Abstract
Background
Neonicotinoids are a group of synthetic insecticides that are highly effective and have a wide range of insecticidal activities. This group includes acetamiprid, dinotefuran, clothianidin, imidacloprid, sulfoxaflor, nitenpyram, thiamethoxam, and thiacloprid. They are extensively used worldwide, both in rural and urban environments. However, the widespread use of neonicotinoids has led to their accumulation and biomagnification in the environment due to their long half-life. This has resulted in the emergence of toxicological and hazardous pollutants, posing significant risks to humans and non-target animals. Neonicotinoids are a type of insecticides that bind to neuronal nicotinic acetylcholine receptors (nAChRs). This mechanism allows them to effectively activate insect nAChRs while having minimal impact on vertebrate nAChRs. This reduces the risk of toxicity and makes them safer for non-target species. However, the presence of neonicotinoids in the environment can still increase the risk of toxicity and exposure. Although they have low affinity for mammalian nAChRs, concerns arise due to the abundance, diversity, and widespread presence of these receptors, as well as their various functions. These factors raise concerns about the potential impact of these pesticides on unintended species. Therefore, it is crucial to remove neonicotinoids from the environment in a sustainable and methodical manner.
Main body of the abstract
Various techniques can be employed to eliminate neonicotinoid residues in soil and aquatic habitats. These techniques include physiochemical remediation methods such as advanced oxidation processes, adsorption, oxidation, Fenton technology, photocatalysis, and activated persulfate-based oxidation. Additionally, microbial remediation techniques involving bacteria, fungi, and microalgae can also be utilized. This review aims to focus on the scientific foundation, advancements, and key topics related to microbial remediation technologies for neonicotinoids. Proper implementation of bioremediation techniques can significantly reduce the harmful effects of neonicotinoids on the environment and human health.
Short conclusion
The main focus of this review is the new studies on the bioremediation of neonicotinoids by bacteria, fungi, and microalgae, and the role of their enzymes. This topic is gaining importance as pesticide bioremediation techniques become increasingly significant.
Similar content being viewed by others
Background
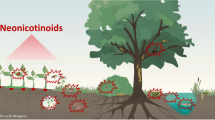
Worldwide, the pesticides market is growing rapidly due to the increasing demand for food and high-yielding crops (Dhuldhaj et al. 2023). Neonicotinoids, often known as insecticides, are frequently employed to protect crops (such as wheat, rice, maize, soybeans, cotton, apple, sugar, beetroot, and potato) against a variety of pests and illnesses carried by insects, hence enhancing production (Simon-Delso et al. 2015; Katić et al. 2021). In order to enhance their insecticidal qualities, neonicotinoids are created and synthesized using the findings of investigations on the structure of nicotine (Humann-Guilleminot et al. 2019). Neonicotinoids, or synthetic nicotinoids with enhanced insecticidal qualities, include clothianidin, acetamiprid, dinotefuran, nitenpyram, imidacloprid, thiamethoxam, and thiacloprid, which have a structure similar to nicotine (Fig. 1). These chemicals are primarily used to control harmful pests, thereby safeguarding crops. They primarily target nicotinic acetylcholine receptors, which have endocrine-disrupting properties. Neonicotinoids, a family of insecticides, work by selectively binding to neuronal nicotinic acetylcholine receptors (nAChRs). This mechanism explains why these insecticides are effective, as they strongly activate insect nAChRs while having little affinity for vertebrate nAChRs. This characteristic reduces the risk of toxicity and enhances safety for non-target species. Although neonicotinoids are generally considered harmless, their presence in the environment can increase the risk of toxicity and exposure. Despite their low affinity for mammalian nAChRs, the abundance, diversity, and widespread presence of these receptors, along with their various functions, raise concerns about the potential impact of these pesticides on unintended species (Casida 2018). The mode of action of neonicotinoid and nicotinic acetylcholine receptor activity when (a) acetylcholine and (b) a neonicotinoid are present is illustrated in Fig. 2. Neonicotinoid environmental contaminants have skyrocketed during the last two decades as a result of their widespread use. Neonicotinoids have minimal soil adsorption (logKoc 1.4–2.3), high water solubility and minimal logKow (0.55–1.26). Partitioning properties and large consumption volume led to the pesticides prolonged persistence in soil as well as their enhanced leaching ability and transport via surface and subterranean runoff, which polluted both water and soil systems (Morrissey et al. 2015; Hladik et al. 2018; Gautam and Dubey 2022). The entry of neonicotinoids into the environment increases toxicological and hazardous pollutants, causing major health and environmental issues (Yu et al. 2020; Wu et al. 2021). Numerous studies have demonstrated the presence of persistent neonicotinoids in the environment, the detrimental effects of neonicotinoids on a variety of species, including mammals, and the potential routes of human exposure to neonicotinoids depicted in Fig. 3 (Cimino et al. 2017). Thus, it is critical to investigate operational and sustainable strategies for remediating polluted habitats caused by this pollutant (Gautam and Dubey 2023). Several methods have been developed to remove neonicotinoids and their leftovers from soil and water sources (Pang et al. 2020). Conventional physiochemical cleanup methods are difficult, costly and time-consuming.
Generally, a variety of techniques can be used to reduce hazardous materials in the environment, such as physical adsorption and sophisticated oxidant processes (Ye et al. 2019). It is important to develop effective methods that are both cost-effective and environmentally friendly. In conclusion, microbial remediation techniques are a promising option for remediating neonicotinoids, both on-site and off-site, due to their low cost, minimal environmental impact, low risk of secondary contamination, and compatibility with other technologies (Li et al. 2020). Microorganisms, including bacteria, fungi, and microalgae, are used in the environmentally friendly process of biodegradation, which eliminates harmful substances from the environment (Bala et al. 2022). Microbial breakdown of neonicotinoids is considered to be the most effective and environmentally friendly on-site remediation process (Hamada et al. 2019). Microorganisms with the ability to degrade substances and survive in high-stress pesticide concentrations can be employed for the remediation of polluted environments, including toxic waste sites and hazardous areas (Rana et al. 2015; Bhatt et al. 2019). These microorganisms produce enzymes that break down xenobiotic compounds, such as agrochemicals, into less environmentally harmful molecules when exposed to moderate conditions like temperature, pH, medium, and salinity (Parte et al. 2017a, b).
Numerous microbes that can degrade neonicotinoids have been isolated and identified (Ahmad et al. 2021). In order to investigate the potential for biodegradation of neonicotinoids in polluted soil and water environments, a wide variety of microorganisms have been used (Hamada et al. 2019). However, further research is needed to understand the specific enzymes and genes involved in degradation. Currently, only a few studies have focused on the genetic and enzymatic basis of organisms that can degrade neonicotinoids, with the aim of developing more sustainable degradation methods (Parween et al. 2016). The main purpose of this review is to highlight the relevance, impact, and significant applications of microbial remediation and enzymatic techniques in efficiently eliminating harmful neonicotinoids from the environment.
Main text
Microbial degradation of neonicotinoids
Numerous enzymes produced by microorganisms play a crucial role in the biotransformation and biodegradation processes involved in microbial remediation of non-engineering organic pollutants known as neonicotinoids (Anjos et al. 2021). Among these pollutants, imidacloprid and acetamiprid are extensively studied for microbial remediation due to their widespread use and commercialization. Research has shown that fungi, bacteria, and microalgae are capable of breaking down neonicotinoids, but bacteria are the most commonly studied organisms in this regard. Degradation rates for neonicotinoids by microbial consortia and isolated strains range from 20 to 100% (Wei et al. 2023). However, the chemical composition and properties of neonicotinoids, the specific strains of microorganisms employed, and the surrounding environment all significantly influence the degradation rates and pathways. Therefore, it is crucial to identify the specific strains capable of degrading particular neonicotinoids under specific conditions. The subsequent subsections will focus on the degradation of neonicotinoids by various microbes, including bacteria, fungi, and microalgae, and the utilization of their enzymes (Fig. 4).
Bacterial biodegradation of neonicotinoids
Bacteria are excellent candidates for microbiological remediation of non-emergent organic pollutants due to their wide distribution and abundance in water and soil. In fact, more than forty bacterial strains have been identified as capable of degrading or modifying various neonicotinoids (Wei et al. 2023). Some commonly reported bacterial strains involved in efficient neonicotinoid removal include Acinetobacter, Ochrobactrum, Pseudomonas, Stenotrophomonas, Ensifer, Pseudoxanthomonas, Variovorax, Bacillus, and Partitioniphaga (Tang et al. 2012). Degradation rates of neonicotinoids can range from 24.2 to 100% depending on the initial concentrations (Wei et al. 2023). Figure 5 illustrates the biodegradation pathways of certain neonicotinoids (thiacloprid, acetamiprid, clothianidin, imidacloprid), while Table 1 provides more specific information on the microorganisms involved in neonicotinoid degradation and the characteristics that contribute to their effectiveness.
Biodegradation pathway of thiacloprid by Stenotrophomonas maltophilia CGMCC1.178, Pseudomonas sp. RPT 52 (Zhao et al. 2009; Gupta et al. 2016), Stenotrophomonas sp. cleave and hydrolyze the N-cyanoimine group of acetamiprid to create the metabolite N-methyl-(6-chloro-3-pyridyl)methylamine (Tang et al. 2012; Wang et al. 2013a, b), clothianidin using WRF Phlebia brevispora in conjunction with Enterobacter sp. TN3W-14 and Pseudomonas sp. TN3W-8, Ochrobactrum anthropi (Wang et al. 2019c; Harry-Asobara and Kamei 2019) aldehyde oxidase (nitro reduction of imidacloprid), by Pseudomonas sp. 1G, amidase (cleavage of N-cyanoimine group) using Pigmentiphaga sp. (bacterial strain) (Pandey et al. 2009; Yang et al. 2013)
Biodegradation of neonicotinoids by fungi
White-rot fungi (WRF) are highly effective organisms that have been widely utilized for the decomposition of various stubborn pollutants, including persistent organic pollutants (POPs), pesticides, endocrine disruptors, and dyes. Their efficient enzymatic activity and strong capability for external degradation are the reasons behind their success in this regard (Mir-Tutusaus et al. 2018). Studies conducted by Mir-Tutusaus et al. (2014) have demonstrated that Trametes versicolor (a type of WRF) can effectively break down agrochemicals such as carbofuran, imiprothrin, cypermethrin, and oxytetracycline, thereby reducing their toxic effects while maintaining cell viability. Moreover, WRFs have shown promising results in bioremediating soil and water contaminated with polychlorinated biphenyls (PCBs), petroleum, chlorophenol, polychlorinated dioxins, and polychlorinated dibenzo-p-furans (PCDD/Fs) (Anasonye et al. 2014; Shahi et al. 2016; Stella et al. 2017). Additionally, research has revealed that the white-rot fungus Phanerochaete sordida can degrade neonicotinoid pesticides such as nitenpyram, clothianidin, dinotefuran, and acetamiprid into compounds with minimal neurological toxicity (Mori et al. 2021). Therefore, the application of this ubiquitous white-rot fungus shows great promise in reducing the pollution caused by neonicotinoid pesticides.
Table 2 provides an overview of the degradation rates and durations of neonicotinoids by various fungi. The transformation ranges from 31% to complete degradation and takes place over a period of 5 days to 4 weeks. The white-rot fungi group, which includes Trametes versicolor, Phlebia brevispora, Phanerochaete sordida, and Phanerochaete chrysosporium, consists of the majority of fungal strains responsible for breaking down neonicotinoids.
Filamentous fungi known as “white-rot” are commonly found in decaying wood and can cause the wood to appear whitened (Yin et al. 2020; Zhuo and Fan 2021). These fungi possess a distinctive ligninolytic system, which includes extracellular enzymes such as laccase (Lac), lignin peroxidase (Lip), manganese peroxidase (MnP), and versatile peroxidase (VP). These enzymes are highly effective in breaking down a wide range of organic pollutants and exhibit low substrate specificity.
The fungal breakdown of neonicotinoids is primarily carried out by internal enzymes such as cytochrome P450 and peroxidase (POD), as well as extracellular enzymes like lignin peroxidase, manganese peroxidase, laccase, and apoenzymes produced by fungal mycelia. The metabolic mechanisms used by bacteria and fungi to break down neonicotinoids are similar. However, reports suggest that cytochrome P450 plays a more significant role in the breakdown of neonicotinoids by fungus. In the case of the commonly studied WRF, Phanerochaete chrysosporium, the inhibition of cytochrome P450 results in a slower degradation rate of acetamiprid (Wang et al. 2019a).
The main enzymes in P. chrysosporium that break down the chloropyridinyl group and target the side chains of acetamiprid, imidacloprid, and thiamethoxam through N-dealkylation are two cytochrome P450 isozymes: CYP5147A3 and CYP5037B3 (Mori et al. 2021). Most WRF strains convert neonicotinoids into metabolites that are either less toxic or non-toxic. However, certain modified products exhibit increased toxicity compared to the original substances. For example, Phanerochaete sordida YK-624 converted dinotefuran into a more hazardous metabolic by-product, while Trametes versicolor transformed imidacloprid and dinotefuran into hydroxylated derivatives with higher toxicity (Wang et al. 2019b; Hu et al. 2022). Harry-Asobara and Kamei (2019) proposed a potential method to enhance the decomposition of acetamiprid and clothianidin by using the WRF Phlebia brevispora in combination with Enterobacter sp. TN3W-14 and Pseudomonas sp. TN3W-8, two bacteria that promote mycelial morphology and proliferation. This approach utilizes a combination of high- and low-neonicotinoid metabolism fungi to effectively remediate neonicotinoids. Furthermore, there are only a few fungal strains currently capable of breaking down neonicotinoids (Table 2), and large-scale fermentation is time-consuming, making it challenging to employ these strains for industrial bioremediation purposes.
Biodegradation of neonicotinoids by microalgae
Aquatic creatures called microalgae are fast-developing and naturally found in various environments throughout the ocean, including freshwater and saltwater. These microalgae play a crucial role in maintaining the balance of the aquatic ecosystem as they are the foundation of the food pyramid (Goswami et al. 2022). Recent research has focused on the ability of microalgae to recover essential nutrients like nitrogen and phosphorus from secondary effluents, which helps prevent eutrophication and addresses environmental issues caused by certain pollutants (Nguyen et al. 2021; Abdelfattah et al. 2022; Mahari et al. 2022). Additionally, microalgae can be used for tertiary treatment in wastewater and produce biomass with various industrial applications (Salama et al. 2017). The biomass generated from microalgae growth can also serve as feedstock for industrial goods and be used in the commercial production of bioenergy. Microalgae have been successfully used to treat pesticides, dyes, heavy metals, and medications from sources like home wastewater, agricultural runoff, and pharmaceuticals (Ashour et al. 2021; Arutselvan et al. 2022; Bhatt et al. 2022). They are capable of degrading and detoxifying a wide range of organic and inorganic pollutants through bioadsorption, bioaccumulation, and biodegradation processes (Sutherland and Ralph 2019; Mustafa et al. 2021) (Table 3). These mechanisms offer promising approaches for removing pesticides at various points of entry. According to Nie et al. (2020), microorganisms can remove pesticides through metabolic processes.
Eukaryotic, photosynthetic microorganisms that are commonly found in aquatic environments are known as microalgae (Goswami et al. 2022). Removing xenobiotics from wastewater treatment plants has shown potential with them. In order for Nannochloropsis sp. to break down imidacloprid by around 50% in the first 20 h, Encarnação et al. (2021) state that light aeration and the presence of UV radiation were crucial. It has been proven possible to enhance imidacloprid elimination by mixing microalgae and bacteria in a biofilm reactor. In wastewater with visible light, Cheng et al. (2022a) demonstrated that the algae-bacteria biofilm reduced the toxicity of the wastewater and eliminated 74.9% of the inorganic particles. The steps involved in the breakdown process of imidazole include ring opening of imidazole and pyridine, hydrolysis, hydroxylation, and the loss of nitro and chlorine groups. Nevertheless, the mineralization was not complete. Nutrient recovery, carbon dioxide sequestration, and the removal of various pollutants, such as pesticides, endocrine disruptors, heavy metals, and medications, are among the multifunctional benefits of microalgae-mediated remediation (Sharma et al. 2022). Microalgae-mediated remediation may be used to complex pollution situations due to its versatility. To fully explore its potential to transform poisons into valuable items that will conserve resources, more study is necessary.
Enzyme-mediated degradation of neonicotinoids
Table 4 provides a summary of the most recent research on the enzymes that degrade imidacloprid. According to Pang et al. (2020), the ABC transporter, which facilitates the detoxification of foreign organisms also known as xenobiotic detoxification, is partly responsible for insects’ ability to detoxify pesticides. ABCG3, an anti-imidacloprid gene derived from Bemisia tabaci, is more highly expressed in females than in males. The suppression of ABCG3 mediated by RNA interference can significantly increase the mortality rate of adult Bemisia tabaci (He et al. 2019). 44 DcitABC transporters have been identified using the Diaphorina citri transcriptome and genome database. In addition to metabolic enzyme genes, DcitABC transporters are well characterized in several D. citri species and play a role in the detoxification of imidacloprid (Liu et al. 2020). Detoxification enzymes in insects, such as glutathione S-transferase, esterase, and P450s, are responsible for catabolizing toxins and pesticides. These enzymes have the ability to increase the gene copy number, mRNA levels, and coding sequence variety by introducing point mutations in the targeted pesticide gene (Li et al. 2007). One well-known family of monooxygenases is cytochrome P450, which has been recognized for its capability to utilize molecular oxygen for substrate oxidation and hydroxylation. This family of enzymes shows promises in various industrial processes (Scott et al. 2008). Cytochrome P450 enzymes are involved in multiple processes, including the metabolism and biosynthesis of foreign substances. Studies have revealed that insects possess a wide range of P450 genes, ranging from 46 to over 150, and each gene may encode a distinct P450 enzyme.
Bioinformatics analysis has demonstrated that BtCPR is a transmembrane protein. With a molecular weight of 76.73 kDa, it consists of NADPH, FAD, and FMN, three conserved binding domains. The highest concentrations of BtCPR are found in the tissues of the adult male whitefly’s head. Its sensitivity to imidacloprid is significantly increased when BtCPR is inhibited (He et al. 2020). Furthermore, Nilaparvata lugens does not show imidacloprid and pyrazine cross-resistance. Nilaparvata lugens has the potential to develop resistance to imidacloprid due to the efficient metabolism of the drug by two P450s, CYP6AY1 and CYP6ER1 (Yang et al. 2016). In the Chinese lizard Eremias argus, CYP2C9 and aldehyde oxidase play a significant role in the reduction of nitro groups, whereas CYP3A4 controls the hydroxylation and desaturation processes (Raby et al. 2018).
Microbial degradation mechanism of neonicotinoids
The microbial breakdown of neonicotinoids is primarily carried out by metabolic processes. These processes involve a specific chemical group called the “magic nitro group.” This group consists of a nitrile group (–C≡N) for acetamiprid and thiamethoxam, and a nitro group (=N–NO2) for imidacloprid, clothianidin, thiamethoxam, nitenpyram, and dinotefuran. The breakdown process begins with the transformation of the nitro group, which can be achieved through denitration, nitro reduction, cleavage, and hydrolysis of the N-cyanoimine group. Subsequent metabolic reactions include dichlorination, hydrolysis, ring opening, oxidation or reduction, and oxidative or reductive cleavage. Throughout the early and middle stages of degradation, various responses can occur. Gautam and Pandey (2022) have identified two bacterial strains, Sphingobacterium sp. and Agrobacterium sp., capable of degrading imidacloprid in pesticide-contaminated farming soils. These strains have the ability to modify imidacloprid, reducing its lethality by lowering the nitro group and producing a less harmful imidacloprid guanidine compound. The metabolic pathways of other neonicotinoids, including imidacloprid, have also been studied.
According to Tang et al. (2012), Stenotrophomonas sp. can cleave and hydrolyze the N-cyanoimine group of acetamiprid, resulting in the formation of the metabolite N-methyl-(6-chloro-3-pyridyl)methylamine. Similarly, Wang et al. (2013a) also observed that Pigmentiphaga sp. AAP-1 produces the same metabolic product (N-methyl-(6-chloro-3-pyridyl)methylamine) by hydrolyzing and cleaving the N-cyanoimine group of acetamiprid. This particular metabolite is considered to be minimally harmful to bees and mammals. In another study, Zhou et al. (2013) found that the bacterial strain Ensifer adhaerens TMX-23 transforms the N-nitroimino group of thiamethoxam into urea and N-nitrosoimine. Furthermore, Sharma et al. (2014) conducted a comprehensive study on the metabolism of imidacloprid using Bacillus alkalinitrilicus obtained from sugarcane cultivation soil. Their study revealed three metabolic products: 6-chloronicotinic acid, guanidine imidacloprid, and nitroso imidacloprid. These results suggest the presence of two different metabolic pathways. In the first pathway, imidacloprid is reduced to nitroso imidacloprid and guanidine imidacloprid, while in the second pathway, it is oxidized to 6-chloronicotinic acid. Both pathways involve the conversion of intermediates into CO2.
In a study conducted by Shettigar et al. (2012), it was discovered that the bacterial strain Bradyrhizobiaceae SG-6C has the ability to convert 6-chloronicotinic acid to CO2, despite being identified in previous studies as a dead-end metabolite (DEM) of imidacloprid and acetamiprid breakdown. This finding suggests a potential method for fully degrading neonicotinoids. Neonicotinoids, such as imidacloprid and thiamethoxam, rely on hydroxylation for their metabolism, with the resulting metabolites exhibiting varying levels of toxicity. Stenotrophomonas maltophilia CGMCC 1.1788 was found to hydroxylate both imidacloprid and thiamethoxam, but Zhao et al. (2009) discovered that the metabolic products of imidacloprid (5-hydroxy and olefin imidacloprid) were more toxic than the parent molecule, while the metabolic compounds of thiamethoxam (4-hydroxy thiamethoxam and the resulting 4-ketone thiamethoxam imine) were less toxic. Despite a limited number of studies proposing enzymatic and genetic mechanisms, as well as metabolic pathways, for the microbial degradation of neonicotinoids, it has been demonstrated that a variety of bacterial enzymes are involved in different metabolic activities. Aldehyde oxidase, carried out by Pseudomonas sp. 1G, is responsible for the nitro reduction of imidacloprid, while Pigmentiphaga sp. utilizes amidase to cleave the N-cyanoimine group of acetamiprid. Nitrile hydratase (NHase) and cytochrome P450 are two other enzymes that assist in the degradation of neonicotinoids (Pandey et al. 2009; Yang et al. 2013). NHase and cytochrome P450 are known to play a significant role in breaking down specific neonicotinoids. Various species have demonstrated that cytochrome P450 enzymes actively facilitate the degradation of neonicotinoids. Research has shown a close connection between hydroxylation and P450 in bacteria. For example, in a study conducted by Guo et al. (2020) using Hymenobacter latericoloratus CGMCC 16346 to reduce imidacloprid levels in surface water, it was found that piperonyl butoxide, a suppressor of cytochrome P450 monooxygenase, hindered the removal of imidacloprid hydroxyl group, resulting in the formation of 5-hydroxy and olefin imidacloprid. Additionally, NHase plays a crucial role in the mineralization of two neonicotinoids, acetamiprid and thiamethoxam, by catalyzing the synthesis of amides from nitriles. NHase can aid in the reaction of acetamiprid hydration to (E)-N2-carbamoyl-N1-[(6-chloro-3-pridyl)methyl]. A number of bacteria, such as Pseudaminobacter salicylatoxidans CGMCC and Variovorax boronicumulans CGMCC 4969 1.17248, require N1-methylacetamidine (IM-1-2) in order to break down acetamiprid (Sun et al. 2017; Guo et al. 2021).
Conclusions
The previous sections provided an overview of neonicotinoids, which are commonly used insecticides in agriculture due to their strong insecticidal properties. Neonicotinoids target the nicotinic acetylcholine receptors (nAChRs) in insects neurons, specifically activating them without affecting vertebrates. However, there is still a possibility of toxicity and exposure to neonicotinoids in the environment. Although these insecticides have a reduced affinity for mammalian nAChRs, the abundance and distribution of these receptors raise concerns. Therefore, it is crucial to consider the long-term negative impacts of neonicotinoids on the environment and work toward removing these residues from polluted ecosystems. Using efficient neonicotinoid-degrading bacteria for remediation is considered a viable approach in contaminated areas. The microbial degradation processes and toxicity of specific neonicotinoids have been extensively studied, while others have received less attention. Many breakdown intermediates of neonicotinoid pesticides, especially imidacloprid, pose a greater risk than the original compound.
The researchers studied microorganisms found in wastewater, rhizosphere soils, neonicotinoid-polluted agricultural soils, and microbial preservation centers. None of the isolated bacteria were able to completely metabolize thiacloprid, acetamiprid, imidacloprid, clothianidin, and thiamethoxam. However, different bacteria were found to break down imidacloprid and acetamiprid simultaneously, producing carbon dioxide and water. The long-term effectiveness of imidacloprid and other neonicotinoids in causing environmental degradation depends on the isolation or cultivation of such bacteria. Only a limited number of studies have determined the enzymes and breakdown pathways involved. Further research is needed to understand the process of bioremediation through the breakdown of microorganisms and enzymes in order to develop an organized approach to treating neonicotinoids.
In order to comprehend the degradation processes in a polluted environment, it is crucial to examine how genes and enzymes function. Different microorganisms that can break down neonicotinoid compounds have been found, but research on the enzymes and functional genes of these species has been limited. Therefore, before extensively utilizing neonicotinoid-degrading microorganisms for bioremediation, a significant amount of fundamental research is needed. High-throughput sequencing techniques have been proposed as a potential tool for thoroughly annotating the genes and metabolites generated during microbial breakdown.
Moreover, the development of modified degrading microbes and the utilization of degrading genes and enzymes are necessary. Additionally, it is important to examine whether the collaborative breakdown of microbial consortia helps prevent the accumulation of hazardous compounds. Recent advancements in omics-based technologies have provided new insights into pesticide biodegradation, which could contribute to the formulation of an effective bioremediation strategy for neonicotinoid-polluted environments.
Availability of data and materials
Not applicable.
Abbreviations
- ACh :
-
Acetylcholine
- CAT :
-
Catalase
- CO2 :
-
Carbon dioxide
- DEM :
-
Dead-end metabolite
- Lac :
-
Laccase
- Lip:
-
Lignin peroxidase
- MnP :
-
Manganese peroxidase
- nAChR :
-
Nicotinic acetylcholine receptors
- NHase :
-
Nitrile hydratase
- PCBs :
-
Polychlorinated biphenyls
- PCDDs :
-
Polychlorinated dibenzo-p-dioxins
- PCDFs :
-
Polychlorinated dibenzo-p-furans
- POD :
-
Peroxidase
- POPs :
-
Persistence organic pollutants
- UV :
-
Ultraviolet
- VP :
-
Versatile peroxidase
- WRF :
-
White-rot fungi
References
Abdelfattah A, Ali SS, Ramadan H, El-Aswar EI, Eltawab R, Ho SH, Elsamahy T, Li S, El-Sheekh MM, Schagerl M (2022) Microalgae-based wastewater treatment: mechanisms, challenges, recent advances, and future prospects. Environ Sci Ecotech 13:100205
Ahmad S, Cui D, Zhong G, Liu J (2021) Microbial technologies employed for biodegradation of neonicotinoids in the agroecosystem. Front Microbiol 12:759439
Akoijam R, Singh B (2015) Biodegradation of imidacloprid in sandy loam soil by Bacillus aerophilus. Int J Environ Anal Chem 95(8):730–743
Anasonye F, Winquist E, Kluczek-Turpeinen B, Rasanen M, Salonen K, Steffen KT, Tuomela M (2014) Fungal enzyme production and biodegradation of polychlorinated dibenzo-p-dioxins and dibenzofurans in contaminated sawmill soil. Chemosphere 110:85–90. https://doi.org/10.1016/j.chemosphere.2014.03.079
Anjos CS, Lima RN, Porto ALM (2021) An overview of neonicotinoids: biotransformation and biodegradation by microbiological processes. Environ Sci Pollut Res Int 28:37082–37109. https://doi.org/10.1007/s11356-021-13531-3
Arutselvan C, Narchonai G, Pugazhendhi A, Seenivasan HK, LewisOscar F, Thajuddin N (2022) Phycoremediation of textile and tannery industrial effluents using microalgae and their consortium for biodiesel production. J Clean Prod 367:133100
Ashour M, Alprol AE, Heneash AMM, Saleh H, Abualnaja KM, Alhashmialameer D, Mansour AT (2021) Ammonia bioremediation from aquaculture wastewater effluents using Arthrospira platensis NIOF17/003: impact of biodiesel residue and potential of ammonia-loaded biomass as rotifer feed. Materials 14:5460
Bala S, Garg D, Thirumalesh BV, Sharma M, Sridhar K, Inbaraj BS, Tripathi M (2022) Recent strategies for bioremediation of emerging pollutants: a review for a green and sustainable environment. Toxics 10(8):484
Bhatt P, Huang Y, Zhan H, Chen S (2019) Insight into microbial applications for the biodegradation of pyrethroid insecticides. Front Microbiol 10:457788
Bhatt P, Bhandari G, Bhatt K, Simsek H (2022) Microalgae-based removal of pollutants from wastewaters: occurrence, toxicity and circular economy. Chemosphere 306:135576
Casida JE (2018) Neonicotinoids and other insect nicotinic receptor competitive modulators: progress and prospects. Annu Rev Entomol 63:125–144
Chen A, Li W, Zhang X, Shang C, Luo S, Cao R, Jin D (2021) Biodegradation and detoxification of neonicotinoid insecticide thiamethoxam by white-rot fungus Phanerochaete chrysosporium. J Hazard Mater 417:126017
Cheng Y, Wang J, Quan L, Li D, Chen Y, Zhang Z, Wu L (2022a) Construction of microalgae-bacteria consortium to remove typical Neonicotinoids Imidacloprid and Thiacloprid from municipal wastewater: difference of algae performance, removal effect and product toxicity. Biochem Eng J 187:108634
Cheng Y, Wang H, Deng Z, Wang J, Liu Z, Chen Y, Ma Y, Li B, Yang L, Zhang Z, Li Wu (2022b) Efficient removal of imidacloprid and nutrients by algaebacteria biofilm reactor (ABBR) in municipal wastewater: performance, mechanisms and the importance of illumination. Chemosphere 305:135418
Cimino AM, Boyles AL, Thayer KA, Perry MJ (2017) Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ Health Perspect 125(2):155–162. https://doi.org/10.1289/EHP515
Dai ZL, Yang WL, Fan ZX, Guo L, Liu ZH, Dai YJ (2021) Actinomycetes Rhodococcus ruber CGMCC 17550 degrades neonicotinoid insecticide nitenpyram via a novel hydroxylation pathway and remediates nitenpyram in surface water. Chemosphere 270:128670
Deng Z, Zhu J, Yang L, Zhang Z, Li B, Xia L, Wu L (2022) Microalgae fuel cells enhanced biodegradation of imidacloprid by Chlorella sp. Biochem Eng J 179:108327
Dhuldhaj UP, Singh R, Singh VK (2023) Pesticide contamination in agro-ecosystems: toxicity, impacts, and bio-based management strategies. Environ Sci Pollut Res 30:9243–9270. https://doi.org/10.1007/s11356-022-24381-y
Elango D, Siddharthan N, Alaqeel SI, Subash V, Manikandan V, Almansour AI, Kayalvizhi N, Jayanthi P (2023) Biodegradation of neonicotinoid insecticide acetamiprid by earthworm gut bacteria Brucella intermedium PDB13 and its ecotoxicity. Microbiol Res 268:127278
Elzaki MEA, Miah MA, Wu M, Zhang H, Pu J, Jiang L, Han Z (2017) Imidacloprid is degraded by CYP353D1v2, a cytochrome P450 overexpressed in a resistant strain of Laodelphax striatellus. Pest Manag Sci 73(7):1358–1363
Encarnação T, Santos D, Ferreira S, Valente AJM, Pereira JC, Campos MG, Burrows HD, Pais AACC (2021) Removal of imidacloprid from water by microalgae Nannochloropsis sp. and its determination by a validated RP-HPLC method. Bull Environ Contam Toxicol 107(1):131–139
Erguven GO, Yildirim N (2019) The evaluation of imidacloprid remediation in soil media by two bacterial strains. Curr Microbiol 76(12):1461–1466
Ferreira L, Rosales E, Danko AS, Sanromán MA, Pazos MM (2016) Bacillus thuringiensis a promising bacterium for degrading emerging pollutants. Process Saf Environ Prot 101:19–26
Fusetto R, Denecke S, Perry T, O’Hair RA, Batterham P (2017) Partitioning the roles of CYP6G1 and gut microbes in the metabolism of the insecticide imidacloprid in Drosophila melanogaster. Sci Rep 7(1):11339
Gautam P, Dubey SK (2022) Biodegradation of imidacloprid: molecular and kinetic analysis. Bioresour Technol 350:126915
Gautam P, Dubey SK (2023) Biodegradation of neonicotinoids: current trends and future prospects. Curr Pollut Rep 9:1–23
Ge F, Zhou LY, Wang Y, Ma Y, Zhai S, Liu ZH, Dai YJ, Yuan S (2014) Hydrolysis of the neonicotinoid insecticide thiacloprid by the N2-fixing bacterium Ensifer meliloti CGMCC 7333. Int Biodeterior Biodegrad 93:10–17
Goswami RK, Agrawal K, Shah MP, Verma P (2022) Bioremediation of heavy metals from wastewater: a current perspective on microalgae-based future. Lett Appl Microbiol 75:701–717. https://doi.org/10.1111/lam.13564
Guo L, Dai Z, Guo J, Yang W, Ge F, Dai Y (2020) Oligotrophic bacterium Hymenobacter latericoloratus CGMCC 16346 degrades the neonicotinoid imidacloprid in surface water. AMB Express 10:7. https://doi.org/10.1186/s13568-019-0942-y
Guo L, Fang WW, Guo LL, Yao CF, Zhao YX, Ge F, an Dai YJ, (2019) Biodegradation of the neonicotinoid insecticide acetamiprid by actinomycetes Streptomyces canus CGMCC 13662 and characterization of the novel nitrile hydratase involved. JAgric Food Chemi 67(21):5922–5931
Guo L, Yang W, Cheng Xi, Fan Z, Chen X, Ge F, Dai Y (2021) Degradation of neonicotinoid insecticide acetamiprid by two different nitrile hydratases of Pseudaminobacter salicylatoxidans CGMCC 1.17248. Int Biodeterior Biodegrad 157:105141
Gupta M, Mathur S, Sharma TK, Rana M, Gairola A, Navani NK, Pathania R (2016) A study on metabolic prowess of Pseudomonas sp. RPT 52 to degrade imidacloprid, endosulfan and coragen. J Hazard Mater 301:250–258
Hamada A, Wahl GD, Nesterov A, Nakao T, Kawashima M, Banba S (2019) Differential metabolism of imidacloprid and dinotefuran by Bemisia Tabaci CYP6CM1 variants. Pestic Biochem Phys 159:27–33. https://doi.org/10.1016/j.pestbp.2019.05.011
Harry-Asobara JL, Kamei I (2019) Indirect bacterial effect enhanced less recovery of neonicotinoids by improved activities of white-rot fungus Phlebia brevispora. J Microbiol Biotechn 29:809–812. https://doi.org/10.4014/jmb.1809.09051
He C, Liang J, Liu S, Wang S, Wu Q, Xie W, Zhang Y (2019) Changes in the expression of four ABC transporter genes in response to imidacloprid in Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pestic Biochem Physiol 153:136–143
He C, Liang J, Liu S, Zeng Y, Wang S, Wu Q, Xie W, Zhang Y (2020) Molecular characterization of an NADPH cytochrome P450 reductase from Bemisia tabaci Q: potential involvement in susceptibility to imidacloprid. Pestic Biochem Phys 162:29–35
Hegde DR, Manoharan T, Sridar R (2017) Identification and characterization of bacterial isolates and their role in the degradation of neonicotinoid insecticide thiamethoxam. J Pure Appl Microbiol 11:393–400
Hirata K, Jouraku A, Kuwazaki S, Kanazawa J, Iwasa T (2017) The R81T mutation in the nicotinic acetylcholine receptor of Aphis gossypii is associated with neonicotinoid insecticide resistance with differential effects for cyano- and nitro-substituted neonicotinoids. Pestic Biochem Physiol 143:57–65
Hladik ML, Main AR, Goulson D (2018) Environmental risks and challenges associated with neonicotinoid insecticides. Environ Sci Technol 52(6):3329–3335. https://doi.org/10.1021/acs.est.7b063883339-3335
Hu K, Barbieri MV, López-García E, Postigo C, López de Alda M, Caminal G, Sarrà M (2022) Fungal degradation of selected medium to highly polar pesticides by Trametes versicolor: Kinetics, biodegradation pathways, and ecotoxicity of treated waters. Anal Bioanal Chem 414(1):439–449
Hu G, Zhao Y, Liu B, Song F, You M (2013) Isolation of an indigenous imidacloprid-degrading bacterium and imidacloprid bioremediation under simulated in situ and ex situ conditions. J Microbiol Biotechn 23(11):1617–1626
Humann-Guilleminot S, Binkowski ŁJ, Jenni L, Hilke G, Glauser G, Helfenstein F (2019) A nation-wide survey of neonicotinoid insecticides in agricultural land with implications for agri-environment schemes. J Appl Ecol 56:1502–1514. https://doi.org/10.1111/1365-2664.13392
Huo-Yong J, Hong-Kai Wu, Yuan P-P, Guo J-J, Wang Li, Dai Y-J (2022) Biodegradation of sulfoxaflor by Pseudomonas stutzeri CGMCC 22915 and characterization of the nitrile hydratase involved. Int Biodeterior Biodegrad 170:105403
Kandil MM, Trigo C, Koskinen WC, Sadowsky MJ (2015) Isolation and characterization of a novel imidacloprid-degrading Mycobacterium sp. strain MK6 from an Egyptian soil. J Agric Food Chem 63(19):4721–4727
Kang K, Yang P, Pang R, Yue L, Zhang W (2017) Cycle affects imidacloprid efficiency by mediating cytochrome P450 expression in the brown planthopper Nilaparvata lugens. Insect Mol Biol 26(5):522–529
Kanjilal T, Bhattacharjee C, Datta S (2016) Utilization of S. aureus strain 502A in biodegradation of insecticide acetamiprid from wetland wastewater. Desalin Water Treat 57(28):13190–13206
Katić A, Kašuba V, Kopjar N, Lovaković BT, Čermak AMM, Mendaš G, Micek V, Milić M, Pavičić I, Pizent A, Žunec S (2021) Effects of low-level imidacloprid oral exposure on cholinesterase activity, oxidative stress responses, and primary DNA damage in the blood and brain of male Wistar rats. Chem Biol Interact 338:109287
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52:231–253
Li H, Qiu Y, Yao T, Ma Y, Zhang H, Yang X, Li C (2020) Evaluation of seven chemical pesticides by mixed microbial culture (PCS-1): degradation ability, microbial community, and Medicago sativa phytotoxicity. J Hazard Mater 389:121834
Liu X, Jiang H, Xiong Y, Peng P, Li HF, Yuan GR, Dou W, Wang JJ (2019) Genome-wide identification of ATP-binding cassette transporters and expression profiles in the Asian citrus psyllid, Diaphorina citri, exposed to imidacloprid. Comp Biochem Physiol Part D 30:305–311
Liu XQ, Jiang HB, Liu Y, Fan JY, Ma YJ, Yuan CY, Lou BH, Wang JJ (2020) Odorant binding protein 2 reduces imidacloprid susceptibility of Diaphorina citri. Pestic Biochem Physiol 168:104642
Ma Y, Zhai S, Mao SY, Sun SL, Wang Y, Liu ZH, Dai YJ, Yuan S (2014) Co-metabolic transformation of the neonicotinoid insecticide imidacloprid by the new soil isolate Pseudoxanthomonas indica CGMCC 6648. J Environ Sci Health APart B 49(9):661–670
Mahari WAW, Razali WAW, Manan H, Hersi MA, Ishak SD, Cheah W, Chan DJC, Sonne C, Show PL, Lam SS (2022) Recent advances on microalgae cultivation for simultaneous biomass production and removal of wastewater pollutants to achieve circular economy. Bioresour Technol 364:128085
Mir-Tutusaus JA, Masis-Mora M, Corcellas C, Eljarrat E, Barcelo D, Sarra M, Caminal G, Vicent T, Rodriguez-Rodriguez CE (2014) Degradation of selected agrochemicals by the white rot fungus Trametes versicolor. Sci Total Environ 500–501:235–242. https://doi.org/10.1016/j.scitotenv.2014.08.116
Mir-Tutusaus JA, Baccar R, Caminal G, Sarra M (2018) Can white-rot fungi be a real wastewater treatment alternative for organic micropollutants removal? A review. Water Res 138:137–151. https://doi.org/10.1016/j.watres.2018.02.056
Mohammed YM, Badawy ME (2017) Biodegradation of imidacloprid in liquid media by an isolated wastewater fungus Aspergillus terreus YESM3. J Environ Sci Health Part B 52(10):752–761
Mori T, Ohno H, Ichinose H, Kawagishi H, Hirai H (2021) White-rot fungus Phanerochaete chrysosporium metabolizes chloropyridinyl-type neonicotinoid insecticides by an N-dealkylation reaction catalyzed by two cytochrome P450s. J Hazard Mater 402:123831. https://doi.org/10.1016/j.jhazmat.2020.123831
Mori T, Sugimoto S, Ishii S, Wu J, Nakamura A, Dohra H, Nagai K, Kawagishi H, Hirai H (2024) Biotransformation and detoxification of tetrabromobisphenol A by white-rot fungus Phanerochaete sordida YK-624. J Hazard Mater 465:133469
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303
Mustafa S, Bhatti HN, Maqbool M, Iqbal M (2021) Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: prospects, challenges and opportunities. J Water Process Eng 41:102009
Nguyen HT, Yoon Y, Ngo HH, Jang A (2021) The application of microalgae in removing organic micropollutants in wastewater. Crit Rev Environ Sci Technol 51:1187–1220
Nie J, Sun Y, Zhou Y, Kumar M, Usman M, Li J, Shao J, Wang L, Tsang DCW (2020) Bioremediation of water containing pesticides by microalgae: mechanisms, methods, and prospects for future research. Sci Total Environ 707:136080
Pandey G, Dorrian SJ, Russell RJ, Oakeshott JG (2009) Biotransformation of the neonicotinoid insecticides imidacloprid and thiamethoxam by Pseudomonas sp. 1G. Biochem Biophys Res Commun 380:710–714. https://doi.org/10.1016/j.bbrc.2009.01.156
Pang R, Chen M, Liang Z, Yue X, Ge H, Zhang W (2016) Functional analysis of CYP6ER1, a P450 gene associated with imidacloprid resistance in Nilaparvata lugens. Sci Rep 6(1):34992
Pang S, Lin Z, Zhang W, Mishra S, Bhatt P, Chen S (2020) Insights into the microbial degradation and biochemical mechanisms of neonicotinoids. Front Microbiol 11:868
Parte SG, Kharat AS, Mohekar AD (2017a) Isolation and characterization of dichlorovos degrading bacterial strain Pseudomonas stutzeri smk. Res J Life Sci Bioinform 2(5):283–288
Parte SG, Mohekar AD, Kharat AS (2017b) Microbial degradation of pesticide: a review. Afr J Microbiol Res 11(24):992–1012
Parween T, Bhandari P, Jan S, Raza SK (2016) Interaction between pesticide and soil microorganisms and their degradation: a molecular approach. Plant Soil Microbes 2(23):43. https://doi.org/10.1007/978-3-319-29573-2_2
Phugare SS, Jadhav JP (2015) Biodegradation of acetamiprid by isolated bacterial strain Rhodococcus sp. BCH2 and toxicological analysis of its metabolites in silkworm (Bombax mori). Clean Soil Air Water 43(2):296–304
Phugare SS, Kalyani DC, Jadhav GYB, JP, (2013) Microbial degradation of imidacloprid and toxicological analysis of its biodegradation metabolites in silkworm (Bombyx mori). J Chem Eng 230:27–35
Quan L, Cheng Y, Wang J, Chen Y, Li D, Wang S, Wu L (2023) Efficient removal of thiamethoxam by freshwater microalgae Scenedesmus sp. TXH: removal mechanism, metabolic degradation and application. J Environ Manag 332:117388
Raby M, Zhao X, Hao C, Poirier DG, Sibley PK (2018) Chronic effects of an environmentally-relevant, short-term neonicotinoid insecticide pulse on four aquatic invertebrates. Sci Total Environ 639:1543–1552
Racar M, Dolar D, Karadakić K, Čavarović N, Glumac N, Ašperger D, Košutić K (2020) Challenges of municipal wastewater reclamation for irrigation by MBR and NF/RO: physico-chemical and microbiological parameters, and emerging contaminants. Sci Total Environ 722:137959
Rana S, Jindal V, Mandal K, Kaur G, Gupta VK (2015) Thiamethoxam degradation by Pseudomonas and Bacillus strains isolated from agricultural soils. Environ Monit Assess 1871:9
Sabourmoghaddam N, Zakaria MP, Omar D (2015) Evidence for the microbial degradation of imidacloprid in soils of Cameron Highlands. J Saudi Soc Agric Sci 14(2):182–188
Salama ES, Kurade MB, Abou-Shanab RA, El-Dalatony MM, Yang IS, Min B, Jeon BH (2017) Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew Sustain Energy Rev 79:1189–1211
Scott C, Pandey G, Hartley CJ, Jackson CJ, Cheesman MJ, Taylor MC, Pandey R, Khurana JL, Teese M, Coppin CW, Weir KM (2008) The enzymatic basis for pesticide bioremediation. Indian J Microbiol 48:65–79
Shahi A, Aydin S, Ince B, Ince O (2016) The effects of white-rot fungi Trametes versicolor and Bjerkandera adusta on microbial community structure and functional genes during the bioaugmentation process following biostimulation practice of petroleum contaminated soil. Int Biodeterior Biodegr 114:67–74. https://doi.org/10.1016/j.ibiod.2016.05.021
Sharma S, Singh B, Gupta VK (2014) Assessment of imidacloprid degradation by soil-isolated Bacillus alkalinitrilicus. Environ Monit Assess 186:7183–7193. https://doi.org/10.1007/s10661-014-3919-y
Sharma R, Mishra A, Pant D, Malaviya P (2022) Recent advances in microalgae-based remediation of industrial and non-industrial wastewaters with simultaneous recovery of value-added products. Bioresour Technol 344:126129. https://doi.org/10.1016/j.biortech.2021.126129
Shettigar M, Pearce S, Pandey R, Khan F, Dorrian SJ, Balotra S, Russell RJ, Oakeshott JG, Pandey G, Lespinet O (2012) Cloning of a novel 6-chloronicotinic acid chlorohydrolase from the newly isolated 6-chloronicotinic acid mineralizing Bradyrhizobiaceae strain SG-6C. PLoS ONE 7(11):e51162
Shi Z, Dong W, Xin F, Liu J, Zhou X, Xu F, Lv Z, Ma J, Zhang W, Fang Jiang M (2018) Characteristics and metabolic pathway of acetamiprid biodegradation by Fusarium sp. strain CS-3 isolated from soil. Biodegradation 29:593–603
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Stella T, Covino S, Cvancarova M, Filipova A, Petruccioli M, D’Annibale A, Cajthaml T (2017) Bioremediation of long-term PCB-contaminated soil by white-rot fungi. J Hazard Mater 324(Pt B):701–710. https://doi.org/10.1016/j.jhazmat.2016.11.044
Sun SL, Yang WL, Guo JJ, Zhou YN, Rui X, Chen C, Ge F, Dai YJ (2017) Biodegradation of the neonicotinoid insecticide acetamiprid in surface water by the bacterium Variovorax boronicumulans CGMCC 4969 and its enzymatic mechanism. RSC Adv 7(41):25387–25397
Sun S, Fan Z, Zha Y, Guo L, Dai Y (2018) A novel nutrient deprivation-induced neonicotinoid insecticide acetamiprid degradation by Ensifer adhaerens CGMCC 6315. J Agric Food Chem 67(1):63–71
Sutherland DL, Ralph PJ (2019) Microalgal bioremediation of emerging contaminants—opportunities and challenges. Water Res 164:114921
Talpur FN, Unar A, Bhatti SK, Alsawalha L, Fouad D, Bashir H, Bashir MS (2023) Bioremediation of neonicotinoid pesticide, imidacloprid, mediated by Bacillus cereus. Bioengineering 10(8):951
Tang H, Li J, Hu H, Xu P (2012) A newly isolated strain of Stenotrophomonas sp. hydrolyzes acetamiprid, a synthetic insecticide. Process Biochem 47:1820–1825. https://doi.org/10.1016/j.procbio.2012.06.008
Tiwari S, Tripathi P, Mohan D, Singh RS (2023) Imidacloprid biodegradation using novel bacteria Tepidibacillus decaturensis strain ST1 in batch and in situ microcosm study. Environ Sci Pollut Res 30(22):61562–61572. https://doi.org/10.1007/s11356-022-24779-8
Toolabi A, Malakootian M, Ghaneian MT, Esrafili A, Ehrampoush MH, Tabatabaei M, AskarShahi M (2017) Optimization of photochemical decomposition acetamiprid pesticide from aqueous solutions and effluent toxicity assessment by Pseudomonas aeruginosa BCRC using response surface methodology. AMB Express 7:1–12
Wang G, Yue W, Liu Y, Li F, Xiong M, Zhang H (2013a) Biodegradation of the neonicotinoid insecticide acetamiprid by bacterium Pigmentiphaga sp. strain AAP-1 isolated from soil. Bioresour Technol 138:359–368. https://doi.org/10.1016/j.biortech.2013.03.193
Wang G, Zhao Y, Gao H, Yue W, Xiong M, Li F, Zhang H, Ge W (2013b) Co-metabolic biodegradation of acetamiprid by Pseudoxanthomonas sp. AAP-7 isolated from a long-term acetamiprid-polluted soil. Bioresour Technol 150:259–265
Wang J, Ohno H, Ide Y, Ichinose H, Mori T, Kawagishi H, Hirai H (2019a) Identification of the cytochrome P450 involved in the degradation of neonicotinoid insecticide acetamiprid in Phanerochaete chrysosporium. J Hazard Mater 371:494–498
Wang J, Tanaka Y, Ohno H, Jia J, Mori T, Xiao T, Yan B, Kawagishi H, Hirai H (2019b) Biotransformation and detoxification of the neonicotinoid insecticides nitenpyram and dinotefuran by Phanerochaete sordida YK-624. Environ Pollut 252:856–862
Wang X, Goulson D, Chen L, Zhang J, Zhao W, Jin Y, Yang S, Yi Li, Zhou J (2020) Occurrence of neonicotinoids in Chinese apiculture and a corresponding risk exposure assessment. Environ Sci Technol 54(8):5021–5030
Wang X, Xue L, Chang S, He X, Fan T, Wu J, Niu J, Emaneghemi B (2019c) Bioremediation and metabolism of clothianidin by mixed bacterial consortia enriched from contaminated soils in Chinese greenhouse. Process Biochem 78:114e122. https://doi.org/10.1016/j.procbio.2018.12.031
Weaver LM, Alexander NL, Cubeta MA, Knappe DR, Aziz TN (2023) Degradation of imidacloprid by Phanerodontia chrysosporium on wood chips for stormwater treatment. Environ Sci Water Res Technol 9(12):3333–3343
Wei J, Wang X, Tu C, Long T, Bu Y, Wang H, Jeyakumar P, Jiang J, Deng S (2023) Remediation technologies for neonicotinoids in contaminated environments: current state and future prospects. Environ Int 178:108044
Wang J, Hirai H, Kawagishi H (2012) Biotransformation of acetamiprid by the white-rot fungus Phanerochaete sordida YK-624. Appl Microbiol Biotechnol 93:831–835
Wondji CS, Irving H, Morgan J, Lobo NF, Collins FH, Hunt RH, Coetzee M, Hemingway J, Ranson H (2009) Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res 19(3):452–459
Wu Y, Xu H, Pan Y, Gao X, Xi J, Zhang J, Shang Q (2018) Expression profile changes of cytochrome P450 genes between thiamethoxam susceptible and resistant strains of Aphis gossypii Glover. Pestic Biochem Phys 149:1–7
Wu C, Wang Z, Ma Y, Luo J, Gao X, Ning J, Mei X, She D (2021) Influence of the neonicotinoid insecticide thiamethoxam on soil bacterial community composition flux, oxidative stress, and detoxification. Environ Sci Technol 46(14):7818–7825. https://doi.org/10.1021/es301006j
Yang H, Wang X, Zheng J, Wang G, Hong Q, Li S, Li R, Jiang J (2013) Biodegradation of acetamiprid by Pigmentiphaga sp. D-2 and the degradation pathway. Int Biodeterior Biodegrad 85:95–102
Yang Y, Huang L, Wang Y, Zhang Y, Fang S, Liu Z (2016) No cross-resistance between imidacloprid and pymetrozine in the brown planthopper: status and mechanisms. Pestic Biochem Phys 130:79–83
Yang WL, Dai ZL, Cheng X, Guo L, Fan ZX, Ge F, Dai YJ (2020) Sulfoxaflor degraded by Aminobacter sp. CGMCC 117253 through hydration pathway mediated by nitrile hydratase. J Agric Food Chem 68(16):4579–4587
Yang W, Fan Z, Jiang H, Zhao Y, Guo L, Dai Y (2021) Biotransformation of flonicamid and sulfoxaflor by multifunctional bacterium Ensifer meliloti CGMCC 7333. J Environ Sci Health Part B 56(2):122–131
Ye J, Chen X, Chen C, Bate B (2019) Emerging sustainable technologies for remediation of soils and groundwater in a municipal solid waste landfill site—a review. Chemosphere 227:681–702
Yin R, Liu Y, Wang J, Xiao T (2020) A review on biodegradation of persistent organic pollutants by white-rot fungi. Earth Environ 48:747–757. https://doi.org/10.14050/j.cnki.1672-9250.2020.48.080
Yu B, Chen Z, Lu X, Huang Y, Zhou Y, Zhang Q, Wang D, Li J (2020) Effects on soil microbial community after exposure to neonicotinoid insecticides thiamethoxam and dinotefuran. Sci Total Environ 725:138328. https://doi.org/10.1016/j.scitotenv.2020.138328
Zamule SM, Dupre CE, Mendola ML, Widmer J, Shebert JA, Roote CE, Das P (2021) Bioremediation potential of select bacterial species for the neonicotinoid insecticides, thiamethoxam and imidacloprid. Ecotoxicol Environ Saf 209:111814
Zhan H, Wan Q, Wang Y, Cheng J, Yu X, Ge J (2021) An endophytic bacterial strain, Enterobacter cloacae TMX-6, enhances the degradation of thiamethoxam in rice plants. Chemosphere 269:128751
Zhang HJ, Zhou QW, Zhou GC, Cao YM, Dai YJ, Ji WW, Shang GD, Yuan S (2012) Biotransformation of the neonicotinoid insecticide thiacloprid by the bacterium Variovorax boronicumulans strain J1 and mediation of the major metabolic pathway by nitrile hydratase. J Agric Food Chem 60(1):153–159
Zhao YJ, Dai YJ, Yu CG, Luo J, Xu WP, Ni JP, Yuan S (2009) Hydroxylation of thiacloprid by bacterium Stenotrophomonas maltophilia CGMCC1.1788. Biodegradation 20(6):761–768
Zhao YX, Chen KX, Wang L, Yuan PP, Dai YJ (2023) Biodegradation of sulfoxaflor and photolysis of sulfoxaflor by ultraviolet radiation. Biodegradation 34:1–15
Zhao YX, Jiang HY, Cheng X, Zhu YX, Fan ZX, Dai ZL, Guo L, Liu ZH, Dai YJ (2019) Neonicotinoid thiacloprid transformation by the N2-fixing bacterium Microvirga flocculans CGMCC 1.16731 and toxicity of the amide metabolite. Int Biodeter Biodegr 145:104806
Zhou GC, Wang Y, Zhai S, Ge F, Liu ZH, Dai YJ, Yuan S, Jy H (2013) Biodegradation of the neonicotinoid insecticide thiamethoxam by the nitrogen-fixing and plant-growth-promoting rhizobacterium Ensifer adhaerens strain TMX-23. Appl Microbiol Biotechnol 97(9):4065–4074
Zhuo R, Fan F (2021) A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci Total Environ 778:146132. https://doi.org/10.1016/j.scitotenv.2021.146132
Zhou GC, Wang Y, Ma Y, Zhai S, Zhou LY, Dai YJ, Yuan S (2014) The metabolism of neonicotinoid insecticide thiamethoxam by soil enrichment cultures, and the bacterial diversity and plant growth-promoting properties of the cultured isolates. J Environ Sci Health A, Part B 49(6):381–390
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval is not required for this study.
Consent for publication
Not required for this study.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Randhawa, J.S. Microbial-assisted remediation approach for neonicotinoids from polluted environment. Bull Natl Res Cent 48, 70 (2024). https://doi.org/10.1186/s42269-024-01227-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-024-01227-w