Abstract
Background
Two cymothoid isopod species, Livoneca redmanii Leach, 1818 and Anilocra alloceraea Koelbel, 1878, have been discovered throughout the research period. These species have been morphologically compared to other closely related species. Their species sequences have been determined using mitochondrial cytochrome c oxidase subunit I (COI) gene fragments and compared to previously identified Livoneca and Anilocra species. The present study aims to provide a detailed morphological description along with parasitological indices of the L. redmanii species in the Mediterranean, which has previously been described in Egypt by several researchers using various misidentifications.
Results
According to the study findings, Livoneca redmanii was accidentally introduced into Qarun Lake with fish fry from the Mediterranean Sea. The morphological and parasitological descriptions of Anilocra alloceraea and L. redmanii are discussed. Additionally, A. alloceraea species is considered the first to be documented on the Egyptian marine coast.
Conclusions
Livoneca redmanii species can rapidly adapt to favorable conditions and be found among the most prevalent species in Egyptian marine environments. Our study supports the description of L. redmanii males, as well as some additional morphological features of both cymothoid species females. The discovery of these species in Egyptian waters has extended their geographic distribution. Additionally, this study marks a significant advancement in our knowledge of the dynamics of some parasitic isopod distribution among their preferred species as well as a critical step toward identifying the species that indeed inhabit Egyptian waters.
Similar content being viewed by others
Background
The family Cymothoidae Leach, 1814, of parasitic isopods has gained increasing global attention in recent years due to their ecological and economic significance (Aneesh et al. 2019, 2021; Hadfield and Smit 2020). Members of cymothoid species are protandric hermaphrodites that feed on the blood, flesh, and mucous of several species of freshwater, marine, and estuary fish, they are easily recognizable, but genera and species are often confused and misidentified (Aneesh et al. 2018; Hadfield and Smit 2020). Much of the existing data on the biodiversity, distribution, range, and host records of this family appears inaccurate due to the difficulty in confirming or rejecting existing species reports (Smit et al. 2014). Despite recent studies (Hadfield and Smit 2020), many cymothoid species still require revision to provide reliable data for future research on this critical group of fish parasites from both an ecological and economic perspective. Works on the parasitic cymothoid fauna of Egypt near the Mediterranean are still sparse, and little is known about this understudied group's effects on marine fish in Egypt's waters. Lately, 15 species from the family Cymothoidae have been reported from Morocco, 16 species from Algeria, 11 cymothoid species from Tunisia, and 12 from Egypt (see Geba et al. 2019).
Among the most significant genera of cymothoids is the genus Livoneca Leach, 1818. Since this genus was initially described by Leach in 1818, it has had several taxonomic issues (Bruce 1990). Livoneca sp. is known to prefer the branchial cavities of their host species (Aneesh et al. 2018). Bruce (1986) classified and described Mothocya Hope, in 1851, and tried to list Livoneca with similar characteristics. He also made note of the inconsistent morphology of the species that are now classified as belonging to Livoneca. Only three species were accepted, and the remaining have problems in their taxa, as well as around 11 species that have been accepted for other genera. According to WoRMS, 2023, there are now 26 species in this genus, of which three are recognized (L. redmanii, L. bowmanii, and L. ovali), although the majority of species within this genus were improperly assigned after its founding.
Livoneca redmanii is one of the cymothoids that are most frequently seen on the Gulf and Atlantic Coasts of the USA, according to previous reports and a reasonably high number of publications. L. papernea Trilles, Colorni, and Golani, 1999 and Lironeca desterroensis have both been described from the Red Sea and Brazil since Bruce (1990) genus revision, respectively. Since the males of this species have not yet been described, there have been various recent attempts to characterize L. redmanii, e.g., Bakenhaster's 2004 description of L. redmanii juveniles. Furthermore, they have been recorded several times from Lake Qarun, the Suez Canal, and the Mediterranean with different misidentifications by many researchers (see Geba et al. 2019). Researchers have been perplexed by the difficulties of differentiating between species as a result of these taxonomic overlaps between these genera and one another, and in an effort to clear up these overlaps, we offered a supplementary morphological description of Livoneca redmanii females as well as the description of males.
Anilocra Leach, 1818, is known to be the most diverse species of the family Cymothoidae, attached to the body surface of its hosts. According to Aneesh et al. (2021), this genus involves 56 accepted species known worldwide. Thus far, there are 62 valid species within this genus, with two species not accepted (one has been synonymized with species in the genus Nerocila, and one is considered nomen nudum), according to WoRMS, 2023. Anilocra species from Egyptian waters have not been well studied from a morphological perspective; other than three species of the genus Anilocra have been reported within our countries, these species have not been confirmed by any of the modern classification methods. Therefore, these species need a taxonomic review (see Table 1). Koelbel 1878, described Anilocra alloceraea (Koelbel 1878) as a new species from the Sumatra Sea, but the host was not named. From specimens obtained on Stolephorus indicus (van Hasselt 1833) in Indonesia and Australia, and later reported from Singapore by Bruce (1987), this species was re-described (Bruce and Harrison-Nelson 1988). However, the illustrations provided by both suggest that they belong to the same species.
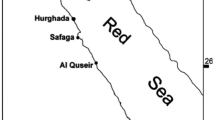
Recently, specifically in Qarun Lake, several attempts have appeared to determine the cymothoid species (see Table 1) which has invaded and drastically depleted the fish population and lost its role as a foundation of the fish industry in Egypt. Besides these attempts, cymothoid fauna did not receive any attention from Egyptian taxonomists, particularly other than what was presented by parasitologists such as Hellal and Youssef (2018), Ali and Aboyadak (2018) , Khalaf-Allah and Youssef (2019), Mahmoud et al. (2019), Geba et al. (2019), Abdullah and Hamouda (2022) who confirmed that cymothoids belong to one species of Livoneca redmanii Leach, 1818, but without any support illustrations. Thus far, studies on these parasitic isopods (Cymothoidae) are very scarce on marine fish from the Egyptian coasts generally and the Alexandria coasts particularly. As a result of this investigation, two species of cymothoids, Livoneca redmanii and Anilocra alloceraea were reported on the Egyptian coast. Therefore, the current study aims to use Camera Lucida to determine the diagnostic and morphological characteristics of these species that were discovered on some economic significance fish from different localities in Egyptian waters. Additionally, it aims to evaluate the degree of similarity between the cymothoid species found in Lake Qarun and those found in the Mediterranean Sea. To achieve this objective, Actinopterygian fish samples were collected seasonally from several locations inside Qarun Lake and the Mediterranean Sea coast of Alexandria.
Methods
Fish sampling
The current investigation is undergoing seasonal work from January to December 2021. Throughout the study, 2949 live Actinopterygii fish samples, representing nine distinct species, were collected from two separate water localities in Egypt. Of these, 1337 fish samples were gathered from various coastal areas of Alexandria, which constitutes 357 specimens from Sardina pilchardus (Walbaum, 1792), with a total length of 15.23 ± 2.18 cm; 234 of Dicentrarchus labrax (Linnaeus, 1758), (16.45 ± 2.57 cm); 231 specimens of Mugil cephalus Linnaeus, 1758, (21.01 ± 5.57 cm); 160 specimens of Pomatomus saltatrix (Linnaeus, 1766), (19.09 ± 3.79 cm); 210 specimens of Scomberornorus commerson (Lacepède, 1800), (26.45 ± 2.91 cm), and 156 specimens of Umbrina cirrose (Linnaeus, 1758), (17.66 ± 1.36 cm). And 1612 fish samples were collected from Qarun Lake, which constitutes 549 specimens of Tilapia zilli (Gervais, 1848), with a total length of 12.62 ± 1.14 cm; 576 specimens of M. cephalus, (17.35 ± 2.13 cm); and 487 specimens of Solea solea (Linnaeus, 1758), (13.95 ± 2.14 cm). Their parasite infections, clinical signs, and any apparent abnormalities were assessed immediately.
Parasitological investigations and parasitic identification
A total of 860 fresh specimens of two cymothoid species were discovered among their hosts. Of them, 139 Anilocra alloceraea specimens were gathered from the host species, Sardina pilchardus. Livoneca redmanii accounts for the remaining 721 cymothoid individuals; 547 of these were documented on three host species found in Qarun Lake, whereas 174 individuals were reported on five host species that were gathered along the Alexandria coast. In the MiTA Lab at the Faculty of Science, the cymothoid parasites width and length were measured to the nearest millimeter (mm), photographed, microscopically examined, and then preserved in 95% ethanol for DNA analysis, while the remaining specimens were stored in 70% ethanol for morphological research and treated using the methods outlined in Aneesh et al. (2019, 2020). The procedures used for appendage dissection, mounting, and drawing were those described by Aneesh et al. (2019). Using a stereo zoom microscope (OPTIKA-SFX-33), and a compound microscope (06AAGPV4541F1ZO), both equipped with drawing tubes, and using methods from Hadfield and Smit (2020) the observed mouthparts and appendages were drawn. Using the multi-focusing stereomicroscope and image-capturing software (Leica Application Suit, V, 04), the specimens were microphotographed. Taxonomy and host nomenclature are employed, according to Fish-Base (Froese and Pauly 2021) and Fricke et al. (2021).
Molecular analysis
Cymothoid isopod specimens were maintained in cold 96% ethanol for molecular analysis and kept there until DNA extraction. Using the D-Neasy Tissue Kit (QIAGEN), total genomic DNA was extracted under the manufacturer's protocol.
The partial fragment of COI mtDNA was amplified with the forward primer Loc1490F 5′-TAA CTTCAGGGTGACCAAAAAATCA-3′ and HCO2198R 5′-GGTCAACAATCATAA AGATATTGG-3′ (Menabit et al. 2022) using Polymerase Chain Reaction (PCR) on a mini thermal cycler (TechGene, USA).
PCR was carried out using GoTaq® Green Master Mix (Promega Corporation-Madison, WI, USA). Following were the thermocycling conditions for both sets of primers: 95 °C for 5 min, followed by 35 cycles of 95 °C for 1.30 min, 51 °C for 1.30 min, 72 °C for 3 min, 72 °C for 7 min, and finally held at 4 °C. PCR outputs. By electrophoresis on a 1 percent agarose gel with TAE 1 buffer supplemented with 2 l of ethidium bromide and UV light, the PCR products were examined. The QIAquick PCR Purification Kit was used to clean the PCR products under the manufacturer's instructions. ABI 3730XLs sequencer was used to sequence the PCR products' two strands (Sigma Lab, Egypt). Sequences were put together. Using the BLASTn program, the obtained COI sequences were utilized to find homologous sequences in GenBank (Table 2). With the help of MAFFT 7's online version, sequences were aligned (Katoh et al. 2019). Using Partition Finder v. 1.1.1's "greedy" search method, the optimum substitution model and partitioning scheme for each DNA partition were determined using the Bayesian Information Criterion (BIC; Schwarz 1978) (Lanfear et al. 2014). With the proper nucleotide substitution model established for each codon location, the barcode fragment dataset was divided into first-, second-, and third-codon positions. For the first codon position, TrNef + G was used, for the second, F81 + G, and for the third, TrN, the proper nucleotide substitution model was adopted.
Phylogenetic reconstruction was carried out using MrBayes 3.2.3 and Bayesian Inference (BI) (Ronquist et al. 2012). To calculate the posterior probability (PP) distribution, phylogenetic trees were built using two concurrent analyses using Metropolis-Coupled Markov Chain Monte Carlo (MCMC) for 20 million generations each. According to Ronquist et al. (2012), topologies were sampled every 1,000 generations, and the average standard deviation of split frequencies was found to be less than 0.01 after the run. By utilizing Bayesian posterior probability (PP), which classifies PP > 0.90 as highly supported, the resilience of the clades was evaluated. After eliminating the first 25% of sampled trees as "burn-in," a majority consensus tree with branch lengths was rebuilt for each iteration. Additionally, MEGA X was used to compute the uncorrected p-distance. GenBank received newly created sequences.
Results
Molecular analysis
Two COI mtDNA sequences were obtained, one from Livoneca redmanii with 642 bp and one from Anilocra alloceraea with 297 bp. The phylogenetic tree (Fig. 1) was constructed with a matrix of 42 taxa based on 702 bp. The specimen recognized by morphology as L. redmanii clustered with the sequences of the species deposited in GenBank in a node with high posterior probability (PP = 1.00). The other isopod, A. alloceraea, clustered in a node with other Anilocra spp. The position of this species is weak; the node does not have great support. The genetic distances (Table 3) show a small difference between L. redmanii found in this study and those sequences reported from Rhode River in the USA (KX360234, KT959449, and KT959417) and one (MZ208985) reported from Egypt from an unknown collection site. The sequence of A. alloceraea shows a bigger similarity with Anilocra clupei (p value = 0.06) (Table 3 and Fig. 1).
Morphological identification and taxonomy
Order: Isopoda Latreille, 1816
Suborder: Cymothoida Wagele, 1989.
Superfamily: Cymothooidea Leach, 1814.
Family: Cymothoidae Leach, 1814.
Genus: Livoneca Leach, 1818.
Livoneca redmanii Leach, 1818 (Figs. 2, 3, 4, 5, 6).
Body weakly vaulted (Fig. 3A,B), twisted to one side. Width 5.5–11.0 mm, length 16.9–29.2mm (N = 310); for both non-ovigerous and ovigerous females. Color generally, light brown (in alcohol); dorsal surface with randomly scattered chromatophores.
Locality: Mediterranean Sea off Alexandria coast and Qarun Lake, Egypt.
Hosts: Dicentrarchus labrax, Mugil cephalus, Pomatomus saltatrix, Scomberornorus commerson, and Umbrina cirrose from the Mediterranean Sea. Mugil cephalus, Solea solea, and Tilapia zillii from Qarun Lake.
Material examined: 8 ovigerous females, 14.0–22.0mm, 10 non-ovigerous females, 12.0–20.0mm, and 9 males, 10.0–19.0mm, off Egyptian coast of the Mediterranean Sea parasitized of different five hosts; and 10 ovigerous females 16.0–22.0mm, 4 non-ovigerous females, 14.0–21.0mm, and 16 males, 9.0 –19.0mm, from Qarun Lake parasitized of three different hosts (Table 2).
Sites of infection: Gills and body surface
Parasitological indices: In the Mediterranean Sea, 174 individuals of Livoneca redmanii were discovered among 118 host individuals from five distinct host species, which accounted for 980 fish that were investigated. These host species included Dicentrarchus labrax, Mugil cephalus, Pomatomus saltatrix, Scomberornorus commerson, and Umbrina cirrose. This isopod exhibits a higher prevalence on S. commerson (P = 27.1%) compared with its prevalence on another host (see Table 2). In contrast, out of 1612 fish specimens that were investigated from three different species, Mugil cephalus, Solea solea, and Tilapia zillii from Lake Qarun. Of these, 547 individuals of Livoneca redmanii were discovered among 472 host individuals. L. redmanii shows a higher overall prevalence in M. cephalus (P = 56.99%) compared to other host species (see Table 2).
For further details on parasitological indices regarding the seasonal and sexual prevalence of Livoneca redmanii and Anilocra alloceraea on their host species in the Mediterranean Sea, please review the paper provided by Zayed et al. (2023). As well as the parasitological indices of Livoneca redmanii parasites on their host species in Qarun Lake which were provided by Hellal and Youssef (2018), Khalaf-Allah and Yousef (2019).
Remarks
A lack of descriptive information for the type species contributed to the unthinking placement of taxa into Livoneca. In our findings, the cymothoid species are categorized as belonging to the Livoneca redmanii, Leach 1818, since the diagnostic features used to identify it agreed with the description provided by Bruce (1990). However, previously the species males were not described, some further morphological diagnoses can be added to Bruce 1990 description to completely support the species. Although there are some generic characters and infection-site distinctions between the genus Livoneca and other genera, it has never been obvious how to distinguish Livoneca from its related genera (Trilles 1981). Livoneca looks to be closely linked to other genera morphologically, such as Catoessa (Schiodte and Meinert 1884), Elthusa (Schiodte and Meinert 1884), Ichthyoxenus (Herklots 1870) and Enispa (Schiodte and Meinert 1884).
In general, cephalic, pereopodal, pleopodal, and pleonal features clearly of Livoneca show that it belongs to the family Anilocrinae (Bruce 1987). Based on morphological characteristics, this may have witnessed a significant divergence from the lineages suggested by Brusca (1981), but it still fits. The following were clarified by comparing the genus Livoneca to other genera:
There was little rationale, according to Bowman and Tareen (1983), for dividing Catoessa and Livoneca. Despite having similar pleopod shapes, Livoneca and Catoessa vary greatly in light of the additional information on Livoneca that is provided here (Figs. 3, 4, 5, 6). Catoessa is distinguished from Livoneca by the presence of a truncate rostrum coupled with a broadly rounded pleotelson, however, some species of Catoessa have a triangular pleotelson, also C. scabricauda has an acute rostral point and C. gruneri has a rounded rostral point and posteriorly narrowed pleotelson. Livoneca and Elthusa genera may be easily separated from one another. The extremely slender pereonite 1 and the antennule being bigger than the antenna indicate that it may be reallocated to another, potentially new genus, therefore it is obvious that it cannot be kept in Livoneca. At that time, neither Livoneca nor Ichthyoxenus had a description of their type species. Schiodte and Meinert (1884) described Livoneca redmanii, the genus type species, but the original specimen has never been identified. The merus of L. redmanii pereopods 6 and 7 are more clearly lobbed (Fig. 5), the lateral border of the pereonites is not thickened, and the pleonites overlap one another laterally (Fig. 3). This species varies from Ichthyoxenus species in these ways. However, the figures given by Menzies et al. (1955) of Livoneca appear identical to the material at the hand of Enispa, the only difference being in the supination of the maxilla of ovigerous females. The isopod parasite from the Mediterranean Sea was therefore firmly identified as L. redmanii. Regarding variations within the genus Livoneca, Livoneca redmanii is distinguishable from its congeners by having the uropod rounded apices, which are shapedly as opposed to acute in L. bowmanii, and exopod shorter and broader than endopod unlike the one in L. desterroensis which having exopod longer than endopod. As a result, our work supports the description of the males, additionally, some supplementary diagnoses for the females, as follows.
Maxilliped with two terminal spines, and one subterminal spine (Fig. 4C, D); Maxillule with four terminal spines (Fig. 4I, J); Maxilla pectinate scales, with two spines on inner lobe and two spines on the outer lobe (Fig. 4E, F); Mandible tapering distally with acute terminal spine as figured (Fig. 4G). Pereonite 1 longest one; pereonites 6 and 7 shortest; coxae 2–7 with acute posterior angles, 2 and 3 may be rounded; 2–8 extended beyond the posterior border of respective pereonites (in dorsal aspect). Pereopods length posteriorly steadily increases, bases lack carinae, carpus and propodus inside border lack spines, but merus outside border may or may not have spines (Fig. 5). Pleonites not equal in length and width (Fig. 3A, C); basis of pleopods 1–5 without stout setae on inner margin; basis lateral margin of pleopod 1 without lamellar accessory gill (Fig. 6A); basis lateral margin of pleopods 2–5 possess dendritic accessory gills and endopod appendix with lamellar accessory gill on medio-proximal region; masculinum differ in size; endopod pleopod 5 with 3–5 proximal folds (Fig. 6E, I, G); posterior margin of pleotelson sub-acuminate with shield-shaped, length slightly smaller than width; exopod and endopod uropodal tapering distally, reached beyond posterior margin of pleotelson; endopod longer than exopod; uropod rami extending beyond posterior margin of pleotelson (Fig. 3E).
Male description
Body length 9.0–21.0mm; width 3.5–6.0 mm (N = 336). Like females except for the following, cephalon explicit from the first pereonite; weakly immersed in the larger specimen. all coxae reaching approximately 3/4 length of their respective pereonites; pereopods 1–6 with spines on propodus and merus (Fig. 4G–J); Pereonites 4–6 widest (Fig. 3C); pereopod 7 with spines on propodus, merus and carpus (Fig. 4K); pereopods 1–7 with spine on external margin of ischium. Pleonite 1 lateral margin rarely concealed by pereonite 7; exopod and endopod of pleopods 1–5 with stout setae on inner margin; pleopods 1–3 with five spines on peduncle region (basis); pleopods 1–5 with dendritic accessory gills on lateral part of basis; pleopods 4 and 5 (Fig. 5H) with dendritic accessory gills on medio-proximal region of endopod.
Anilocra alloceraea Koelbel, 1878 (Figs. 7, 8, 9).
Anilocra alloceraea, Koelbel, 1878; Female ♀♀, A General habitus, dorsal view; B General habitus, lateral view; C Antenna; D Antennule; E Maxilla; F Mandible; G Maxillule with Maxillule apex, lateral view; H Maxillule apex, ventral view; I Pleotelson and uropod; J Uropod (Endopod and Exopod); K Cephalon dorsal view. Scale bars: a = 2.5 mm (A, B, I, K); b = 0.5 mm (C, D, E, F, G, J) and c = 0.2 mm (H)
Body elongates (Fig. 8A, B); length four times longer than the width; length 2.3–2.9 mm; width 0.5–0.7mm; length–width ratio 4.1–4.5 (N = 139); in dorsal aspect the lateral margin slightly convex, dorsum is gently vaulted, coxae discernible. Body widest at between pereonites 5 and 6; and narrowest at pleonites 5.
Locality: Mediterranean Sea off Alexandria coast, Egypt.
Hosts: Sardina pilchardus.
Material examined: 6 ovigerous females, 23.0–29.6mm, 10 non-ovigerous females, 1.80–2.80mm, and 3 males, 1.61–2.56mm, off the Egyptian coast of the Mediterranean Sea.
Sites of infection: Mian Micro-niche on the body surface (particularly between the pectoral fin).
Parasitological indices: The host species, Sardina pilchardus was found to have 139 Anilocra alloceraea individuals on its exterior body surface. The parasite's overall prevalence was rather high (P = 23.0%) and had a mean intensity of 1.7 (Table 2), accounting for 24.1% of female hosts and 21.6% of male hosts, respectively.
Remarks
Koelbel (1878) described Anilocra alloceraea from Sumatra, and the host was not mentioned. The morphological characters of A. alloceraea are very close to A. alloceraea Bariche and Trilles 2006; A. clupei Williams & Williams, 1986; A. leptosoma Bleeker, 1857 and A. caudata Bovallius, 1887. A. alloceraea differs from A. alloceraea by having the cephalon abruptly narrowed at the level of the antenna (the cephalon gradually narrowed), maxillule with four terminal spines (three terminal spines), maxilla with two spines on both the medial and lateral lobes (maxilla medial lobe with 2 recurved small spines and a few small setae, and the maxilla lateral with one spine). Compared to A. clupei, this species has a body length–weight ratio of 3.3–3.6 (rather than 4.0–4.5 in A. alloceraea); antenna extending into pereonite 1 (antenna extending into pereonite 2); anterior margin of head truncate (anterior margin of head narrowly rounded); inner lobe of maxilla with one large spine and one small recurved spine, outer lobe with 2 spines (rather than inner lobe with 2 recurved large spines; outer lobe with 2 recurved small spines; outer margin provided with very small folds), furthermore, the molecular analysis showed 152 base pair differences and only 89% similarity.
The important characteristics to distinguish A. leptosoma from A. alloceraea are body less than four times as long as wide; antennule article 3 antero-distal margin weakly produced, 1.2–1.4 times as wide as long; pleonite 1 with lateral margin posteriorly produced; Pleotelson ovate; lateral margins converging smoothly to the caudomedial point, in addition, pereopods spines absent. When comparing A. alloceraea to A. caudata the following suit of characters can be noted; pleotelson of A. caudata lateral margins weakly convex and posterior margin biconcave, distinct from lateral margin; in addition, pleonite 1 lateral margin not posterioriy produced (Bruce 1986). The description given by Bruce (1986) is completely accurate, but there are a few morphological identifications that can be added in addition to the characters already mentioned as head (Fig. 8K) sub-triangular; anterior margin partly rounded with big eyes; eyes about half width of head; distance between eyes about 31.1% of head width. Antennule (Fig. 8D) with 8 articles, extending to the middle of the eye; article 3 with strongly produced anterodistal angle; articles 4–8 with few terminal setules. Antenna (Fig. 8C) with 10 articles, extending to pereonite 1; article 5 slightly longer than other articles; each anterodistal angle provided few setules at articles 7–10. Mandible palp (Fig. 8F) article 3 with about 7–11 setae on lateral margin and distal segment decreasing in size to the last setae. Maxillule (Fig. 8G&H) with 4 very slightly recurved terminal spines at distal end; maxilla (Fig. 8G) with 2 lobes; inner lobe with 2 recurved large spines; outer lobe with 2 recurved small spines; outer margin provided with very small folds; maxilliped palp with 3 articles; provided with few setae on the outer margin; distal segment with 3 slightly recurved spines. Pereon (Fig. 8A) is widest between pereonites 5 and 6, and narrowest at pereonite 1; pereonites 2 and 3 are shortest, 6 longest; posterolateral margins of pereonite 7 rounded, slightly produced ventrally and extending more than a quarter of pleonite 1. Pleonites 1–5 gradually narrower toward posterior; pleonite 1 slightly longest and widest with partly covered by pereonite 7, pleonite 5 narrowest. Pereopods size gradually increases to pereopod 7 (Fig. 9A–G). Pereopod 1–4 (Fig. 9A–D) dactyls with swelling on the anterior and posterior margin (both sides), but swellings on outer margin higher than those on inner margin. Pereopods 1–7 (Fig. 9A–C, G) carpus with spines on postero-margin; pereopod 5 to 7 (Fig. 9E–G) with spines on anterior margin of the carpus and propodus. Pleotelson with lateral margins straight, strongly turned up; posterior margin scarcely bisinuate with broad caudomedial lobe, not provided with setae. Pleopods (Fig. 9H–L) slender, elongate (exopods 2.3–2.5 times as long as wide), endopod of all pleopods shorter than exopod; endopods of pleopods 3–5 with simple proximomedial lobe; endopod of pleopod 5 with complexy folded proximomedial lobes with sinuses. These additional characteristics support the traits described by Bruce (1986) along with some minor morphological differences that prevent this species from being classified as another species.
Discussion
The findings unequivocally established the parasite species under examination as members of the family Cymothoidae. These cymothoid species are classified as belonging to the genera Livoneca and Anilocra after the generic characteristics used to diagnose them. It fully agrees with the description provided by Brusca (1990) and Bruce (1986). With its congeners in the same family, the genus Livoneca did, however, cause generic confusion. Livoneca has been restricted since its establishment among the cymothoid genera. This main discovery was reinforced by our drawing of fully developed males and females, as well as by genetic research that produced findings that are entirely consistent with those of Brusca (1981 and 1990) and Bruce (1986).
Since these species were unintentionally brought from the waters of Alexandria by fish fry to the Qarun Lake region, it was necessary to confirm reports of species that had been introduced. To achieve this aim, many cymothoid samples were gathered from Lake Qarun and the Egyptian coast of Alexandria for molecular and morphological investigation. Subsequently, genetic analysis and the application of modern systematic categorization techniques enabled Livoneca redmanii parasites inhabiting the Mediterranean Sea to be identified as those from Lake Qarun.
Here, will list some previous records of cymothoid species within Egyptian environments. Nerocila orbignyi (Guérin-Méneville, 1832) was found to be the parasitic isopod studied by Younes et al. (2016) on Tilapia zilli (Gervais, 1848) and Solea vulgaris Quensel, 1806. In their study of parasitic isopods, Mahmoud et al. (2016); Rashed et al. (2021); Ali and Aboyadak (2018) recognized two species as N. orbignyi and Renocila thresherorum Williams and Bunkley-Williams, 1980. Additionally, Anilocra, Livoneca, Nerocila, and Renocila are four genera that are represented by the species that Mahmoud et al. (2017) found as part of their investigation into the cymothoid isopod invasion of Lake Qarun with infected fish. He discovered just female Livoneca redmanii pre-adult isopods, all of which were detected by him. Elgendy et al. (2018) focused on hematological and histological alterations while examining the large parasite infection on 150 T. zillii captured in June 2016. He further identified all recovered isopods as N. bivittata. The identifications of isopods made by Mahmoud et al. (2016, 2017, 2019); Elgendy et al. (2018), Younes et al. (2016), and other researchers (see Table 1) may be inaccurate and at odds with the current data. This might be because earlier researchers were unable to use Camera Lucida drawings for more specific information used in species identification, and the detected parasite species may not have referred to a taxonomist who is knowledgeable in isopods. Additionally, they lacked a thorough understanding of the target species' natural history and likely life cycle. Geba et al. (2019) confirmed that the parasitic species inhabiting Lake Qarun is L. redmanii using molecular identification. They also noted that only two recent attempts to diagnose Livoneca have been made outside of our countries, Bruce (1986) and Brusca (1981), but that this genus has been recorded many times within our countries with synonyms for other species (See Geba, et al. 2019). Hellal and Yousef (2018) correctly described this species in Lake Qarun; however, they made no assertions and did not provide any data or visual illustrations, however, genome studies provided further validation and our genetic findings agree with that presented by Geba et al. (2019). Following a review of the diagnostic characteristics of the specimens gathered the entire drawings provided in this study are in perfect accordance with the description provided by Brusca (1990) for Livoneca redmanii, and Bruce (1986) for Anilocra alloceraea.
Based on the findings of this investigation, it was discovered that Livoneca redmanii parasitizes a broad variety of commercially significant fish species; in contrast, Anilocra alloceraea parasitizes just one host species from the Mediterranean Sea. The fish fry probably brought additional cymothoid species into the lake. However, this study makes it abundantly evident that the parasite Livoneca redmanii can grow, adapt extremely well, and withstand environmental circumstances that its family Cymothoidae peers would not be able to.
The present study provides information on previously undocumented fish hosts, together with further taxonomic information that verified these species and gave an in-depth understanding of them, indicating a connection between the cymothoid species from Lake Qarun and species from the Mediterranean. Furthermore, it provided additional taxonomic details that validated these species and expanded our understanding of them. From an economic perspective, a large consortium of Egyptian experts worked hard to come up with quick fixes for the issue of salvaging Qarun Lake and getting it back in balance following the growing issues inside it. By establishing a connection between the species studied from the lake and the species found in the Mediterranean Sea, this study has helped to clarify some of the issues surrounding it. Careful research is necessary to understand the processes involved in bringing fish seed to Lake Qarun, particularly in the case of some fish farms, to prevent additional financial losses.
Conclusions
In conclusion, the transportation of fish fry from the Mediterranean Sea to Lake Qarun not only introduced new species to the ecosystem but also facilitated the establishment and rapid adaptation of Livoneca redmanii. This particular species proved to be highly successful in its acclimation to the favorable conditions of Egyptian marine habitats, emerging as one of the most prevalent species. Furthermore, this study not only provided additional insights into the morphology of female Livoneca redmanii but also successfully diagnosed the complete set of male morphological characteristics. Additionally, the research findings unveiled the presence of Anilocra alloceraea as the first species identified on the Egyptian coast, further expanding our understanding of marine biodiversity in the region. These findings highlight the significant impact of species transportation and shed light on the dynamic nature of ecosystems in response to environmental changes.
Availability of data and material
Raw data were generated at the faculty of science at Al-Azhar University. Derived data supporting the findings of this study are available from the corresponding author on request.
Abbreviations
- BIC:
-
Bayesian information criterion
- BI:
-
Bayesian inference
- MCMC:
-
Markov Chain Monte Carlo
- NEF:
-
No. of examined fish
- NIF:
-
No. of infested fish
- MI:
-
Mean intensity
References
Abd El Aal AMI, El Ashrum AMM (2011) A morphological study (SEM) on a parasitic marine isopod, Cymothoa spinipalpa (isopoda: Cymothoidae). Egypt J AquacultureV 1:17–26
Abdallah ESH, Hamouda AH (2022) Livoneca redmanii Leach, 1818 (Cymothoidae) a parasitic isopod infesting the gills of the European seabass, Dicentrarchus labrax (Linnaeus, 1758): morphological and molecular characterization study. BMC Veterinary Res 18:330–316
Abdel-Latif HM (2016) Cymothoid parasite, Nerocila orbigni inflects great losses on Tilapia zilli in lake Qarun at Fayoum province. Int J Innovative Studies Aqua Biol Fish 2(3):1–9
Abdelmageed AA, Khalifa U, Elsaied HE, Hamouda AH, El Gelani SS (2022) Lake Qarun between entangled history and blurred future: Retrospectives and prospective. Egypt J Aqua Res 48:299–306
Angyal D, Chávez-Solís EM, Liévano-Beltrán LA, Magaña B, Simoes N, Mascaró M (2020) New distribution records of subterranean crustaceans from cenotes in Yucatan (Mexico). ZooKeys 911:21–49. https://doi.org/10.3897/zookeys.911.47694
Ali NG, Aboyadak IM (2018) Histopathological alterations and condition factor deterioration accompanied by isopod infestation in Tilapia zilli, Mugil capito, and Solea aegyptiaca from Lake Qaroun. Egypt J Aquat Res 44(2018):57–63
Aneesh PT, Helna AK, Trilles JP, Chandra K (2018) A taxonomic review of the genus Joryma Bowman and Tareen, 1983 (Crustacea: Isopoda: Cymothoidae) parasitizing the marine fishes from Indian waters, with a description of a new species. Mar Biodivers. https://doi.org/10.1007/s12526-018-0920-7
Aneesh PT, Helna AK, Trilles JP, Chandra K (2019) Occurrence and redescription of Anilocra leptosoma bleeker, 1857 (Crustacea: isopoda: Cymothoidae) parasitizing the clupeid fish Tenualosa toli (valenciennes) from the Arabian Sea. India Mar Biodivers 49:443–450
Aneesh PT, Helna AK, Kumar AB, Trilles JP (2020) A taxonomic review of the branchial fish parasitic genus Elthusa Schioedte and Meinert, 1884 (Crustacea: isopoda: Cymothoidae) from Indian waters with the description of three new species. Mar Biodivers 50:e65
Aneesh PT, Hadfield KA, Smit NJ, Biju-Kumar A (2021) Morphological description and molecular characterization of a new species of Anilocra Leach, 1818 (Crustacea: Isopoda: Cymothoidae) from India. Int J Parasitol Parasites Wildl 14:321–328
Bariche M, Trilles JP (2006) Anilocra alloceraea n. sp., a new parasitic cymothoid isopod from off Lebanon (Eastern Mediterranean). Syst Parasitol 64:203–214
Bowman TE, Tareen IV (1983) Cymothoidae from fishes of Kuwait (Arabian Gulf) (Crustacea, Isopoda). Smithson Contrib Zool 382:1–30
Bruce NL (1986) Revision of the isopod crustacean genus Mothocya Costa, in Hope, 1851 (Cymothoidae: Flabellifera), parasitic on marine fishes. J Nat Hist 20(5):1089–1192
Bruce NL (1987) Australian Pleopodias Richardson, 1910, and Anilocra Leach, 1818 (Isopoda: Cymothoidae), crustacean parasites of marine fishes. Rec Aust Mus 39(2):85–130
Bruce NL (1990) The genera Catoessa, Elthusa, Ichthyoxenus, Idusa, Livoneca, and Norileca n. gen. (Isopoda, Cymothoidae), crustacean parasites of marine fishes, with descriptions of eastern Australian species. Rec Aust Mus 42(3):247–300
Bruce NL, Harrison-Nelson EB (1988) New records of fish parasitic marine isopod Crustaceans (Cymothoidae, subfamily Anilocrinae) from the Indo-West Pacific. Proceedings
Brusca RC (1981) A monograph on the Isopoda Cymothoidae (Crustacea) of the eastern Pacific. Zool J Linn Soc 73(2):117–199
Brusca NL (1990) The Genera Catoessa, Elthusa, Enispa, Ichthyoxenus, Idusa, Livoneca, and Norileca n. gen. (Isopoda, Cymothoidae), crustacean parasites of marine fishes, with descriptions of Eastern Australian species. Rec Aust Mus 42:247–300
Eissa IAM, Ismail MM, Abd El-Wahab MM, Dessouki AA, Abdel-Mawla HI, Qorany RA (2020) Mixed parasitic infestation in Dicentrarchus labrax, Dicentrarchus punctatus, and Sparus aurata in Suez Canal. Int J Fish Aquat Res 5(3):01–11
Elgendy MY, Hassan AM, Abdel Zaher MF, Abbas HH, Soliman WS, Bayoumy EM (2018) Nerocila bivittata massive infestations in Tilapia zillii with emphasis on hematological and histopathological changes. Asian J Sci Res 1(11):134–144
Fricke R, Eschmeyer WN, van der Laan R (2021) Catalog of fishes: genera, species, references. http://research.calacademy.org/research/ichthyology/catalog/fishca. tmain.asp accessed January 2021
Froese R, Pauly D (2021) FishBase. Version (02/2015). World Wide Web electronic publication. Available from. http://www.fishbase.org. Accessed January 2021
Fujita H, Kawai K, Deville D, Umino T (2023) Molecular and Morphological Characterizations of the Fish Parasitic Isopod Mothocya parvostis (Crustacea: Cymothoidae) Parasitizing Optional Intermediate Hosts: Juveniles of the Cobaltcap Silverside Hypoatherina tsurugae and Yellowfin Seabream Acanthopagrus latus. Zool Stud 62:21. https://doi.org/10.6620/ZS.2023.62-21
Fujita H, Umino T, Saito N (2021) Molecular identification of the aegathoid stage of Anilocra clupei (Isopoda: Cymothoidae) parasitizing sweeper Pempheris sp. (Perciformes: Pempheridae). Crustacean Res 50:29–31
Geba KM, Sheir SK, Aguilar R, Ogburn MB, Hines AH, Khalafallah HJ, El-Kattan A, Hassab El-Nabi SE, Galal-Khallaf A (2019) Molecular and morphological confirmation of an invasive American isopod; Livoneca redmanii Leach, 1818, from the Mediterranean region to Lake Qaroun, Egypt. Egypt J Aquat Biol Fish 23(4):251–273
Hadfield KA, Smit NJ (2020) Review of the global distribution and hosts of the economically important fish parasitic isopod genus Ceratothoa (Isopoda: Cymothoidae), including the description of Ceratothoa springbok n. sp. from South Africa. Int J Parasitol 50:899–919
Hata H, Sogabe A, Tada S, Nishimoto R, Nakano R, Kohya N, Takeshima H, Kawanishi R (2017) Molecular phylogeny of obligate fish parasites of the family Cymothoidae (Isopoda, Crustacea): evolution of the attachment mode to host fish and the habitat shift from saline water to freshwater. Mar Biol 164:105. https://doi.org/10.1007/s00227-017-3138-5
Hellal AM, Yousef OEA (2018) Infestation Study of Livoneca redmanii (Isopoda, Cymothoidae) on Mugil cephalus in Lake Qarun, Egypt. Egypt Acad J Biolog Sci 10(1):1–17
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service multiple sequence alignment, interactive sequence choice, and visualization. Brief Bioinform. https://doi.org/10.1093/bib/bbx108
Khalaf-Allah HMM, Youssef OEA (2019) Infestation study of Livoneca redmanii (Isopoda, Cymothoidae) on Solea solea in Lake Qarun, Egypt. J Egypt Soc Parasitol 49(1):105–114
Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A (2014) Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evol Biol. https://doi.org/10.1186/1471-2148-14-82
Mahmoud NE, Fahmy MM, Abuowarda MM, Khattab MS (2016) Parasitic Cymothoid Isopods and their impacts in commercially important fishes from Lake Qarun, Egypt. Int J Chem Tech Res 9(12):221–229
Mahmoud NE, Fahmy MM, Abuowarda MM (2017) An investigation of cymothoid isopod invasion in lake Qarun fishes with preliminary trial for biological control. Int J ChemTech Res 10(2):409–416
Mahmoud NE, Fahmy MM, Abuowarda MM, Zaki MM, Ismael E, Ismail EM (2019) Influence of Water Quality Parameters on the Prevalence of Livoneca redmanii (Isopoda; Cymothoidae) Infestation of Mediterranean Sea Fishes, Egypt. Egypt Inter J Vet Sci 8(3):174–181
Menabit S, Begun T, Teaca A, Mureşan M, Lavin P, Purcarea C (2022) DNA Barcoding and Distribution of Gastropods and Malacostracans in the Lower Danube Region. Diversity 14:533–549. https://doi.org/10.3390/d14070533
Menzies RJ, Bowman TE, Alverson FG (1955) Studies of the biology of the fish parasite Livoneca convexa Richardson (Crustacea, Isopoda, Cymothoidae). Wasmann J Biol 13:277–295
Rania AA, Rehab RA (2015) Some studies on parasitic isopods of some marine fishes. Egypt J Chem Environ Health 1(1):400–420
Rashed MA, Fadl SE, Elnady AM (2021) Effect of isopoda on the health status of gilthead seabream (Sparus aurata). Egyptian Journal of Animal Health 1(1):34–43
Ronquist F, Teslenkovan M, Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) Mr Bayes 3.2: efficient Bayesian phylogenetic inference and model choice across large model space. Syst Biol. https://doi.org/10.1093/sysbio/sys029
Samn AAM, Metwally KM, Zeina AF, Khalaf-Allah HMM (2014) First occurrence of Nerocila bivittata: parasitic isopods (skin shedders) on Lithognathus mormyrus (Osteichthyes, Sparidae) from Abu-Qir Bay, Alexandria, Egypt. J Am Sci 10(7):171–179
Schiodte C, Meinert FR (1884) Symbolae ad monographium cymothoarum crustaceorum isopodum familiae. IV. Cymothoidae Trib. II. Cymothoinae. Trib. III. Lironecinae. Naturhistorisk Tidsskrift Ser III 14:221–454 (plates 6–13)
Shaheen AA, Abd El Latif AM, Elmadawy RS, Noor Eldeen AI (2017) Isopodiosis in some fishes from Egyptian Qaroun Lake: prevalence, identification, pathology, and in vitro trials to get rid of it. Res J Pharma Biol Chem Sci 8(1):1971–1978
Shalloof K, Aly W, El-Far A, Fetouh MA, El-Ganiny A, Amin A (2022) Distribution of Fish Species Infested by Livoneca redmanii (Isopoda, Cymothoidae) in Lake Manzala, Egypt during Dredging Operations. Egypt J Aqua Biol Fish 26(6):803–811
Smit NJ, Bruce NL, Hadfield KA (2014) Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int J Parasitol Parasites Wildl 3:188–197. https://doi.org/10.1016/j.ijppaw.2014.03.004
Welicky RL, Hadfield KA, Sikkel PC, Smit NJ (2017) Molecular assessment of three species of Anilocra (Isopoda, Cymothoidae) ectoparasites from Caribbean coral reef fishes, with the description of Anilocra brillae sp. n. ZooKeys 663:21–43
Welicky RL, Smit NJ (2019) Redescription and molecular characterisation of the fish ectoparasite Anilocra capensis Leach, 1818 (Isopoda: Cymothoidae), with description of six new species of Anilocra Leach, 1818 from Africa. Parasites Vectors 12:387–421
Younes AM, Noor El-din A, Abdel-Latif MA (2016) A contribution of crustacean isopodoa, bacterial infections and physicochemical parameters in mass mortalities among fishes in Lake Qarun. Res J Pharm Biol Chem Sci 7(2):1906–1911
Youssef EM, Salam NH, Eissa IAM, Zak MS (2014) Parasitological studies on the isopoda (Cymothoidae) parasites infesting some marine fishes at Suez Canal area at Ismailia Province, Egypt with a key to the cymothoid genera. Life Sci J 11(1):227–231
Zayed OE, Hellal AM, Zeina AF, Tayel SI (2023) Prevalence of parasitic isopods (Cymothoidae and Gnathiidae) on actinopterygii fishes from the Alexandria coast off the Mediterranean Sea, Egypt. Egypt J Aquat Biol Fish 27(4):847–866
Acknowledgements
This paper is based on work funded by the Science, Technology, and Innovation Funding Authority (STDF), under the Graduate Support Grant (PGSG) grant. Project No. 45018/2021, Academy of Scientific Research and Technology (ASRT), Egypt. We would also like to sincerely thank the anonymous referees for their helpful comments that enabled us to improve the quality of the manuscript.
Funding
This manuscript is subject to funding from the Science, Technology, and Innovation Funding Authority (STDF) of the Ministry of Scientific Research in Egypt.
Author information
Authors and Affiliations
Contributions
AMH served as the study overall supervisor while it was being prepared. AFZ is a member of the research outputs and methodology stewardship supervision group. AFZ and SIT conceptualized and designed the study. OEZ edited all drawings and morphological, and descriptive data while writing the first draft of the manuscript. AFZ with OEZ were the main contributors to writing the manuscript. MAME investigated and analyzed the species under study genetically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could influence the work presented in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zayed, O.ES., Hellal, A.M., Zeina, A.F. et al. Morphological and molecular assessment of Livoneca redmanii Leach, 1818, and Anilocra alloceraea Koelbel, 1878 (Isopoda: Cymothoidae) from Egyptian waters. Bull Natl Res Cent 48, 3 (2024). https://doi.org/10.1186/s42269-023-01158-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-023-01158-y