Abstract
Background
Medicinal plants play a major role in the delivery of healthcare, particularly among the rural population of Ethiopia. Plant extracts and their bioactive compounds have been utilized for the treatment of several diseases. This study was aimed at evaluating the antibacterial activity, antioxidant capacity, and phytochemical content of selected medicinal plants used in Dibatie district, western Ethiopia.
Methods
Study plants were collected, shade dried, pulverized, extracted by maceration in 80% ethanol, and subjected to antibacterial, antioxidant, and phytochemical tests. Minimum inhibitory concentration (MIC) was determined using 96-well microplates and nutrient broth microdilution. Antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. Phytochemical screening was conducted using standard test methods.
Results
The ethanolic extract of Polystachya steudneri Rchb.f. pseudobulbs was the most active against gram-negative Proteus mirabilis, Salmonella typhimurium, Klebsiella pneumoniae, Escherichia coli, and Shigella flexneri, with MIC values of 8 ± 0, 11 ± 5, 3 ± 1, 3 ± 1, and 2 ± 0 mg/mL, respectively. The ethanolic extract of P. steudneri was also the most effective against gram-positive Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus agalactiae, and Enterococcus faecalis, with MIC values of 8 ± 0, 8 ± 0, 3 ± 1, and 16 ± 0 mg/mL, respectively. Ethanolic extracts of Gnidia involucrata Steud. ex A.Rich. stems and roots were effective antioxidants, with respective 50% DPPH free radical inhibitory concentrations (IC50) of 168.68 and 181.79 µg/mL, followed by that of P. steudneri (IC50 = 203.11 µg/mL). The study plants contained alkaloids, anthocyanins, anthraquinones, cardiac glycosides, coumarins, flavonoids, phenols, saponins, steroids, tannins, and terpenoids.
Conclusions
This study confirmed the antibiotic, antioxidant, and phytochemical constituents of the investigated plants and suggested further investigations that may lead to bioactive lead compounds.
Similar content being viewed by others
Background
Infectious diseases are the most common causes of mortality and morbidity among human beings throughout the world [1]. Recently, the rapid emergence and spread of multidrug-resistant pathogens have been considered major challenges for the treatment of several infectious diseases [2, 3]. The multiple drug resistance mechanisms include drug uptake limitation, drug target modification, drug inactivation, and active drug efflux, and the resistance processes differ based on microbial types [4]. Besides, certain pathogenic bacteria form biofilms through quorum sensing and develop drug resistance [5]. Hence, there is a need for the discovery of new drugs against multidrug-resistant pathogenic microorganisms.
Plant extracts and their bioactive compounds have been utilized for the treatment of several diseases since ancient times [5]. Medicinal plants were the major sources of bioactive compounds that could be used as potential alternatives to conventional antimicrobials [6]. The antibacterial activity of the plants could be ensured either by inhibiting the growth of bacteria or by disturbing the cell-to-cell communication system between the bacteria through anti-quorum sensing (AQS) [7], in which the latter is currently preferable, especially against antimicrobial-resistant bacteria. Hence, plant-derived medicines have been considered convenient therapies due to their fewer side effects and greater pharmacological efficacy [8]. Medicinal plants contain natural phytochemicals such as alkaloids, flavonoids, saponins, tannins, terpenoids, steroids, resins, cardiac glycosides, coumarins, and phenolic compounds, among others, that could have a multitude of biological activities [9].
Moreover, medicinal plants are potent antioxidants and play an important role in sequestering reactive oxygen species (ROS) in living cells owing to the presence of various phytochemicals [10]. Polyphenols from plants scavenge free radicals and inhibit enzymes that are responsible for the formation and accumulation of reactive oxygen species (ROS) [11]. Antioxidant phenolic compounds reduce the level of free radicals in living cells, thereby preventing the oxidation of cellular components by donating hydrogen atoms to free radicals and forming stable, nontoxic compounds like phenoxyl radicals [12]. These compounds prevent or treat diseases related to oxidative stress, such as cancer, diabetes, cardiovascular diseases, inflammatory joint diseases, dementia, asthma, eye diseases, and atherosclerosis [10]. Additionally, plant-derived phenolic compounds capture and neutralize free radicals in human cells to protect them from aging [13].
Ethiopia is a center for plant diversity, diverse topography, and multiple ethnic groups, languages, cultures, and beliefs, which enhance the practice of using medicinal plants. Particularly, the Metekel zone in Benishangul Gumuz Regional State has various ethnic groups (e.g., Agaw, Amhara, Gumuz, Oromo, and Shinasha), multiple cultures, and a diversity of medicinal plants. For instance, Asparagus flagellaris (Kunth) Baker, Brucea antidysenterica J. F. Mill., Celosia trigyna L., Crepis rueppellii Sch. Bip., Gnidia involucrata Steud. ex A.Rich., Polystachya steudneri Rchb.f., and Sauromatum venosum (Aiton) Kunth are traditionally used to treat toothache, leishmaniasis, tapeworm, diarrhea, gonorrhea, wounds, and amoeba, respectively, in the Dibatie district of the Metekel zone, western Ethiopia. However, there are limited reports yet on the ethnomedicine, antimicrobial activity, antioxidant properties, and phytochemical profiles of these plants in Ethiopia as a whole and in the Dibatie district of the Metekel zone in particular. Therefore, the current study was aimed at evaluating the antibacterial activity, antioxidant potential, and phytochemical constituents of the above medicinal plants.
Methods
Study period, study design and area
This study involved a preliminary ethnomedicinal survey through a semi-structured interview [14], which was conducted from April 2021 to June 2022. Following the ethnomedicinal investigation, the medicinal plants were selected, and the laboratory samples were collected in November 2022. Then, the laboratory work was carried out from February to June 2023. The field ethnobotanical data and sample collection were conducted in the Dibatie district of the Metekel zone, Benishangul Gumuz Regional State, western Ethiopia. Residents from eleven kebeles (sub-districts) such as Berber, Dibatie-02, Donben, Galessa, Gipho, Jan, Lega-buna, Parzeyit, Qorqa, Sombo-sire, and Tuski-gambela participated in the interview process. The plant material preparation, extraction, antibacterial test, antioxidant assay, and phytochemical screening were carried out at the laboratory of the Directorate of Modern and Traditional Medicine Research at the Ethiopian Public Health Institute, Addis Ababa, Ethiopia.
Plants selection
Medicinal plants were selected for laboratory work depending on the prior ethnobotanical survey of their traditional medicinal uses. The selection criteria were mainly based on the medicinal plants traditionally used to treat human ailments such as amoeba, diarrhea, gonorrhea, leishmaniasis, tapeworm, toothache, and wounds. Because these diseases were caused or aggravated due to the infestation by bacteria, protozoans, or helminthes. The selection of the study plants also emphasized the relative curing potential of each plant species (percentage of fidelity level) to heal the above ailments. The percentage of fidelity level helps to give a hint for further investigations on the medicinal efficacy of bioactive constituents. The percentage of fidelity level (FL%) was calculated using the formula: FL% = Ip/Iu × 100, where Ip is the number of respondents who indicated the use of a species for the same major ailments and Iu is the total number of respondents who mentioned the plant for any major ailments indicated [15]. Accordingly, plants with a fidelity level greater than 45% were selected for the laboratory investigations (Table 1). In addition, the selected plants were prioritized since they were not studied using the same methods as the present study so far.
Sample collection and preparation
Based on the above criteria, roots of Asparagus flagellaris, fruits of Brucea antidysenterica, inflorescence having seeds of Celosia trigyna, roots of Crepis rueppellii, roots and stems of Gnidia involucrata, pseudobulbs of Polystachya steudneri, and tubers of Sauromatum venosum were collected from Berber, Galessa, Jan, Lega-buna, Sombo-sire, and Tuski-gambela sub-districts. The collected plants were identified by Dr. Ermias Lulekal and Mr. Baressa Anbessa in the department of plant biology and biodiversity management at Addis Ababa University. In addition, the scientific names were checked by referring to the website Plants of the World Online (POWO). Voucher specimens were deposited at the National Herbarium of Addis Ababa University (ETH).
Fruits, seeds, and inflorescences of the indicated plants (Table 1) were shade dried without washing since their dust content was negligible. Roots, stems, and pseudobulbs were washed with tap water, rinsed with distilled water to remove dust, and shade dried in a solar drier. Dried samples were pulverized using an electric grinder to a moderately fine powder and kept in the refrigerator at 4 ºC until extraction.
Extraction process
As the local community usually uses water as a solvent, aqueous 80% ethanol was used for effective extraction of bioactive compounds from medicinal plant materials. The reason is that aqueous-alcoholic (80% ethanol) extracts are better in phytochemical (e.g., phenolics, flavonoids, tannins, etc.) content and antioxidant activity [16, 17]. The extraction was carried out by macerating 50 g of powdered plant parts in 500 mL of 80% ethanol and continuously shaking for 24 h using a magnetic stirrer. The mixture was filtrated using Whatman number 1 filter paper. The residue was re-macerated for 24 h and filtered. The filtrates were combined and concentrated in vacuo using a rotary evaporator (BUCHI R-300 Rotavapor, Switzerland). The concentrated extracts were dried in a water bath at 40 ºC and kept in desiccators with active silica gel until they dried well.
Antibacterial assay
The test microorganisms were from the American Type Culture Collection (ATCC). Ethanolic extracts of each sample were tested in vitro against the active pathogenic bacterial strains existing in the laboratory. These include the gram-negative (Proteus mirabilis ATCC-35,659, Salmonella typhimurium ATCC-13,311, Klebsiella pneumoniae ATCC-700,603, Escherichia coli ATCC-25,922, and Shigella flexneri ATCC-12,022) and the gram-positive (Staphylococcus aureus ATCC-25,923, Staphylococcus epidermidis ATCC-12,228, Streptococcus agalactiae ATCC-12,386, and Enterococcus faecalis ATCC-1,829,212) bacterial strains.
Nutrient broth and Mueller-Hinton agar were used for microorganism sub-culturing and growing. For that purpose, 13 g of nutrient broth was dissolved in 1000 mL of distilled water, well mixed, and autoclaved at 121 °C and 15 pounds per square inch (psi) for 15 min. Mueller-Hinton agar (38 g) was also dissolved in 1000 mL of distilled water, well mixed, boiled on a hot plate, and autoclaved.
Mueller-Hinton agar for bacteria was used for the subculturing of microorganisms. In this regard, 3–5 well-isolated colonies of the same morphological type from the refreshed agar plate culture were selected. The bacterial colonies were inoculated on sterilized plates containing Mueller-Hinton agar, followed by incubation at 37 °C for 24 h. Later, the bacterial colonies were transferred to the nutrient broth using the sterilized inoculating loop.
The minimum inhibitory concentration (MIC) was determined using 96-well microplates by the nutrient broth microdilution method. Tween 80 was used to dissolve the extracts since it is a low-toxicity surfactant that increases the solubility of bioactive phytochemicals [18]. The ethanolic extract of each sample was dissolved in 5% Tween 80 to an end concentration of 32 mg/mL, which needs to be engaged in serial dilutions. An aliquot of 100 µL of each extract was subjected to serial dilutions in nutrient broth to concentrations of 16, 8, 4, 2, 1, 0.50, 0.25, and 0.13 mg/mL. A standard reference (ciprofloxacin) was taken as a positive control in concentrations of 10, 5, 2.50, 1.25, 0.63, 0.31, 0.16, and 0.08 µg/mL. Tween 80 (5%) was used as a negative control. The microorganism suspension was standardized to 1 × 108 CFU/mL (0.08 to 1.00 turbidity) using a UV-Vis spectrophotometer at 625 nm. An aliquot of 100 µL of standardized microorganisms was inoculated into each well containing serially diluted extracts and controls (positive, negative, and growth controls), except for sterility control. Then, plates were incubated at 37 °C for 18–24 h. In order to read the microorganism growth, 40 µL of 2, 3, 5 tripenyl tetrazolium chloride (TTC) with a concentration of 0.40 mg/mL was added into each well and incubated at 37 °C for 30 min. The development of pink color in the microplate well indicated the presence of living cells (microorganisms), and the reverse result showed inhibition of microbial growth. The lowest concentration of each extract displaying no visible pink color was recorded as the MIC.
During the antibacterial test, we followed various safety practices to avoid any potential hazards. Bacterial cultures were treated as potential pathogens. All materials, media, tubes, plates, loops, needles, pipettes, and other items used were sterilized by autoclaving or using commercially sterilized products. Work spaces were thoroughly cleaned using 70% ethanol or 10% bleach both before and after usage. Mouth pipetting was avoided by staying away from food and drink in the laboratory and washing hands with disinfectant soap before and after working. Labeling everything clearly, autoclaving or disinfecting all waste material, and cleaning up spills with care were also the other safety precautions that we followed during the experiments. Additionally, all necessary personal protective equipment and biological safety cabinets (class II) were used to avoid contamination.
Antioxidant (2,2-diphenyl-1-picrylhydrazyl (DPPH)) assay
The free radical scavenging ability of ethanolic extracts was determined by using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [19]. Briefly, a fresh 0.1 mM DPPH solution was prepared in 80% ethanol. Ethanolic extracts and ascorbic acid (a positive control) were kept in test tubes at different concentrations (15.63–500 µg/mL) through serial dilution in 80% ethanol. Then, 1 mL of DPPH solution was mixed with 1 mL of each extract and a positive reference in the test tube. The mixtures were shaken thoroughly and incubated in the dark for 30 min at room temperature. The mixture of 1 mL of 80% ethanol and 1 mL of DPPH solution was considered a blank. The absorbance of each mixture was measured at 517 nm against a blank using a UV-VIS spectrophotometer (UV-1800 SHIMADZU).
The percentage of inhibition was calculated using the formula: % Inhibition = [(Ab - As) / Ab] x 100, where Ab is the absorbance of the blank and As is the absorbance of the sample. Later, the 50% inhibition concentration (IC50) was calculated for ascorbic acid and extracts of medicinal plants by using the slope equation: Y = mx + c [10].
Phytochemical screening
The ethanolic extracts were employed for preliminary screening of phytochemicals such as alkaloids, anthocyanins, anthraquinones, cardiac glycosides, coumarins, flavonoids, phenols, saponins, steroids, tannins, and terpenoids following the standardized protocols [5, 13, 20,21,22]. The results were expressed as (+) for the presence and (-) for the absence of phytochemical compounds.
Statistical data analysis
The percentage of fidelity level was computed based on the ethnobotanical data to assess the healing potential of each plant species against the corresponding disease. The minimum inhibitory concentration (MIC) data were described as the means ± standard deviation of triplicate analyses. Depending on the MIC values, the principal component analysis (PCA) was computed to indicate variations in the antibiotic effect of medicinal samples and the susceptibility of bacterial strains using R-statistical packages (ggplot2 and grid). The DPPH free radical scavenging activity and the 50% inhibition concentration (IC50) were expressed as means of triplicate determinations. Qualitative phytochemical profiles were expressed as the presence (+) and absence (-) of phytochemical constituents. Microsoft Excel version 2013 was also used for the data analysis.
Results
Antibacterial activities of medicinal plants
Minimum inhibitory concentration (MIC) values of selected medicinal plants were evaluated against gram-negative (Proteus mirabilis, Salmonella typhimurium, Klebsiella pneumoniae, Escherichia coli, and Shigella flexneri) and gram-positive (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus agalactiae, and Enterococcus faecalis) bacterial strains at concentrations less than or equal to 16 mg/mL.
MIC values of plant extracts against gram-negative bacteria
The ethanolic extract of P. steudneri pseudobulbs showed the highest antibacterial activity against gram-negative bacterial strains by inhibiting P. mirabilis, S. typhimurium, K. pneumoniae, E. coli, and S. flexneri with MIC values of 8 ± 0, 11 ± 5, 3 ± 1, 3 ± 1, and 2 ± 0 mg/mL, respectively. Whereas the ethanolic extract of A. flagellaris roots exhibited the lowest antibacterial activity as it inhibited both S. typhimurium and E. coli at MIC values of 16 ± 0 mg/mL, and P. mirabilis, K. pneumoniae, and S. flexneri at MIC values > 16 mg/mL each (Table 2).
MIC values of plant extracts against gram-positive bacteria
The ethanolic extracts of P. steudneri pseudobulbs and G. involucrata stems were the most active against gram-positive bacterial strains. The extract of P. steudneri inhibited S. aureus, S. epidermidis, S. agalactiae, and E. faecalis at MIC values of 8 ± 0, 8 ± 0, 3 ± 1, and 16 ± 0 mg/mL, and that of G. involucrata stems inhibited these bacteria at MIC values of 3 ± 1, 16 ± 0, 2 ± 0, and 16 ± 0 mg/mL, respectively. On the other hand, ethanolic extracts of A. flagellaris roots and S. venosum tubers were active only against S. agalactiae, with MIC values of 4 ± 0 and 2 ± 0 mg/mL, respectively (Table 3).
Coordinates of study plants and bacterial strains based on MIC values
The results of principal component analysis (PCA) revealed that the ethanolic extract of P. steudneri pseudobulb was the most effective antibacterial, closest to the positive reference (ciprofloxacin). The extracts of A. flagellaris root and S. venosum tuber were the least effective antibacterials among the studied medicinal plant species. The results of PCA also showed that S. agalactiae was the most susceptible bacterial strain, illustrated with the longest arrow, while P. mirabilis was found to be the least susceptible (Fig. 1).
Antioxidant activity of the study plants
DPPH free radical scavenging ability
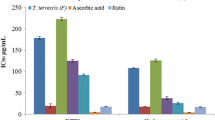
The deep purple color of the DPPH solution changed to colorless when it was mixed with plant extracts having antioxidant properties and ascorbic acid (positive reference). In contrast, the purple color was retained when the DPPH solution was mixed with extracts of plants with less antioxidant activity and the negative control. In this perspective, following the ascorbic acid, ethanolic extracts of G. involucrata stems and roots exhibited the highest DPPH free radical scavenging activity, while extracts of C. rueppellii roots showed the lowest (Fig. 2).
The 50% inhibitory concentration (IC50)
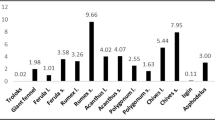
Relative to the positive reference (IC50 = 53.76 µg/mL), ethanolic extracts of G. involucrata stems showed IC50 value of 168.68 µg/mL, followed by extracts of G. involucrata roots (IC50 = 181.79 µg/mL). Ethanolic extracts of the remaining plants, such as P. steudneri, B. antidysenterica, C. trigyna, S. venosum, A. flagellaris, and C. rueppellii, exhibited IC50 values of 203.11, 293.56, 366.15, 387.82, 459.55, and 527.57 µg/mL, respectively (Fig. 3).
Phytochemical screening of medicinal plants
Qualitative phytochemical screening was carried out to determine the presence or absence of alkaloids, anthocyanins, anthraquinones, cardiac glycosides, coumarins, flavonoids, phenols, saponins, steroids, tannins, and terpenoids in the ethanolic extracts of the studied medicinal plants, and a summary of the findings is presented in Table 4. Accordingly, root extracts of A. flagellaris were confirmed to have the tested compounds apart from anthraquinones and steroids. Extracts of B. antidysenterica fruits contained all the other tested phytoconstituents except anthocyanins, anthraquinones, cardiac glycosides, and saponins. Except for anthraquinones, the tested phytochemicals were detected in extracts of C. trigyna inflorescence with seeds. The root extracts of C. rueppellii were observed to contain alkaloids, cardiac glycosides, coumarins, and terpenoids. Extracts of G. involucrata roots and stems contained almost similar phytochemicals (anthocyanins, anthraquinones, cardiac glycosides, flavonoids, phenols, tannins, and terpenoids) except for the presence of saponins in roots but not in stems, and the reverse was true for steroids. The tested phytochemicals were observed in the extracts of P. steudneri pseudobulbs, except for alkaloids, saponins, and terpenoids. Tubers of S. venosum contained the other tested constituents except anthocyanins, anthraquinones, saponins, and terpenoids.
Discussion
In the ethnomedicinal perspective, leaves and stems of A. flagellaris were reported to be used against gonorrhea and syphilis in Nigeria [23], its fruits for eye diseases, and its roots for measles in Uganda [24]. Leaves of B. antidysenterica were used to treat wounds in Zuway Dugda district [25] and diarrhea surrounding the Gullele Botanic Garden in central Ethiopia [26]. Whole parts of Celosia trigyna were reported to heal arthritis, diarrhea, and dysentery in Kafa Zone [27], and seeds were used to treat tapeworm in Libo Kemkem district, northwest Ethiopia [28]. Leaves and roots of Crepis rueppellii were used to cure dysentery by residents on the Dek Island of Lake Tana, northwest Ethiopia [29]. Roots of Gnidia involucrata were reported to treat gonorrhea and ascaris in the Bule Hora district of southern Ethiopia. The tubers of S. venosum were traditionally used to treat ascaris [29] and hemorrhoids [30] in northwest Ethiopia. Hence, the literature supports the ethnomedicinal data in the present study and the effectiveness of the study plants against several infectious diseases, except for P. steudneri, which has not been studied yet.
In the current study, it was observed that the minimum inhibitory concentrations (MIC) of the investigated medicinal plants were dependent on the types of bacterial strains. The investigated medicinal plants exhibited various MIC values against different gram-negative and gram-positive bacterial strains. Similar to the current findings, the previous studies reported P. mirabilis as an antimicrobial-resistant bacterial strain [31, 32]. In line with the present study, other previous studies also reported the antimicrobial resistance of S. epidermidis among gram-positive bacteria [33, 34]. This indicates the multiple antibiotic resistance of both P. mirabilis and S. epidermidis.
An earlier study conducted by Taye et al. [35] reported that the methanolic extracts of B. antidysenterica root showed a MIC value of 15.63 mg/mL against S. aureus, while the ethanolic extracts of its fruits inhibited the same bacterial strain at a MIC value of 4.00 mg/mL in the present study. Here, variation in the antibacterial activity of B. antidysenterica might be due to differences in the extraction solvents used or the bioactivity of the tested plant parts. A similar study conducted by Kalbessa et al. [36] reported the highest efficacy of the ethyl acetate extracts of G. involucrata root bark against S. aureus compared to the other bacterial strains. However, the current findings revealed the most sensitive bacteria, S. agalactiae, to the ethanolic extracts of G. involucrata roots than S. aureus. On the other hand, the study conducted by Zakerifar et al. [37] showed that S. agalactiae was reported to be resistant to certain antibiotics like erythromycin, levofloxacin, ofloxacin, quinupristin, and tetracycline and susceptible to chloramphenicol, gentamicin, linezolid, penicillin, and vancomycin. In this respect, there are variations in the antibacterial efficacy of medicinal plants and differences in the degree of susceptibility of bacterial strains. Thus, MIC values varied among the extracts of different medicinal plants in the present study. This might be linked to the difference in the biologically active phytochemicals they contain [38]. Besides, MIC values differed among different bacterial strains owing to their variation in antibiotic resistance.
The study conducted by Odeja et al. [23] showed that the leaf essential oil of A. flagellaris had high antioxidant activity, with 90.74% inhibition of DPPH free radicals at a concentration of 500 µg/mL. In the present findings, however, the ethanolic extracts of its root exhibited 51.28% inhibition at the same concentration. In this case, the variation in the antioxidant activity of A. flagellaris might be because of the extraction methods employed or the excess of phytoconstituents in leaves rather than roots. The other study conducted by Kalbessa et al. [36] indicated that ethyl acetate extracts of G. involucrata root bark and its isolated compound exhibited 70.70 and 85.80% inhibition at concentrations of 100 µg/mL, respectively. This is slightly comparable with the current findings, in which the ethanolic extracts of G. involucrata stem showed 68.91% inhibition at 125 µg/mL. This confirms the antioxidant potential of different parts of G. involucrata to reduce risks related to free radicals.
The antibacterial and antioxidant activities of medicinal plants depend on their phytochemical constituents. This is due to the fact that the phytochemical constituents of the medicinal plants are associated with their antioxidant and antibacterial activities [9, 39]. Medicinal plants contain mainly phenolic antioxidants like ß-carotene, flavonoids, phenolic acids, terpenes, tocopherols, vitamin C, and so on [40]. Antioxidant phenolic compounds scavenge free radicals and prevent oxidation of cellular components either by donating hydrogen atoms to free radicals to form stable, harmless compounds [12] or by inhibiting enzymes responsible for the production of reactive oxygen species [11]. Thus, they take part in the prevention or treatment of oxidative stress-related diseases, for example, atherosclerosis, biliary diseases, cancer, dementia, diabetes, hypertension, kidney disease, macular degeneration, neurodegenerative diseases, and obesity [41].
The phytochemical screening of ethanolic extracts showed that the selected medicinal plants contain important phytochemicals, which could play crucial roles in their bioactivities. Phytochemical constituents, mainly bioactive secondary metabolites, play significant roles in the bioactivities of medicinal plants by eliciting a definite and specific action on the human body [42]. The current results showed the presence of steroids in the ethanolic extracts of B. antidysenterica fruits, C. trigyna inflorescence with seeds, G. involucrata stems, P. steudneri pseudobulbs, and S. venosum tubers. Steroids are used to treat rheumatism, asthma, allergies, skin infections, and inflammations [43] and to relieve inflammation and swelling in cancer patients [42, 43]. Cardioactive steroids, for example, cardenolides, improve heart function, although they are highly toxic and received at a therapeutic dose of 60% of the lethal dose [44]. Results of phytochemical screening revealed the presence of alkaloids in the extracts of A. flagellaris, B. antidysenterica, C. trigyna, C. rueppellii, and S. venosum. Isoquinoline alkaloids are found in higher plants and are known to have antispasmodic, antiviral, antifungal, anticancer, antioxidant, and enzyme inhibitory activities [45]. Besides, diterpenoid alkaloids are potent to treat various cancers as new drugs [46].
Flavonoids, tannins, and phenols were identified from the ethanolic extracts of all investigated medicinal plants except that of C. rueppellii. Flavonoids have antioxidant, anti-inflammatory, and antimicrobial activities; hence, they attribute to the medicinal properties of different medicinal plants [43]. For instance, plants like Zingiber, Curcuma, and Acorus were reported as sources of antibacterial and antiseptic agents owing to their flavonoid content [47]. Tannins were described as healing agents for inflammation, hemorrhoids, and gonorrhea [42] and were known to have anticancer [47] and antidiabetic [48] effects. Polyphenolic compounds have been beneficial as antioxidants, anti-inflammatory, and antibacterial agents and reduce blood pressure and heart disease [47]. Out of the examined plants, saponins were found in extracts of A. flagellaris roots, C. trigyna inflorescence with seeds, and G. involucrata roots. Saponins were stated to treat different human diseases, such as skin infections, liver diseases, trauma, chronic venous insufficiency, and kidney diseases [49].
The tested medicinal plants were positive for cardiac glycosides, except for B. antidysenterica. In agreement with the current findings, the studies conducted by Liu et al. [50] and Ravi et al. [51] reported the existence of cardiac glycosides in many medicinal plants. Cardiac glycosides have beneficial effects for the heart [47] in that they treat congestive heart failure and cardiac arrhythmia by inhibiting the Na+/K+ pump and increasing the level of calcium ions (Ca+), which enhances the contraction of heart muscles and reduces swelling [13]. Terpenoids were detected in ethanolic extracts of A. flagellaris roots, B. antidysenterica fruits, C. trigyna inflorescence with seeds, C. rueppellii roots, and G. involucrata roots and stems. Medicinally, they provide significant actions such as antiviral, antibacterial, antimalarial, anti-inflammatory, anticancer, and inhibition of cholesterol synthesis [42]. Coumarins are among the essential phytochemical compounds found in medicinal plants [42]. In this regard, results from the current study indicated the presence of coumarins in ethanolic extracts of the investigated medicinal plants, except in the roots and stems of G. involucrata. Medicinally, coumarins were appreciated to treat microbial infections, cancers, tuberculosis, inflammatory diseases, malaria, and AIDS-acquired immunodeficiency syndrome [52].
The qualitative phytochemical screening revealed that anthraquinones were identified from ethanolic extracts of G. involucrata roots and stems and P. steudneri pseudobulbs. The plant-derived natural anthraquinones were reported to have antiviral potential against different infectious viruses [53]. Results also showed that the examined medicinal plants, such as A. flagellaris, C. trigyna, G. involucrata, and P. steudneri, were positive for the anthocyanins test. Plant-based anthocyanins have antioxidant properties that play important roles in health and therapeutic effects [54]. Furthermore, the presence of phytochemicals such as alkaloids, flavonoids, glycosides, phenolic compounds, saponins, tannins, and triterpenoids promotes the anthelmintic properties of medicinal plants [55].
In the present study, a comparative investigation was carried out between the extracts of G. involucrata roots and stems, though local people traditionally use its roots. The extract of G. involucrata stems showed even higher antibacterial activity, antioxidant capacity, and phytochemical contents than the root extract. This might be due to the distribution of secondary metabolites from the areas of synthesis (leaves) to the areas of sink (roots and stems) via phloem tissue. Hence, results from the current study suggest the use of G. involucrata stems instead of its roots since root harvesting is usually destructive for the sustainable use of medicinal plants.
Conclusions
Ethanolic extracts of the investigated medicinal plants were active against different gram-negative and gram-positive bacterial strains at various concentrations. Additionally, the ethanolic extracts exhibited considerable antioxidant activity compared to ascorbic acid. The qualitative phytochemical screening revealed the presence of important bioactive compounds in the tested medicinal plants. Hence, the findings from this study support the traditional medicinal use of the investigated plants. The study was restricted to their 80% ethanolic extracts. Thus, further investigations using different solvents of various polarities will be required to extract lead compounds for the development of appropriate drugs. Besides, toxicity studies will be necessary to encourage their further use.
Availability of data and materials
The data that support the findings reported herein are available from the corresponding author upon reasonable request.
Abbreviations
- ATCC:
-
American type culture collection
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- FL:
-
Fidelity level
- IC50 :
-
50% inhibition concentration
- MIC :
-
Minimum inhibitory concentration
- PCA :
-
Principal Component Analysis
- UV-Vis :
-
Ultra-Violet Visible
References
Teka A, Rondevaldova J, Asfaw Z, Demissew S, Van Damme P, Kokoska L, et al. In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south central Ethiopia. BMC Complement Altern Med. 2015;15:286.
Acharjee M, Zerin N, Ishma T, Mahmud MR. In vitro antibacterial activity of medicinal plants against Urinary Tract Infection (UTI) causing bacteria along with their synergistic effects with commercially available antibiotics. New Microbes New Infect. 2023;51:101076.
Singh A, Kumar J, Sharma VK, Singh DK, Kumari P, Nishad JH, et al. Phytochemical analysis and antimicrobial activity of an endophytic Fusarium proliferatum (ACQR8), isolated from a folk medicinal plant Cissus quadrangularis L. South Afr J Bot. 2021;140:87–94.
Qadri H, Haseeb Shah A, Mudasir Ahmad S, Alshehri B, Almilaibary A, Ahmad Mir M. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J Biol Sci. 2022;29:103376.
Prabu SL, Umamaheswari A, GF SJ. Investigation on the biofilm eradication potential of selected medicinal plants against methicillin-resistant Staphylococcus aureus. Biotechnol Rep. 2020;28:e00523.
Rajput M, Kumar N. Medicinal plants: a potential source of novel bioactive compounds showing antimicrobial efficacy against pathogens infecting hair and scalp. Gene Rep. 2020;21:100879.
Baloyi IT, Cosa S, Combrinck S, Leonard CM, Viljoen AM. Anti-quorum sensing and antimicrobial activities of South African medicinal plants against uropathogens. S Afr J Bot. 2019;122:484–91.
Nazar S, Hussain MA, Khan A, Muhammad G, Bukhari SNA. Alkaloid-rich plant Tylophora indica; current trends in isolation strategies, chemical profiling and medicinal applications. Arab J Chem. 2020;13:6348–65.
Mapfumari S, Nogbou N-D, Musyoki A, Gololo S, Mothibe M, Bassey K. Phytochemical screening, antioxidant and antibacterial properties of extracts of Viscum Continuum E. Mey. Ex Sprague, a South African mistletoe. Plants. 2022;11:2094.
Jafri SAA, Khalid ZM, Khan MR, Ashraf S, Ahmad N, Karami AM, et al. Evaluation of some essential traditional medicinal plants for their potential free scavenging and antioxidant properties. J King Saud Univ Sci. 2023;35:102562.
Atta S, Waseem D, Naz I, Rasheed F, Phull AR, Ur-Rehman T, et al. Polyphenolic characterization and evaluation of multimode antioxidant, cytotoxic, biocompatibility and antimicrobial potential of selected ethno-medicinal plant extracts. Arab J Chem. 2023;16:104474.
Alu’datt MH, Rababah T, Alhamad MN, Gammoh S, Al-Mahasneh MA, Tranchant CC, et al. Pharmaceutical, nutraceutical and therapeutic properties of selected wild medicinal plants: thyme, spearmint, and rosemary. In: Ther Probiotic Unconv Foods. 2018. p. 275–90. https://linkinghub.elsevier.com/retrieve/pii/B9780128146255000145. Accessed 10 May 2023.
Iqbal E, Salim KA, Lim LBL. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus Velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ Sci. 2015;27:224–32.
Martin GJ. Ethnobotany: a methods manual. 1st ed. London, New York: Chapman & Hall; 1995.
Tahir M, Asnake H, Beyene T, Van Damme P, Mohammed A. Ethnobotanical study of medicinal plants in Asagirt District, Northeastern Ethiopia. Trop Med Health. 2023;51:1.
Gonfa T, Teketle S, Kiros T. Effect of extraction solvent on qualitative and quantitative analysis of major phyto-constituents and in vitro antioxidant activity evaluation of Cadaba rotundifolia Forssk leaf extracts. Yildiz F, editor. Cogent Food Agric. 2020;6:1853867.
Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80.
Iswandana R, Olisia S, Adriyani M, Jufri M, Munim A. Application of tween 80 and tween 20 for microwave-assisted extraction of oxyresveratrol from mulberry (Morus alba L.) twigs. J Appl Pharm Sci. 2020;10:93–100.
Kızıl G, Kızıl M, Yavuz M, Emen S, Hakimoğlu F. Antioxidant activities of ethanol extracts of Hypericum triquetrifolium. and Hypericum scabroides. Pharm Biol. 2008;46:231–42.
Bargale SS, Tripathy TB, Shashirekha HK. Phyto physicochemical profile of Withania somnifera Dunal (Solanaceae). J Drug Deliv Ther. 2019;9:263–8.
Mechqoq H, Hourfane S, Yaagoubi ME, Hamdaoui AE, Msanda F, Almeida JRG da S, et al. Phytochemical screening, and in vitro evaluation of the antioxidant and dermocosmetic activities of four Moroccan plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics. 2022;9:94.
Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud. 2020;8:603–8.
Odeja OO, Ibok MG, Okpala EO. Composition and biological assays of the leaf essential oil of Asparagus flagellaris (Kunth) Bak. Clin Phytosci. 2021;7:12.
Masters ET. Medicinal plants of the upper Aswa River catchment of northern Uganda - a cultural crossroads. J Ethnobiol Ethnomed. 2023;19:48.
Megersa M, Nedi T, Belachew S. Ethnobotanical study of medicinal plants used against human diseases in Zuway Dugda District, Ethiopia. Gorzalczany S, editor. Evid Based Complement Alternat Med. 2023;2023:1–22.
Woldeamanuel MM, Geda MK, Mohapatra S, Bastia TK, Rath P, Panda AK. Ethnobotanical study of endemic and non-endemic medicinal plants used by indigenous people in environs of Gullele botanical garden Addis Ababa, central Ethiopia: a major focus on Asteraceae family. Front Pharmacol. 2022;13:1020097.
Gosa BB, Wana TW. Evaluation of the phytochemical and antibacterial activity of four selected plant extracts against some pathogenic bacteria. Int J Environ Agric Biotechnol. 2022;7:265–76.
Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-Gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11:4.
Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124:69–78.
Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-Awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–25.
Liu L, Dong Z, Ai S, Chen S, Dong M, Li Q, et al. Virulence-related factors and antimicrobial resistance in Proteus mirabilis isolated from domestic and stray dogs. Front Microbiol. 2023;14:1141418.
Tumbarello M, Trecarichi EM, Fiori B, Losito AR, D’Inzeo T, Campana L, et al. Multidrug-resistant Proteus mirabilis bloodstream infections: risk factors and outcomes. Antimicrob Agents Chemother. 2012;56:3224–31.
Chabi R, Momtaz H. Virulence factors and antibiotic resistance properties of the Staphylococcus epidermidis strains isolated from hospital infections in Ahvaz, Iran. Trop Med Health. 2019;47:56.
Eladli MG, Alharbi NS, Khaled JM, Kadaikunnan S, Alobaidi AS, Alyahya SA. Antibiotic-resistant Staphylococcus epidermidis isolated from patients and healthy students comparing with antibiotic-resistant bacteria isolated from pasteurized milk. Saudi J Biol Sci. 2019;26:1285–90.
Taye B, Giday M, Animut A, Seid J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac J Trop Biomed. 2011;1:370–5.
Kalbessa A, Dekebo A, Tesso H, Abdo T, Abdissa N, Melaku Y. Chemical constituents of root barks of Gnidia involucrata and evaluation for antibacterial and antioxidant activities. J Trop Med. 2019;2019:1–8.
Zakerifar M, Kaboosi H, Goli HR, Rahmani Z, Peyravii Ghadikolaii F. Antibiotic resistance genes and molecular typing of Streptococcus agalactiae isolated from pregnant women. BMC Pregnancy Childbirth. 2023;23:43.
Guadie A, Dakone D, Unbushe D, Wang A, Xia S. Antibacterial activity of selected medicinal plants used by traditional healers in Genta Meyche (Southern Ethiopia) for the treatment of gastrointestinal disorders. J Herb Med. 2020;22:100338.
El Hachlafi N, Benkhaira N, Al-Mijalli SH, Mrabti HN, Abdnim R, Abdallah EM, et al. Phytochemical analysis and evaluation of antimicrobial, antioxidant, and antidiabetic activities of essential oils from Moroccan medicinal plants: Mentha suaveolens, Lavandula stoechas, and Ammi visnaga. Biomed Pharmacother. 2023;164:114937.
Škrovánková S, Mišurcová L, Machů L. Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res. 2012:75–139. https://linkinghub.elsevier.com/retrieve/pii/B9780123945983000034. Accessed 20 Nov 2023.
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72.
Agidew MG. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull Natl Res Cent. 2022;46:87.
Mlozi SH. The role of natural products from medicinal plants against COVID-19: traditional medicine practice in Tanzania. Heliyon. 2022;8:e09739.
Nagorny P, Cichowicz N. New strategy based on sequential Michael/Aldol reactions for the asymmetric synthesis of cardenolides. Strateg Tactics Org Synth. 2016:237–67. https://linkinghub.elsevier.com/retrieve/pii/B9780081007563000091. Accessed 24 Jul 2023.
Dey P, Kundu A, Kumar A, Gupta M, Lee BM, Bhakta T, et al. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv Nat Prod Anal. 2020:505–67. https://linkinghub.elsevier.com/retrieve/pii/B9780128164556000159. Accessed 2 Aug 2023.
Shen Y, Liang W-J, Shi Y-N, Kennelly EJ, Zhao D-K. Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids. Nat Prod Rep. 2020;37:763–96.
Sharma T, Pandey B, Shrestha BK, Koju GM, Thusa R, Karki N. Phytochemical screening of medicinal plants and study of the effect of phytoconstituents in seed germination. Tribhuvan Univ J. 2020;35:1–11.
Bouyahya A, El Omari N, Elmenyiy N, Guaouguaou FE, Balahbib A, Belmehdi O, et al. Moroccan antidiabetic medicinal plants: ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci Technol. 2021;115:147–254.
Bailly C, Vergoten G. Esculentosides: insights into the potential health benefits, mechanisms of action and molecular targets. Phytomedicine. 2020;79:153343.
Liu R, Su B, Huang F, Ru M, Zhang H, Qin Z, et al. Identification and analysis of cardiac glycosides in Loranthaceae parasites Taxillus chinensis (DC.) Danser and Scurrula Parasitica Linn. and their host Nerium Indicum Mill. J Pharm Biomed Anal. 2019;174:450–9.
Ravi BG, Guardian MGE, Dickman R, Wang ZQ. High-resolution tandem mass spectrometry dataset reveals fragmentation patterns of cardiac glycosides in leaves of the foxglove plants. Data Brief. 2020;30:105464.
Patil SB. Medicinal significance of novel coumarin analogs: recent studies. Results Chem. 2022;4:100313.
Ntemafack A, Singh RV, Ali S, Kuiate JR, Hassan QP. Antiviral potential of anthraquinones from Polygonaceae, Rubiaceae and Asphodelaceae: potent candidates in the treatment of SARS-COVID-19, a comprehensive review. S Afr J Bot. 2022;151:146–55.
Netravati GS, Pathrose B, N MR, Kuruvila PMJ. Comparative evaluation of anthocyanin pigment yield and its attributes from Butterfly pea (Clitorea Ternatea L.) flowers as prospective food colorant using different extraction methods. Future Foods. 2022;6:100199.
Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB. Preliminary analysis of phytoconstituents and evaluation of anthelminthic property of Cayratia auriculata (in vitro). Maedica. 2019;14:350.
Acknowledgements
The authors are grateful to the Office of Postgraduate Program, Office of the Research Directorate, College of Natural and Computational Sciences, and Department of Plant Biology and Biodiversity Management of Addis Ababa University for making the financial support for this work available through the Thematic Project entitled ‘Ethnobotany of the medicinal and wild edible plants of the Dibatie people, and antimicrobial activity study of plants against infectious diseases of the Dibatie district, Metekel zone, Benishangul Gumuz Regional State, Western Ethiopia’. Likewise, we would like to thank Directorate of the Modern and Traditional Medicine Research of Ethiopian Public Health Institute for part of financial and chemical support, and laboratory facilities. Field assistants, informants and administrative leaders in the study area are acknowledged for their support during ethnobotanical survey and sample collection.
Funding
This study was funded by the Office of Postgraduate Program and Office of the Directorate for Research in Addis Ababa University, and by Directorate of the Modern and Traditional Medicine Research in Ethiopian Public Health Institute.
Author information
Authors and Affiliations
Contributions
B.A. conducted the ethnobotanical survey, selected and identified the plants, collected samples and conducted the laboratory work, analyzed the data, and wrote the original draft. E.L. identified the plants, supervised the field and laboratory works, and reviewed the manuscript. A.H. and A.D. supervised the field and laboratory works, and reviewed the manuscript. E.D. participated in the plant extraction, phytochemical screening, and antioxidant assay. A.A. and S.D. carried out the antibicterial assay.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethnobotanical survey and plant material collection were carried out following the letters of consent given by the Department of Plant Biology and Biodiversity Management at Ababa University (Ref. no. DPBBM/CNS/478/13/2020) and the Office of Dibatie District Administration (Ref. no. 766/
 ).
).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Anbessa, B., Lulekal, E., Hymete, A. et al. Ethnomedicine, antibacterial activity, antioxidant potential and phytochemical screening of selected medicinal plants in Dibatie district, Metekel zone, western Ethiopia. BMC Complement Med Ther 24, 199 (2024). https://doi.org/10.1186/s12906-024-04499-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04499-x