 (2024). https://doi.org/10.1186/s42269-024-01234-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-024-01234-x