Abstract
Background
The convergence of pulmonary hemorrhage, pulmonary amyloidosis, and multiple myeloma is uncommon. Amyloidosis can affect the pulmonary parenchyma in a diffuse, tracheobronchial, or parenchymal pattern and may rarely be associated with pulmonary hemorrhage. Additionally, pulmonary amyloidosis is not a frequent manifestation of multiple myeloma. We present a case of a male patient with pulmonary hemorrhage as the initial manifestation of AL pulmonary amyloidosis, and ultimately, confirmation of multiple myeloma through bone marrow biopsy.
Case presentation
The clinical case involves a 60-year-old male with no significant medical history, who was admitted presenting a clinical picture evolving over 6 months characterized by hemoptoic cough, accompanied by dyspnea, a decrease in functional capacity, and constitutional symptoms. Thoracic CT images revealed multilobar ground-glass opacities with suspected alveolar hemorrhage. In response to this clinical presentation, bronchoalveolar lavage with cytology was performed, revealing the presence of hemosiderin-laden macrophages. Given the complexity of the case, further investigation included a wedge biopsy of the lung. The pathological report indicated an atypical lymphoplasmacytoid proliferation with deposits of eosinophilic amorphous material, suggestive of amyloidosis. Congo red staining confirmed the presence of amyloid material. Elevated Kappa light chains were detected in both serum and urine, with an increased K/L ratio. Immunoglobulins G and M were found to be decreased. As part of the comprehensive assessment, a bone marrow biopsy was conducted, confirming the diagnosis of multiple myeloma with 10% atypical plasma cells. In light of this diagnosis, appropriate treatment has been initiated to address this intricate medical condition effectively.
Conclusion
The present case report provides an illustrative perspective on an uncommon presentation of pulmonary amyloidosis secondary to multiple myeloma, with the initial manifestation being pulmonary hemorrhage. The findings from both the physical examination and laboratory tests were consistent with pulmonary amyloidosis, and definitive confirmation of the multiple myeloma diagnosis was achieved through bone marrow biopsy. This case highlights the significance of considering pulmonary amyloidosis as a potential cause of hemoptysis, especially in patients with associated risk factors for multiple myeloma. Early recognition of this clinical association is pivotal for precise diagnosis and prompt therapeutic intervention. The complexity of this case underscores the importance of a comprehensive diagnostic approach in unraveling intricate medical conditions.
Similar content being viewed by others
Background
Pulmonary hemorrhage as a manifestation of amyloidosis is an infrequent phenomenon (Ogo et al. 2021). Furthermore, pulmonary hemosiderosis is characterized by recurrent episodes of interalveolar hemorrhage, leading to abnormal iron accumulation in the form of hemosiderin within alveolar macrophages (Levy and Wilmott 1986). Systemic amyloidosis can affect the pulmonary parenchyma diffusely, tracheobronchial, or parenchymatous (García et al. 2009). Although the lungs are frequently involved in amyloidosis, pulmonary manifestations seldom present symptomatically, posing challenges to diagnosis (Riehani and Soubani 2023; Milani et al. 2017). Unusual cases of systemic AL amyloidosis with pulmonary involvement have been documented (Milani et al. 2017), and in exceptional instances, vascular involvement can trigger arterial dissection with bronchial hemorrhage, presenting pulmonary hemorrhage as an infrequent manifestation of this amyloid involvement (Road et al. 1985).
In the specific context of AL amyloidosis, a clone of plasma cells or, less commonly, a clone of lymphoplasmacytes or B cells, produces monoclonal immunoglobulins. In approximately 80% of cases, these monoclonal immunoglobulins belong to the lambda (L) light chain, as opposed to the kappa (K) chain (Lacouture-Fierro et al. 2022). This phenomenon can develop within the framework of a plasma cell dyscrasia, such as in the case of multiple myeloma (Milani et al. 2017). We present here the case of a male patient whose pulmonary hemorrhage was the initial manifestation of AL pulmonary amyloidosis with kappa light chain involvement. Confirmation of the presence of multiple myeloma was obtained through a bone marrow biopsy. This case underscores the complexity of the clinical presentation of amyloidosis and its association with multiple myeloma.
Case presentation
The clinical case presents a 60-year-old male patient who requires an emergency care due to a symptomatic presentation that has developed over 6 months. The patient does not disclose any pathological personal history, prior medication use before admission, nor any family medical history. Symptoms include hemoptoic cough associated with dyspnea, a Modified Medical Research Council score of 3, and an approximate weight loss of 8 kg. Over the past two months, there has been a symptomatic worsening with a significant increase in hemoptysis and dyspnea. Upon admission, oxygen saturation is at 85%, with no significant findings on pulmonary auscultation, no cardiac auscultation abnormalities were detected. The abdomen is soft and depressible, with no palpable hepatomegaly or splenomegaly. There is no edema in the lower extremities, and the neurological examination is normal. No lymphadenopathy was palpated. Hemogram results are normal, but biochemistry reveals mild hypercalcemia and an elevated pro-brain natriuretic peptide level. The patient is human immunodeficiency virus-negative.
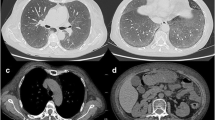
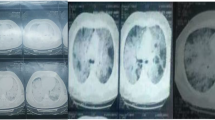
Chest X-ray shows cardiomegaly and extensive parenchymal involvement characterized by diffuse fine reticular opacities in both lung fields, more pronounced in the lower lobes (Fig. 1). A Chest Angiotomography reveals bilateral multilobar ground-glass opacities, diffusely infiltrating the pulmonary parenchyma. Areas of consolidation in the lower lobes, centrilobular and subpleural hypo-densities converging in the middle lobe, with evidence of pulmonary parenchymal destruction, are observed. The interpretation suggests the possibility of small vessel vasculitis with alveolar hemorrhage and emphysematous changes (Fig. 2).
In the chest X-ray, fine reticular infiltrates are observed in both fields, with a basal predominance as indicated by the white arrow.
In the chest angiotomography, ground-glass opacities are observed (white arrow), areas of parenchymal destruction (blue arrow), and basal consolidation areas (orange arrow).
Transthoracic echocardiography shows non-obstructive concentric remodeling of the left ventricle, a dilated right ventricle with reduced function, a dilated right atrium, and mild to moderate tricuspid regurgitation. A high probability of pulmonary hypertension is suggested, with a pulmonary artery systolic pressure of 50 mmHg and an ejection fraction of 57%. This constellation of findings points toward a complex medical condition that necessitates thorough evaluation and a multidisciplinary approach for clinical management.
Autoimmune studies were conducted in response to the clinical and radiological findings, including slightly positive antinuclear antibodies and Russell's viper venom. Additional results showed negative extractable nuclear antigens, negative antineutrophil cytoplasmic antibodies, negative anticardiolipins, and normal complement levels. Tumor markers, prostate-specific antigen, alpha-fetoprotein, and carcinoembryonic antigen, returned negative results, providing additional information in the patient's assessment.
Given the complexity of the clinical picture, a fibrobronchoscopy with bronchoalveolar lavage (BAL) was performed. Macroscopic findings during this procedure suggested the presence of alveolar hemorrhage, which was confirmed by BAL analysis (Fig. 3). A total of 100 cells were observed, with 96% being inflammatory, notably dominated by histiocytes (80%) and hemosiderophages (70%). Staining with Periodic Acid–Schif (PAS), Ziehl–Neelsen, (ZN) and Gomori's stain were negative, excluding the presence of certain pathogens and specific conditions (Fig. 3A), the cellular block is depicted in Fig. 3B.
Shows Papanicolaou staining and the cellular block from BAL. A Papanicolaou at 10× magnification. BAL. Hemosiderophages containing hemosiderin pigments in their cytoplasm are observed in red circles. B Cell block at 40 × magnification, Hematoxylin and Eosin stain reveals histiocytes and hemosiderophages accompanied by an amorphous eosinophilic background interspersed with erythrocytes in blue circles
Due to the persistence of constitutional symptoms and extensive involvement of alveolar hemorrhage, with the imminent risk of ventilatory failure, the decision was made to transfer the patient to the Intensive Care Unit. Given the low probability of autoimmune disease, a pulmonary biopsy by thoracoscopy was chosen as the consecutive course of action.
The macroscopic findings of the pulmonary biopsy revealed an anthracotic lung with grayish lines and a nodular sensation in the lingula. The pathological report indicated an atypical lymphoplasmacytoid proliferation with deposits of eosinophilic amorphous material suggestive of amyloidosis (Fig. 4). Additionally, amyloid nodules were observed (Fig. 5).
The immunohistochemistry results provided additional details about the cellular composition, highlighting a mixed phenotype of lymphocytes with a slight predominance of CD3, CD5, and BCL2-positive T lymphocytes. A minimal population of CD20-positive B lymphocytes was identified, with no expression of CD23 and cyclin D1. The presence of plasma cells was less than 5% of hematolymphoid cellularity, expressing CD38 (Fig. 6) and CD138 but being negative for CD 56 (Fig. 7). No kappa-lambda restriction was observed. The cellular proliferative activity, measured by Ki67, was 10%.
The positive Congo red staining in both interstitial and nodular acellular material (Fig. 8), coupled with the negativity of PAS staining for fungi, led to the diagnosis of diffuse interstitial amyloidosis. Given the amyloid involvement in both the lung and the heart, ATTR amyloid infiltration was ruled out through pyrophosphate scintigraphy and cardiac magnetic resonance imaging.
The investigation to rule out systemic AL amyloidosis revealed normal protein electrophoresis (Fig. 9) and immunofixation (Fig. 10). However, Kappa light chains were found to be elevated in both serum and urine (Figs. 11, 12), with an increased K/L ratio. Additionally, a decrease in immunoglobulins G and M was observed. In light of these findings, a bone marrow biopsy was conducted, confirming the presence of multiple myeloma.
The bone marrow biopsy report indicated a plasma cell population of 10%, with a pathological phenotype monotypic for Kappa light chains, consistent with plasma cell neoplasia involvement. Flow cytometry (Fig. 13) revealed 2.72% plasma cells, of which 0.03% were polyclonal with a normal phenotype, and the remaining 2.69% were monoclonal for Kappa. Additionally, the cells were positive for CD38, CD138, and beta-2 microglobulin.
Flow cytometry performed on bone marrow. The blue population represents the myeloid granulocytic lineage. The pink population represents the erythroid lineage. The violet-colored population denotes the B lymphoid lineage, while the light blue population refers to the monocytic lineage. The red population corresponds to plasma cells with a tumoral phenotype, exhibiting intermediate SSC/FSC expression, negative for CD45 and CD19, and positive for CD138, CD38, and monoclonality for KAPPA. The final result of the flow cytometry analysis concluded the presence of neoplastic plasma cells
Upon the diagnosis of multiple myeloma and evidence of systemic amyloidosis, no leukemic karyotypes were identified. Treatment for multiple myeloma was initiated with Bortezomib (1.3 mg/m2) 2.3 mg SC on days 1, 4, 8, and 11, Lenalidomide 25 mg orally on days 1 to 14 of the cycle, Dexamethasone 40 mg orally on days 1 and 2, 4 and 5, 8 and 9, 11 and 12 of the cycle, and zoledronic acid 4 mg IV monthly, six to nine cycles were defined based on the patient's response and progression. Antiviral prophylaxis included acyclovir, and calcium plus vitamin D supplementation was administered. Underscoring the complexity and the need for comprehensive management to address both medical conditions effectively.
The patient was evaluated by the Hematology Service both in the chemotherapy room and through an external consultation. The informed consent for the start of chemotherapy was signed.
Discussion
In the specific case of AL amyloidosis, light chains can infiltrate virtually any organ, excluding the central nervous system. This leads to variable clinical manifestations, resulting in nonspecific symptoms that hinder early disease recognition. The average time from symptom onset to diagnosis ranges from 6 to 12 months, often requiring multiple assessments by different specialists before reaching a definitive diagnosis (Milani et al. 2017). The most common symptoms include fatigue (80%), dyspnea (70%), peripheral edema (60%), constipation or diarrhea (60%), paresthesias (50%), and weight loss (30%) (Lacouture-Fierro et al. 2022). Within the most common manifestations, hemoptysis or pulmonary hemorrhage are not evident, making the diagnosis even more challenging.
In Japan, a survey conducted on AL amyloidosis patients between 2012 and 2014 included 741 participants with reported primary manifestations, such as proteinuria, renal dysfunction, congestive heart failure, orthostatic hypotension, diarrhea, arrhythmia, hepatomegaly, peripheral neuropathy, and splenomegaly. It is noteworthy that the mentioned manifestations were not present in the case under study. From the entirety of patients, only 12 reported the presence of amyloid in the pulmonary biopsy. Therefore, it is possible that amyloidosis may not be promptly considered in the differential diagnoses of a patient presenting with hemoptysis. (Shimazaki et al. 2018).
This case underscores the importance of considering pulmonary amyloidosis as a potential cause of hemoptysis, especially in patients with multiple myeloma risk factors. The patient presented with pulmonary symptoms, including dyspnea and hemoptoic expectoration, for the six months preceding admission. Initial evaluation suggested the possibility of small-vessel vasculitis with alveolar hemorrhage and emphysematous changes. The confirmation of hemosiderophages through bronchoalveolar lavage (BAL) supported the diagnosis of pulmonary hemorrhage. However, the biopsy indicated the presence of amyloid material. Given the constitutional symptoms, systemic involvement was suspected, leading to an extended investigation with protein electrophoresis, which yielded normal results. Additionally, with elevated levels of Kappa light chains in blood and urine, the diagnosis of multiple myeloma was established, subsequently confirmed through bone marrow biopsy.
In the medical literature, cases of patients with pulmonary amyloidosis associated with multiple myeloma have been documented. These cases have revealed the presence of amyloid deposits in pulmonary biopsies, concomitantly accompanied by hemosiderosis, and have presented symptoms, such as cough and dyspnea. However, hemoptysis and pleuritic pain have been less common in these situations. In our case, the predominant symptoms were hemoptysis and dyspnea, which complicated the diagnosis from the outset (Ogo et al. 2021; Kronen et al. 2022).
Despite the diversity of histopathological forms of pulmonary amyloidosis, diffuse presentations in the lungs tend to be more common in the context of systemic amyloidosis, such as multiple myeloma. This pattern suggests the possibility that pulmonary amyloidosis in our patient may be a manifestation of a systemic pathology (Liu et al. 2018).
In cases of AL amyloidosis, light chains can infiltrate nearly any organ, excluding the central nervous system. This leads to variable clinical manifestations, resulting in nonspecific symptoms that complicate the early recognition of the disease. Generally, the average time from the onset of symptoms to diagnosis ranges from 6 to 12 months, often requiring multiple assessments (Milani et al. 2017).
Given a median survival of 13 months in cases of diffuse pulmonary amyloidosis, it is crucial to achieve a timely diagnosis and promptly initiate treatment to enhance life expectancy. In the case of our patient, treatment commenced immediately under the supervision of the Hematology department (Poletti et al. 2004).
Conclusions
The case presented underscores the importance of including pulmonary amyloidosis in the differential diagnosis of patients with pulmonary hemorrhage and constitutional symptoms, particularly when associated with a plasma cell neoplasm. The complexity of the clinical presentation emphasizes the need for comprehensive evaluation and interdisciplinary collaboration to achieve an early diagnosis and appropriate treatment.
Availability of data and materials
All the data and supporting files have been presented within the case report.
Abbreviations
- BAL:
-
Bronchoalveolar lavage
- PAS:
-
Periodic Acid–Schif
- ZN:
-
Ziehl–Neelsen
- K:
-
Kappa
- L:
-
Lambda
References
García RG, Sanz AH, Martín PN, González SS, Díaz-Lobato S, Pérez-Rodríguez E (2009) Amiloidosis traqueobronquial y nodular múltiple. Revista Patol Respir 12(4):175–177
Kronen R, Ziehr DR, Kane AE, VanderLaan PA, Kholdani CA, Hallowell RW (2022) Pulmonary amyloidosis as the presenting finding in a patient with multiple myeloma. Respir Med Case Rep 37:101626
Lacouture-Fierro JA, Mejía-Buriticá L, Ribero-Vargas DA (2022) Amiloidosis AL: conceptos actuales. Med Lab 26(2):119–139
Levy J, Wilmott RW (1986) Pulmonary hemosiderosis. Pediatr Pulmonol 2(6):384–391
Liu Y, Jin Z, Zhang H, Zhang Y, Shi M, Meng F, Sun Q, Cai H (2018) Diffuse parenchymal pulmonary amyloidosis associated with multiple myeloma: a case report and systematic review of the literature. BMC Cancer 18(1):1–5
Milani P, Basset M, Russo F, Foli A, Palladini G, Merlini G (2017) The lung in amyloidosis. Eur Respir Rev 26(145):170046
Ogo N, Yanagihara T, Nishimura R, Mannoji H, Yoneda R, Hayashi M, Egashira A, Asoh T, Maeyama T (2021) Pulmonary amyloidosis complicated with pulmonary hemosiderosis, diagnosed with bronchoscopy. Respir Med Case Rep 33:101400. https://doi.org/10.1016/j.rmcr.2021.101400
Poletti V, Costabel U, Casoni GL, Bigliazzi C, Drent M, Olivieri D (2004) Rare infiltrative lung diseases: a challenge for clinicians. Respiration 71(5):431–443
Riehani A, Soubani AO (2023) The spectrum of pulmonary amyloidosis. Respir Med 218:107407
Road JD, Jacques J, Sparling JR (1985) Diffuse alveolar septal amyloidosis presenting with recurrent hemoptysis and medial dissection of pulmonary arteries. Am Rev Respir Dis 132(6):1368–1370
Shimazaki C, Hata H, Iida S, Ueda M, Katoh N, Sekijima Y, Ikeda S, Yazaki M, Fukushima W, Ando Y (2018) Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med 57(2):181–187
Acknowledgements
Not applicable.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
CBJ performed the clinical examination and diagnosis of the patient, as well as manuscript writing. MQO and XCJ contributed to literature review and manuscript writing. JPP, MAS, IMC, FHG and SMG contributed to literature review and information gathering. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The informed consent was obtained from the patient, and the case was submitted to the Research Ethics Committee at Universidad del Rosario, Life Sciences Department (DVO005-CV1788). All required measures were undertaken to preserve the information's confidentiality.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Birchenall-Jiménez, C., Perdomo-Polania, J., Serna, M. et al. Pulmonary hemorrhage as a presentation of AL amyloidosis secondary to multiple myeloma: a case report. Bull Natl Res Cent 48, 17 (2024). https://doi.org/10.1186/s42269-024-01173-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-024-01173-7