Abstract
Background
The increase in the demand for synthetic drugs due to the surge in the cases of drug-resistant infectious organisms has led to the search for new medicines in plants. Some plants have phytochemicals that can serve medicinal purposes. This study focuses on the antibacterial activity and antioxidant potential of Allium sativum and Allium cepa extract on bacterial isolates isolated from the wound of diabetic patients. Agar well-diffusion method was used for the antibacterial susceptibility. Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, and Escherichia coli were used for this study. The total phenolic content of the extract was determined using standard Spectrophotometric techniques.
Results
The ethanolic extract of each plant had higher inhibitory effects against the bacterial isolates than the aqueous extracts. The zone of inhibition of each ethanolic section ranged from 3 to 12.5 mm, while the aqueous extracts ranged from 4 to 10 mm. The highest antimicrobial activity was observed at 150 mg/ml in A. sativum ethanolic extract when tested against Staphylococcus aureus, which resulted in a zone of inhibition of 12.5 mm. The aqueous and ethanolic extracts of A. cepa have higher phenolic content than that of A. sativum.
Conclusion
This research showed that the ethanolic and aqueous extracts of both plants vary in their abilities to serve act as antioxidants and antimicrobials.
Similar content being viewed by others
Background
The past decades saw traditional systems of medicine become a topic of global importance. It played a significant role in providing primary health care among the people in developing countries (Mosihuzzaman 2012). It has been estimated that a large proportion of the populace in developing countries relies heavily on medicinal plants and traditional medicine to meet their primary health demands. As direct care services, herbal medicine specialists have made significant contributions to human well-being over the ages, according to historical records (Hou et al. 2010). The compounds of flavonoids, phenols, alkaloids, terpenes, steroids, cardiac glycosides, stilbenes, tannins, saponins, and many more phytochemical constituents of medicinal plants are responsible for the preventive or therapeutic effects attributed to medicinal plants (Raina et al. 2014).
There has been a growth in the use of plant products as medicines for a variety of human ailments as a result of an increasing human population, the lack of synthesized pharmaceuticals, the high cost of therapies, and the array of synthesized drugs' negative impacts, and more. Therefore, phytomedicine involving phytochemical extracts sub-fractions or pure compounds of single phytochemical serve medicinal purposes through single or multiple interactions with the target cells, tissue, or organ to effect pharmacological or physiological changes in the disease conditions where it functions as laxatives, antidiabetics, anticoagulants, anticardiovascular diseases, antibiotics, antimalarial, antioxidants, antihyperlipidemia, antihypertension, and so many others (Rasool and Bassam 2012).
According to Sofowora (2008), a medicinal plant is any plant that possesses therapeutic value in any of its parts, including the leaves, stem, bark, roots, flower, or fruits to be used to prepare medicinal concoctions or as precursors for the production of pharmaceutical drugs. It can also be defined as those plants that are commonly used in traditional, alternative/complementary medicine for the prevention and treatment of specific ailments and diseases (Schulz et al. 2001; Chintamunnee and Mahomoodally 2012). They can be found to grow either as wild plant species naturally present in terrestrial ecosystems habitats devoid of direct human activities. Medicinal plants can also be planted domestically through selected breeding and management for commercial purposes as a source of bioactive compounds required in pharmaceutical drug production (Calixto 2000; Hassan et al. 2010).
Phytochemical constituents of medicinal plants have been the basis to which its therapeutic effects are attributed, and the essential phytochemicals are flavonoids, alkaloids, sterols, terpenoids, phenolic acid, stilbenes, lignin’s, tannins, and saponins (Okigbo et al. 2008). Alkaloids are one of the commonly known phytochemicals that give the bitter-tasting of the plants medicinal extracts and are concentrated in the plant parts, such as the stem, roots, and partly in the leaves or protecting against parasites feeding on the plants (Smith-Hall et al. 2012).
Onion (Allium cepa L.) and garlic (Allium sativum L.) are examples of medicinal herbaceous plants that are among the oldest plants purposefully cultivated to serve as food and also for medicinal uses (Martins et al. 2016). Onion is a vegetable with long medicinal folklore where the onion bulbs serve culinary functions as a food flavoring and therapeutic use. Likewise, the leaves, stalk, and roots do in medicinal applications and can be used in preparing dishes (Guarrera and Savo 2013). The ethno medicinal effects of onion have positively impacted the circulatory system as diuretics, antiarteriosclerosis through lowering blood LDL cholesterol, and formation of blood clotting during tissue injuries. It has been applied to controlling diabetes through its antihyperglycemic effect and prevents fungal and bacterial infections (Hannan et al. 2010).
In case of wounds and wasp sting, onion juice can be applied where it helps to reduce inflammations or swelling, and skin boils. Thiosulfate chemical compound in onion has effectively killed many common bacteria, including Salmonella typhi, Escherichia coli, and Pseudomonas aeruginosa (Shinkafi and Dauda 2013). Onion covers vast medicinal properties of cardiovascular protectives, antioxidant, antimicrobial activity, antihyperglycemic, and prevention of respiratory tract infections. Onion is an essential source of phenols, flavonoids, and volatile organosulfur antioxidant molecules (Gazuwa et al. 2013a, b).
Garlic (A. sativum L.) is also an example of a bulb-producing herbaceous plant that belongs to the family of Amaryllidaceae and is medically effective in treating atherosclerosis and reducing LDL cholesterol triglyceride levels in respiratory infections. The bioactive compound present in garlic is mostly alliin, the primary ingredient in garlic with a similar chemical structure to sulfur-containing amino acid cysteine. Freshly harvested garlic is rich in sulfur-containing compounds diallyl polysulfide, vinyldithiins, alliin, and S-allylcysteine. It also contains non-sulfur-containing compounds of flavonoids, terpenes and cardiac glycosides, and enzymes (Onyeagba et al. 2004).
Garlic has a higher concentration of sulfur compounds (allicin, diallyl disulfide, S-allylcysteine, and diallyl trisulfide), responsible for its therapeutic properties. Garlic sources various biologically active phytomolecules, including organosulfur compounds, phenolic acids, allyl thiosulfinates, flavonoids, vitamins, and minerals (Chen et al. 2013). Garlic contains minerals like germanium, calcium, copper, iron, potassium, magnesium, selenium, zinc, vitamins A, B1, C, fiber, and water (Gebreselema and Mebrahtu 2013). Research has proved that garlic has medicinal effects on a wide range of pathologic conditions of ringworm, Candida, and vaginitis due to its fungicidal, tonic, antiseptic, and parasiticidal activities, which are of high benefit in the management of the ill-health condition (Peni et al. 2012).
The antibacterial effect of garlic showed that it produces growth inhibitory effects on gram-negative typhoid or paratyphoid or enteritis group of bacteria, given scientific evidence that garlic possesses a high capacity to kill germs and reduce the proliferation amoebic enteritis that causes dysentery (Modaresi and Heidari 2015).
Several health benefits of garlic are seen in the area of wounds, flu, ulcers, athlete's foot and skin infections treatment, reduction in viral load, Streptomyces, worms, respiratory ailments, blood thinning, high blood pressure, colic, cancer of the stomach, colds, bladder problems, kidney problems, and many more (Borek 2001; Bhandari 2012). Allicin and Ajuein are the most critical garlic compounds responsible for their smell and medicinal values. It has been observed that garlic reduces cholesterol aggregation, triglyceride, and lipid peroxidation in humans and animals and has antibiotic activities (Kumar et al. 2010).
These compounds can directly or indirectly act as an antioxidant by modulating the oxidative pro-apoptotic pathway or by stimulating the synthesis of endogenous antioxidant molecules or enzymes. The ability of the antioxidants to scavenge free radicals generated during cellular respiration and protect the human body from diseases has led to the increasing interest in the discovery of new antioxidant phytochemicals (Pohare et al. 2017). Clinical studies have shown that regular consumption of garlic powder of about 300 g/day protects the endothelial cells of body organs and tissues from free radicals oxidative damage. It possesses anti-inflammatory, anticardiovascular, anticancer activities, antibacterial effects, and antioxidants (Wang 2017). This study aims to examine the antimicrobial activity and antioxidant potential of A. sativum and A. cepa extract on bacterial isolates from the wound of diabetic patients using the agar diffusion method.
Methods
Collection of plant materials
Fresh A. sativum and A. cepa were purchased from the Ede Market, Osun State, Nigeria, and were transported to the Department of Microbiology Laboratory, Adeleke University, Ede, Osun State. Oyawoye, O.M formally identified the plant specimens by checking their physical features. There are no vouchers attached to these specimens.
Preparation of plant extract
The raw A. sativum and A. cepa were sliced, crushed, dried in the oven, and then pulverized to powder. The extraction was performed by soaking 100 g of the pulverized garlic and onion in 1000 ml of distilled water and 95% ethanol solution to extract bioactive from the garlic clove, according to Shuford et al. (2005). The mixture was kept at room temperature for 48 h in a sterile flask covered with aluminum foil to avoid evaporation. The residue and the filtrate were obtained by filtering the soaked garlic (A. sativum) and onion (A. cepa) using Whatman No. 1 filter paper. The extract evaporated in a rotary evaporator. The residue was dried on a cardboard paper, and the filtrate was obtained as extract and was kept in the refrigerator for biochemical and microbiological studies.
Preparation of extracts serial dilution
Three serial dilutions of each extract were prepared in a sterile test tube. Different concentrations of ethanol and aqueous extract of A. sativum and A. cepa 50 mg/ml, 100 mg/ml, and 150 mg/ml were prepared aseptically. The dilution standard used were 0.5 g of the extract in 10 mls of sterile distilled water to make 50 mg/ml, 1 g in 10 mls of sterile distilled water to make 100 mg/ml, and 1.5 g of the extract in 10 mls of sterile distilled water to make 150 mg/ml.
Collection of bacteria samples
The test organisms used in this study were obtained from the Culture Collections of the Department of Medical Microbiology and Parasitology, University College Hospital, Ibadan, Oyo State. The bacteria used in this study were: Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus epidermidis, and Proteus vulgaris. The test organisms were streaked on the Nutrient Agar slants and were incubated overnight at 37 °C. The cultures were kept under refrigerated conditions.
Screening and determination of total phenolic compound
Total phenol will be extracted by boiling 2 g of sample with 50 ml of diethyl ether in water bath for 15 min. The estimation will be carried out by calorimeter assay. 1 ml of sample will be mixed with 1 ml of Folin–Ciocalteu’s phenol reagent for 15 min which will be then cooled and poured in petri plates under sterilized condition of laminar flow hood. The wells of 6 mm will be bores in each plate and the plates will be inoculated with 30 ul of inoculum. Then, 1000 µg/75 µl of each of sample will be pipetted in each well and plates were incubated at 37 °C for 24 h. After 24 h, ones of inhibition will be measured in millimeter (mm).
Determination of diameter (mm) of zone of inhibition
Onyeagba et al. (2004) revealed that an inoculum suspension was swabbed uniformly to solidify 20 mL, Nutrient Agar for bacteria, and the inoculum was allowed to dry for 5 min. Holes of 6 mm diameter were made in the seeded agar using a micropipette. An aliquot of 0.5 ml from each plant crude extract (50, 100, 150 mg/ml) was added into each well on the seeded medium and allowed to stand on the bench for one h for proper diffusion and after that, incubated at 37 °C for 24 h. The resulting inhibition zones were measured in millimeters (mm).
Results
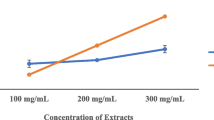
The total phenolic contents of A. cepa and A. Sativum are represented in Figs. 1 and 2 and Table 1. The phenolic contents of A. cepa (onions) and A. sativum (garlic) are interpreted in Table 1 for both the aqueous extract and ethanol extracts. The highest phenolic content was observed in the A. cepa aqueous extract and the lowest in A. sativum ethanol extracts. Garlic, red, brown, and white onions showed a varying number of phytochemicals. The aqueous extract of A. cepa (0.5550 ± 0.36) and A. Sativum (0.2543 ± 0.21) was significantly higher in phenolic content compared to the ethanolic extract (0.1520 ± 0.15) and 0.1322 ± 0.10, respectively, as shown in Figs. 1 and 2.
Table 1 shows the total phenolic content of A. cepa (onions) and A. sativum (garlic) as justified from another study by Onyeagba et al. (2004).
Table 2 shows the antibacterial activity of different concentrations of aqueous/ethanol extracts of A. Sativum against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, and Proteus mirabilis. The zone of inhibition of the organisms ranged from 3.0 to 12.5 mm, and the highest inhibition was observed in Staphylococcus aureus with 12.5 mm at 150 mg/ml for ethanol extract. The lowest inhibition was 3 mm, observed in Pseudomonas aeruginosa at the aqueous extract of 150 mg/ml and Klebsiella pneumonia at ethanol extract of 100 mg/ml.
Table 3 shows the antibacterial activity of different concentrations of aqueous/ethanol extracts of A. cepa against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, and Proteus mirabilis. The zone of inhibition of the organisms ranged from 4.0 to 14 mm. The highest inhibition was observed in Staphylococcus aureus with 14 mm at 150 mg/ml for ethanol extract, and Klebsiella pneumonia had the lowest inhibition at the aqueous extract of 100 mg/ml (Fig. 3).
Discussion
The highest phenolic content was observed in the A. cepa aqueous extract and the lowest in A. sativum ethanol extracts. Garlic, red, brown, and white onions showed a varying number of phytochemicals. This confirmed a similar qualitative work on garlic and onions by Gazuwa et al. (2013a, b) and Micová et al. (2019) who studied samples of garlic to confirm its total polyphenols content, total sulfur content, and antioxidant activity. The high phenolic content of these extract has also proven its antioxidant and medicinal potentials of the plants. The health properties of garlic depend on its bioactive compounds and especially on phenolic compounds, which have interesting pharmacological properties, and are present in relatively high amounts (Chen et al. 2013).
Phenolic compounds are large groups of secondary metabolites that are able to neutralize or quench the free radicals. Flavonoids and their derivatives are the largest group of polyphenols found in plants (Hounsome et al. 2009).
Health benefits of garlic depend on its content of biologically active compounds (polyphenols, sulfur compounds, and antioxidant active ingredient), which differs between cultivars (Micová et al. 2019). Ren et al. (2017) evaluated polyphenolic content and antioxidant activity in two onion varieties grown under organic and conventional production systems and observed that levels of total phenolic and total flavonoids showed a significant year-on-year variation and were significantly different between organic and conventional production systems. Organic cultivation practices resulted in significantly higher levels of potential bioactive compounds in onion.
Benkeblia (2005) evaluates the antimicrobial activity of different phenolic compounds of three types of onions and garlic against two bacteria (Staphylococcus aureus and Salmonella enteritidis) and three fungi (Aspergillus niger, Penicillium cyclopium, and Fusarium oxysporum). Garlic showed the highest inhibition, and green onion showed the lowest inhibition. Fusarium oxysporum shows the most heightened sensitivity. The antimicrobial activities of A. sativum extract obtained in this study agree with those of Zakaria and Astal (2003). They studied the antibacterial effects of garlic extracts on certain Gram-positive and Gram-negative bacteria. A study by Shobana et al. (2009) revealed that alcohol extract of A. sativum has the highest inhibitory activity against all test bacteria, while Zakaria and Astal (2003) also reported the inhibition of the growth of S. aureus, S. Typhi, and E. coli by fresh garlic extract.
Minimum inhibition against Klebsiella pneumonia was in agreement with the study of Enejiyon et al. (2020), where Kneumonia pneumoniae acetone extract showed no antibacterial activity in all the concentrations used. Staphylococcus aureus was the most susceptible test isolate, which still correlates with Enejiyon et al. (2020). Factors responsible for the high susceptibility of these organisms were not confirmed but presumed to be attributed to the presence of phytochemical constituents. Ethanol was the most effective of the three extraction solvents, possibly due to the more excellent solubility of active chemicals in alcohol. As a result, E. coli was the most vulnerable and prone to A. cepa extracts, while S. aureus was particularly prone to A. sativum extracts (Anyawu and Okoye 2017).
However, the presence of phytoconstituents elements in plant extracts is presumed to cause these species' high vulnerability (Bakht et al. 2013). Extracts collected in this study showed that Allium species strongly affected pathogenic bacteria, based on their minimal inhibitory concentrations. There were relatively low inhibitory concentrations for both A. sativum and A. cepa, which indicates that their bioactive components have the best antibacterial capability (Gazuwa et al. 2013a, b).
A single clove of garlic has the potential to cure a man of a large number of diseases by inhibiting the population of different strains of bacteria and fungi. Garlic (A. sativum) use in cardiovascular therapeutics has an even more extended history back over 3000 years to ancient times. Different animal studies have established garlic to have a cholesterol-lowering effect (Enejiyon et al. 2020; Anyawu and Okoye 2017). The active chemical in garlic is allicin, which is produced when raw garlic is crushed, allowing the enzyme alliinase to act on the stable precursor in garlic’s antidiabetic, antibiotic, and perhaps anticancer well-accepted world over because many of scientific literature supports these effects. Garlic also has hepatoprotective, antioxidant, and anthelmintic effects.
Research for new antimicrobials has been fundamental in recent times. Because onion is known to act synergistically with antibiotics, and resistance has not been reported for garlic, more dose–response preclinical studies and eventually clinical studies should be done to assess the use of an antibiotic/garlic combination for bacteria that are difficult to eradicate (Micová et al. 2019).
Conclusions
It is concluded from this study that A. cepa and A. sativum extract has antimicrobial activity against Staphylococcus aureus. It is expected that using natural products as therapeutic agents will probably not elicit resistance in microorganisms. Research must continue to isolate and purify the active components of this natural herb.
Availability of data and materials
Not applicable.
References
Ameh GI, Eze SC, Omeje FU (2012) Phytochemical screening and antimicrobial studies on the methanolic bulb extract of Allium sativum L. Afr J Biotech 12(14):1665–1668
Anyawu MU, Okoye RC (2017) Antimicrobial activity of Nigerian medicinal plants. J Intercult Ethnopharmacol 6(2):240–259
Bakht J, Khan S, Shafi M (2013) Antimicrobial potential of fresh Allium cepa against gram positive and gram negative bacteria and fungi. Pak J Bot 45(1):1–6
Benkeblia N (2005) Antimicrobial activity of essential oil extracts of various Onions (Allim cepa) and Garlic (Allium sativum). Lebensm-Wiss-u-Technol 37:263–268
Bhandari PR (2012) Garlic (Allium sativum L.): a review of potential therapeutic applications. Int J Green Pharm 6:118–129
Borek C (2001) Antioxidant health effects of aged garlic extracts. J Nutr 131:1010–1015
Calixto JB (2000) Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phyto therapeutic agents). Braz J Med Biol Res 33(2):179–189
Chen S, Shen X, Cheng S, Li P, Du J, Chang Y, Meng H (2013) Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 8(11):e79730
Chintamunnee V, Mahomoodally MF (2012) Herbal medicine commonly used against infectious diseases in the tropical island of Mauritius. J Herb Med 2:113–125
Enejiyon SO, Abdulrahman A, Adedeji SA, Abdulsalam R, Oyedum M (2020) Antibacterial activities of the extracts of Allium sativum (Garlic) and Allium cepa (Onion) against selected pathogenic bacteria. Tanzan J Sci 46(3):914–922
Gazuwa SY, Makanjuola ER, Jaryum KH, Kutshik JR, Mafulul SG (2013a) The phytochemical composition of Allium Cepa/Allium sativum and the effects of their aqueous extracts (cooked and raw forms) on the lipid profile and other hepatic biochemical parameters in female albino wistar rats. Asian J Exp Biol Sci 4(3):406–410
Gazuwa SY, Makanjuola ER, Jaryum KH, Kutshik JR, Mufulul SG (2013b) The phytochemical composition of Allium cepa/Allium sativum and the effects of their aqueous extracts (cooked and raw forms) on the lipid profile and other hepatic biochemical parameters in female albino wistar rats. Asian J Exp Biol Sci 4(3):406–410
Gebreselema G, Mebrahtu G (2013) Medicinal values of garlic: a review. Int J Med Med Sci 5(9):401–408
Guarrera PM, Savo V (2013) Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: a review. J Ethnopharmacol 146(3):659–680
Hannan A, Humayun T, Hussain MB, Yasir M, Sikandar S (2010) In vitro antibacterial activity of onion (Allium cepa) against clinical isolates of Vibrio cholera. J Ayub Med Coll Abbottabad 22(2):160–163
Hassan S, Mohammed N, Alyemeni F (2010) Cultivation and domestication study of high value medicinal plant species (its economic potential and linkages with commercialization). Afr J Agric Res 5(18):2462–2470
Hou CC, Chen CH, Yang NS, Chen YP, Lo CP, Wang SY, Tien YJ, Tsai PW, Shyur LF (2010) Comparative metabolomics approach coupled with cell- and gene-based assays for species classification and anti-inflammatory bioactivity validation of Echinacea plants. J Nutr Biochem 21:1045–1059
Hounsome N, Hounsome B, Tomos D, Edwards-Jones G (2009) Changes in antioxidant compounds in white cabbage during winter storage. Postharvest Biol Technol 52:173–179
Kumar KPS, Biswajit DBC, Tiwari P (2010) Allium cepa: a traditional medicinal herb and its health benefits. J Chem Pharm Res 2(1):283–291
Lanzotti V (2006) The analysis of onion and garlic. J Chromatogr A 1112:3–22
Martins N (2016) Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: a review. Food Chem 211:41–50
Micová M, Urminská D, Bystrická J, Kovarovič J, Harangozo L (2019) The influence of variety on the content of bioactive compounds in Garlic (Allium sativum L.). Food Sci Food Sci 8(4):1076–1079
Modaresi M, Heidari M (2015) The effect of Allium sativum extract on pituitary-gonad axis in heat stressed female mice. J Chem Pharm Res 7(6):7–9
Mosihuzzaman M (2012) Herbal medicine in healthcare: an overview. Nat Prod Commun 7(6):807–812
Okigbo RN, Eme UE, Ogbogu S (2008) Biodiversity and conservation of medicinal and aromatic plants in Africa. Biotechnol Mol Biol Rev 3(6):127–134
Onyeagba R, Ugbogu OC, Okeke CU, Iroakasi O (2004) Studies on the antimicrobial effects of garlic (Allium sativum L.), ginger (Zingiber officinale Roscoe) and lime (Citrus aurantifolia L.). Afr J Biotechnol 3:552–554
Peni IJ, Ajagbonna OP, Elinge CM, Agaie BM, Elsa AT, Yusuf H, Ribah IM (2012) Effects of Allium sativum bulb extract, diminazene aceturate and their combination on parasitaemia, and biochemical indices in rats experimentally infected with Trypanosoma brucei. J Appl Pharm Sci 2(6):112–115
Pohare JG, Shendarkar GR, Daswad AK, Shelke DP, Wangawar SN, Dhadwe AK (2017) Physicochemical characterization and antioxidant study of Allium cepa leaf extracts. Int J Pharma Chem Res 1:3–21
Raina H, Soni G, Jauhari N, Sharma N, Bharadvaja N (2014) Phytochemical importance of medicinal plants as potential sources of anticancer agents. Turk J Bot 38:1027–1035
Rasool H, Bassam A (2012) Medicinal plants (importance and uses). Pharm Anal Acta 3(10):139
Ren F, Reilly K, Gaffney M, Kerry JP, Hossain M, Rai DK (2017) Evaluation of polyphenolic content and antioxidant activity in two onion varieties grown under organic and conventional production systems. J Sci Food Agric 97(9):2982–2990
Schulz V, Hänsel R, Tyler VE (2001) Rational phyto therapy: a physician’s guide to herbal medicine, 4th edn. Springer, Berlin, p 306
Shinkafi SA, Dauda H (2013) Antibacterial activity of Allium cepa (onion) on some pathogenic bacteria associated with ocular infections. Sch J Appl Med Sci 1(3):147–151
Shobana S, Vidhya VG, Ramya M (2009) Antibacterial activity of garlic varieties (Ophioscordon and sativum) on enteric pathogens. Curr Res J Biol Sci 1(3):123–126
Shuford JA, Steckelberg JM, Patel R (2005) Effects of fresh garlic extract on Candida albicans biofilms. Antimicrob Agents Chemother 49(1):473
Smith-Hall C, Larsen HO, Pouliot M (2012) "People, plants and health: a conceptual. MolImmunol 42(4):501–510
Sofowora EA (2008) Medicinal plants and traditional medicines in Africa. University of Ife press, Nigeria, pp 1–23
Wang H (2017) Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem 217:773–781
Zakaria Y, Astal AL (2003) Effect of storage and temperature of aqueous garlic extract on the growth of certain pathogenic bacteria. J Al Azhar Univ 6(2):11–20
Acknowledgements
The current work was funded by Taif University Researchers Supporting Project Number (TURSP-2020/310), Taif University, Taif, Saudi Arabia.
Funding
The current work was funded by Taif University Researchers Supporting Project Number (TURSP-2020/310), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
OM conceptualized the research, and TM, SC, JA, TA, SO, IAR, GEB, NI, MA, and HF conducted the research and wrote, edited, and revised the manuscript. All authors read and approved the final manuscript for publications.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
All authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oyawoye, O.M., Olotu, T.M., Nzekwe, S.C. et al. Antioxidant potential and antibacterial activities of Allium cepa (onion) and Allium sativum (garlic) against the multidrug resistance bacteria. Bull Natl Res Cent 46, 214 (2022). https://doi.org/10.1186/s42269-022-00908-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00908-8