Abstract
Background
Lymphangioma is a multi-systemic disease mostly affecting the mesentery, omentum, mesocolon and retroperitoneum and rarely involving bone, with fewer than 30 cases reported so far. Lymphangioma usually has no specific clinical manifestation and could be easily misdiagnosed. Lymphangioma with myxoid degeneration was not reported. We report a histopathologically proved case of lymphangioma of 6th and 7th cervical vertebra (C6–7) with myxoid degeneration.
Case presentation
A 45-year-old male who suffered from pain in the right shoulder for 1 month, aggravated with numbness in the right little finger and ring finger and radiating pain for half a month. CT showed multiple cystic bone destruction areas in C6–7 vertebral right part and appendage with marginal osteosclerosis and surrounded by soft tissue density. The size of the lesion was about 36 mm × 41 mm . MRI: the margin of the lesion appears lobulated. Lesion showed slightly hypointense on T1WI, slightly hyperintense on T2WI and STIR. Multiple patchy T1WI hypointense and T2WI hyperintense shadows were seen within the lesion. No enhancement was observed in the lesion after injection of contrast, and there was no abnormal signal in surrounding soft tissue and bone. The histopathological examination confirmed the diagnosis of lymphangioma with myxoid degeneration after surgical resection. After over 2 years of follow-up, there were no signs of disease recurrence and progression.
Conclusions
To the best of our knowledge, this is the first case reported in English language literature of lymphangioma with myxoid degeneration.
Similar content being viewed by others
Background
Lymphangioma is an uncommon malformation of the lymphatic system (Valakada et al. 2018; Lin et al. 2014). Lymphatic diseases could range from small to systemic lymphangiomas. Lymphangioma, a benign proliferation of lymphatic vessels, is a rare disease with various clinical manifestations (Valakada et al. 2018; Lin et al. 2014; Bozca et al. 2020; Yang and Goo 2006; Ersoy et al. 2013; Puri et al. 1992; Blei 2011). Although the underlying pathogenic factor is unclear, it is generally believed to be congenital malformation and the dynamics of the lymphatic circulation associated with changes in the lymphatic system (Jung et al. 2010). Lymphangioma could occur in any part of the body, but most lesions are located in the abdomen, such as the mesentery, omentum, mesocolon, and retroperitoneum, while bone incidence is very low (Pour et al. 2013; Mifsut et al. 2013; Warin et al. 2010). In a few cases, symptoms documented in the literature were mainly due to compression of surrounding structures, for example, lymphangioma found in neck region may compress the brachial plexus causing pain or numbness.
We describe a case of cervical spine (C-spine) lymphangioma in a 45-year-old male, in which the diagnosis was made after the pathological analysis of the resected tumor. According to the English literatures we know, this is the first case of lymphangioma with myxoid degeneration in vertebrae.
Case presentation
We present a case of a 45-year-old man who was admitted to hospital in February 2017 due to right shoulder pain for 1 month, increased pain accompanied by right little finger and ring finger numbness, and radiating pain for half a month. The patient had no family history, genetic history or history of psycho-social disease. No relevant treatment measures were taken in this patient before.
General medical examination revealed no fever and no special signs and symptoms. The skin temperature and skin color of patient’s right neck was normal, no obvious vascular manifestations were seen on the surface. There was no obvious pain when pressing the lesion and the right upper extremity blood circulation, sensation and movement were good. Physiological reflexes were normal and pathological reflexes were not elicited.
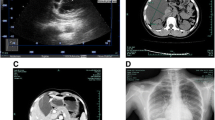
Computed tomography (CT) scan revealed multiple cystic bone destruction areas in C6–7 vertebral right part and appendage with marginal osteosclerosis and surrounded by soft tissue density. The size of the lesion was about 36 mm × 41 mm (Fig. 1). Magnetic Resonance Imaging (MRI) features were shown as follows: (a). The margin of the lesion appears lobulated. (b). Lesion showed slightly hypointense on T1-weighted image (T1WI), slightly hyperintense on T2-weighted image (T2WI) and slightly hyperintense on Short tau inversion recovery (STIR). (c) Multiple patchy T1WI hyposignals and T2WI hypersignals were seen within the lesion. (d). No enhancement was observed in the lesion after injection of contrast, and there was no abnormal signal in surrounding soft tissue and bone (Fig. 2).
(1-1) CT bone window axial image. The intraosseous lesion is microcystic type. The tumor shows multiple cyst-like osteolytic bone destruction and has sclerotic margins. (1-2) CT soft tissue window sagittal image. Tumor shows lower density than surrounding bone and soft tissue. The tumor is lobulated with breakthrough bone cortex projecting outward in an ovoid shape, compressing surrounding soft tissue
(2-1) Sagittal image on T1WI, (2-2) Sagittal image on T2WI, (2-3) Sagittal image on STIR, (2-4) Coronal contrast-enhanced image on T1WI. Tumor margin appears lobulated. Slightly hypointense on T1WI, slightly hyperintense on T2WI and STIR are seen in (2-1, 2-2, 2-3). Multiple patchy T1WI hypointense and T2WI hyperintense shadows were seen within the lesion (red arrow). No enhancement was observed in contrast MRI in (2-4)
Preoperative laboratory tests were normal as follows: routine blood test, electrolyte test, urine analysis, blood coagulation test, erythrocyte sedimentation rate, fasting plasma glucose, and cardiac enzymes were negative. Laboratory tests of tumor markers were normal as follows: Alpha fetoprotein (AFP): 2.78 ng/ml (normal range: 0–7.00 ng/ml), Carcinoembryonic antigen (CEA): 2.82 ng/ml (normal range: 0–5.00 ng/ml), Carbohydrate antigen 19-9 (CA19-9): 10.73 U/ml (normal range: 0–30.00 U/ml), Total prostate specific antigen (tPSA): 1.79 ng/ml (normal range: 0–4.00 ng/ml), Carbohydrate antigen 72-4 (CA72-4): 3.76 U/ml (normal range: 0–6.90 U/ml), and Neuron specific enolase (NSE): 10.50 ng/ml (normal range: 0–16.30 ng/ml).
Histopathological section of cervical tumor (posterior resection specimen) showed fibromuscular tissue with myxoid degeneration, in which lumen like structures of variable size and uneven thickness are visible (Fig. 3). S-100 protein (partial+), cluster of differentiation 34 (CD34) (+), and erythroblast transformation-specific regulated gene-1 (ERG) (+) were tested positive, and cytokeratin (CK) (−) and epithelial membrane antigen (EMA) (−) were negative in immunohistochemical findings. Special staining results: elastic fibers (−).
Histopathological section showing variably sized lumen-like structures with heterogeneous wall thickness. Lumen walls are composed of fibrous connective tissue with a monolayer of flattened endothelial cells in the inner village. Myxoid degeneration can be seen locally in the tumor (Black arrow; H&E, × 100)
Combined with the results of blood biochemical tests, clinical manifestations, imaging data and immunohistochemical tests, the pathological morphology could be consistent with lymphangioma with extensive myxoid degeneration.
After over 2 years of follow-up, there were no signs of disease recurrence and progression. Patient had no right shoulder pain, no right little finger and ring finger numbness.
Discussion
The absence of lymphatic vessels within bone was once believed in the past, but it has been widely accepted that Nixon (Nixon 1970) applied lymphangiography to confirm the presence of intraosseous lymphatic vessels. Bone lymphangiomas are rare and predominate in multiple forms. Most of them believe that it is caused by lymphatic congenital dysplasia or secondary lymphatic injury, resulting in lymphatic reflux obstruction, lymphatic dilatation and lymphangiomatoid hyperplasia (Bellini and Hennekam 2014). Lymphangiomas of the skeletal system can be part of a systemic lesion. Most patients with lymphangioma in bone could also find lymphangiomas in soft tissues. Lymphangiomas were called lymphangiomatosis or multiple lymphangiomas if lesions occur simultaneously in two different organs, or two or more relatively isolated lesions occur within the same organ (Jin et al. 2020). Multiple organs such as lung, liver, spleen, and particularly bone may be involved concurrently in approximately 75% of cases (Faul et al. 2000). In pediatric patients, 60% of lymphangiomas are detected at birth and 90% within 2 years of age. The recurrence rate was 100% in the aspiration and injection groups compared to 33% in surgical resections in pediatric patients (Alqahtani et al. 1999). However, bone lymphangioma is relatively stable and does not require treatment when there are no clinical symptoms, which can be treated surgically with better surgical outcomes if they cause clinical symptoms (Naguib et al. 2008). After 2 years follow-up in our case, there was no recurrence of the lesion.
Histologically, the bone lymphangiomas appear as a multilocular cavity filled with lymphatic fluid and containing endothelial cells. Lymphangioma in bone usually occurs in childhood (Naguib et al. 2008). According to the size of the cyst, bone lymphangiomas are divided into macrocystic type (≥ 2.0 cm), microcystic type (< 2.0 cm), and mixed type (Jin et al. 2020). Microcystic-type lymphangiomas are the most common (Fig. 1-1), followed by mixed type. The margins of the lesion may have osteosclerosis. Lymphangiomas occur most commonly in the thoracic and lumbar vertebrae, whereas lesion in this case was rare that located in the cervical spine. The tumor showed low density on CT (Mifsut et al. 2013; Warin et al. 2010; Jin et al. 2020; Aviv et al. 2001). MRI showed hypointensity on T1WI, hyperintensity on T2WI and STIR sequences (Mifsut et al. 2013; Aviv et al. 2001; Herruela-Suffee et al. 2016; Mi et al. 2020). No enhancement was observed in contrast CT and MRI images.
The male patient in our case was 45 years old. The lesion was located in the cervical vertebra, broke through the bone cortex and extended outwardly, belonging to microcystic-type lymphangioma. CT showed that the lesion was slightly low-density with osteosclerosis at the edge. Lesion signals on different MRI sequences were described as follows: slightly hypointense on T1WI, slightly hyperintense on T2WI and STIR; multiple small patchy T1WI hypointense as well as T2WI hyperintense opacities were seen within the lesion. In our case, the lesion CT density and the MRI signals were not typical, which may related to the presence of intra-lesional myxoid degeneration. A few patchy T1WI hyposignals and T2WI hypersignals (the same signal intensity as cerebrospinal fluid) in this lesion were considered components of lymphatic fluid without myxoid degeneration, while other parts of the lesion showed slightly hyperintense on T2WIconsidered as myxoid degeneration.
Myxoid degeneration is a common occurrence in soft tissue tumors and tumor-like lesions, which essence are mostly hyaluronic acid and acidic mucopolysaccharides. It is not a normal tissue in adults and is actively secreted by the tumor cells in these lesions (Baheti et al. 2015). Certain tumors have distinctive and persistent features of myxoid degeneration, which often serve as a diagnostic clue, such as various myxomas, nodular fasciitis, botryoid rhabdomyosarcoma, and myxoid chondrosarcoma (notochordal sarcoma) (Orlandi et al. 1998). Lymphangioma with myxoid degeneration has not been reported yet.
Conclusions
In conclusion, bone lymphangioma is a rare disease with no specific clinical manifestations. Typical bone lymphangioma, mostly microcystic or mixed type, appears hypodense on CT, hypointense on T1WI, and hyperintense on T2WI without any contrast enhancement. This lesion contained myxoid degeneration with atypical findings on MRI, which was slightly hypointense on T1WI and slightly hyperintenseon T2WI. Myxoid degeneration can also occur in bone lymphangioma, which improving physicians' medical understanding of lymphangioma.
Data availability
Some or all data, models, or code generated or used during the study are available from the corresponding author by request (MX, e-mail: xmqzhuhai@163.com).
Abbreviations
- AFP:
-
Alpha fetoprotein
- CA19-9:
-
Carbohydrate antigen 19-9
- CA72-4:
-
Carbohydrate antigen 72-4
- CD34:
-
Cluster of differentiation 34
- CEA:
-
Carcinoembryonic antigen
- CK:
-
Cytokeratin
- C spine:
-
Cervical spine
- CT:
-
Computed tomography
- EMA:
-
Epithelial membrane antigen
- ERG:
-
Erythroblast transformation specific regulated gene-1
- MRI:
-
Magnetic Resonance Imaging
- NSE:
-
Neuron specific enolase
- STIR:
-
Short tau inversion recovery
- T1WI:
-
T1-weighted image
- T2WI:
-
T2-weighted image
- tPSA:
-
Total prostate specific antigen
References
Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM (1999) 25 years’ experience with lymphangiomas in children. J Pediatr Surg 34(7):1164–1168
Aviv RI, McHugh K, Hunt J (2001) Angiomatosis of bone and soft tissue: a spectrum of disease from diffuse lymphangiomatosis to vanishing bone disease in young patients. Clin Radiol 56(3):184–190
Baheti AD, Tirumani SH, Rosenthal MH, Howard SA, Shinagare AB, Ramaiya NH, Jagannathan JP (2015) Myxoid soft-tissue neoplasms: comprehensive update of the taxonomy and MRI features. AJR Am J Roentgenol 204(2):374–385
Bellini C, Hennekam RC (2014) Clinical disorders of primary malfunctioning of the lymphatic system. Adv Anat Embryol Cell Biol 214:187–204
Blei F (2011) Lymphangiomatosis: clinical overview. Lymphat Res Biol 9(4):185–190
Bozca BC, Ozbudak IH, Alpsoy E (2020) A case of Heck’s disease with primary intestinal lymphangiectasia treated with imiquimod. Indian J Dermatol Venereol Leprol 86(6):724–725
Ersoy O, Akin E, Demirezer A, Yilmaz E, Solakoglu T, Irkkan C, Yurekli OT, Buyukasik S (2013) Evaluation of primary intestinal lymphangiectasia by capsule endoscopy. Endoscopy 45(Suppl 2 UCTN):E61–E62
Faul JL, Berry GJ, Colby TV, Ruoss SJ, Walter MB, Rosen GD, Raffin TA (2000) Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 161(3 Pt 1):1037–1046
Herruela-Suffee C, Warin M, Castier-Amouyel M, Dallery F, Bonnaire B, Constans JM (2016) Whole-body MRI in generalized cystic lymphangiomatosis in the pediatric population: diagnosis, differential diagnoses, and follow-up. Skelet Radiol 45(2):177–185
Jin D, Sun X, Shen W, Zhao Q, Wang R (2020) Diagnosis of lymphangiomatosis: a study based on CT lymphangiography. Acad Radiol 27(2):219–226
Jung SW, Cha JM, Lee JI, Joo KR, Choe JW, Shin HP, Kim KY (2010) A case report with lymphangiomatosis of the colon. J Korean Med Sci 25(1):155–158
Lin RY, Zou H, Chen TZ, Wu W, Wang JH, Chen XL, Han QX (2014) Abdominal lymphangiomatosis in a 38-year-old female: case report and literature review. World J Gastroenterol 20(25):8320–8324
Mi H, Chi J, Zhao X, Lu Q (2020) A case report of generalized lymphangiomatosis with chylopericardium: the crucial role of magnetic resonance lymphangiography. Eur Heart J Case Rep 4(5):1–5
Mifsut D, Renovell P, Gomar F, Saravia M (2013) Percutaneous osteoplasty in treatment of bone lymphangiomatosis. Indian J Orthop 47(5):515–518
Naguib MB, Al-Jazan N, Hashem T (2008) Lymphangiomatosis: a differential diagnosis of lytic lesions of the temporal bone. J Laryngol Otol 122(11):e23
Nixon GW (1970) Lymphangiomatosis of bone demonstrated by lymphangiography. Am J Roentgenol Radium Ther Nucl Med 110(3):582–586
Orlandi A, Bianchi L, Spagnoli LG (1998) Myxoid dermatofibrosarcoma protuberans: morphological, ultrastructural and immunohistochemical features. J Cutan Pathol 25(7):386–393
Pour KG, Moradvaesi B, Nouri M, Khoddami M, Jadali F (2013) Intra-Abdominal lymphangiomatosis with bone marrow involvement in a 7-year old girl: a case report. Oman Med J 28(2):45
Puri AS, Aggarwal R, Gupta RK, Sewatkar AB, Gambhir S, Tandon P, Choudhuri G (1992) Intestinal lymphangiectasia: evaluation by CT and scintigraphy. Gastrointest Radiol 17(2):119–121
Valakada J, Madhusudhan KS, Ranjan G, Garg PK, Sharma R, Gupta AK (2018) Abdominal lymphangiomatosis with intestinal lymphangiectasia diagnosed by magnetic resonance lymphangiography: a case report. Curr Probl Diagn Radiol 47(3):200–202
Warin M, Bonnaire B, Deramond H (2010) Generalized cystic lymphangiomatosis of bone with splenic involvement: minor variant of a systemic disease. J Radiol 91(9 Pt 1):907–910
Yang DH, Goo HW (2006) Generalized lymphangiomatosis: radiologic findings in three pediatric patients. Korean J Radiol 7(4):287–291
Acknowledgements
We greatly appreciate the assistance of professor Liu in the Department of Radiology at Peking University People's Hospital for providing medical records.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.L. was involved in drafting the manuscript. M.Z. and J.Y. were involved in acquisition of data. Y.C. prepared the figures. M.X. reviewed and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Guangdong Hospital of Traditional Chinese Medicine (ZE2021-331-01).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Zhang, M., Ye, J. et al. Cervical lymphangioma with myxoid degeneration: a rare case report and literature review. Bull Natl Res Cent 46, 58 (2022). https://doi.org/10.1186/s42269-022-00736-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00736-w