Abstract
Background
Grain amaranth (Amaranthus spp.) has been cultivated since ancient times in some countries in the world and it is one of the oldest food crops. At present, the crop has gained more importance in the plains of India, especially in parts of Gujarat and Maharashtra. Grain amaranth exhibits an incredible extent of morphological diversity and an extensive adaptability to diverse eco-geographical conditions. Hence, the aim of the recent research was to evaluate the genetic diversity of 19 genotypes from four diverse species of Amaranthus from India using ISSR markers.
Results
The set of 11 polymorphic ISSR primers produced a total of 114 amplicons, among which 98 amplicons were polymorphic. The mean number of polymorphic amplicons per primer was 8.91. Overall, the size of PCR-amplified DNA fragments ranged from 200 to 3702 bp. The average percent polymorphism was 87.15%, and the average PIC value was 0.853, which indicates good selection of primers in the present study for the assessment of genetic diversity. The unique amplicon (marker)-producing primers were also found which can be used for identification of genotypes. The dendrogram grouped 19 grain amaranth genotypes into two major clusters. The groups formed on the principle component analysis (PCA) plot resembles with the results of the dendrogram although some genotypes have been diverted on the PCA plot.
Conclusions
The technique may be used to obtain reasonably precise information on the genetic relationship among grain amaranth genotypes. Such information may be useful for selecting the diverse parents and monitoring the genetic diversity periodically in the breeder’s working collection of grain amaranth.
Similar content being viewed by others
Introduction
Grain amaranth (Amaranthus spp.) has been cultivated since ancient times in several countries in the world, and it is one of the oldest food crops in the new world. Among the three pseudocereals (buckwheat, Fagopyrum spp.; chenopods, Chenopodium spp.; and amaranth, Amaranthus spp.), grain amaranth is most important (Janssen et al. 2017). Grain amaranth (Amaranthus spp.) is traditionally an important edible pseudocereal, which is widely grown in the Himalayas and also in the plains to some extent during the rainy and cool season of the year. The family Amaranthaceae (Dicotyledons, order: Caryophyllales) is composed of 60 genera and about 800 species (Pagi et al. 2017). The genus amaranth is dibasic with X = 16 and X = 17 chromosomes, almost equally distributed in section amaranth (Rastogi and Shukla 2013). Grant (1959) has reported the information regarding the chromosome number of 30 species of Amaranthus. Among these, four have 32 chromosomes, while all others have 34 chromosomes. Among the 30 species, Amaranthus caudatus L. (2n = 32), Amaranthus cruentus L. (2n = 34), and Amaranthus hypocondriacus L. (2n = 32) are domesticated species and grown for grain purpose (Pagi et al. 2017). Grain amaranths are widely scattered in Asia (Pagi et al. 2017). The crop is most important and widespread in India even though it is not clear, when it was introduced from Brazil to India through Malabar Coast by early Portuguese traders after 1500 A.D. At present, the crop has gained more importance in the plains of India, especially in parts of Gujarat and Maharashtra, and it is grown along the edge of the field (Pagi et al. 2017).
Amaranthus species exhibit an incredible amount of morphological diversity and extensive adaptability to diverse eco-geographical conditions (Lee et al. 2008). The evaluation of genetic diversity among populations of diverse phytogeographic areas is essential because the existence, perpetuation, and continuance of a species to fulfill the demands of changing environments mainly depend on the level of variability available in the gene pool of particular species (Ray and Roy 2008). Moreover, the diversity present in particular species is utilized for the development of new varieties using plant breeding. Morphological characters have been used for diversity analysis in Amaranthus spp. (Omondi et al. 2016). The limitations of these characters result from the flexibility of certain characters and alterations caused by environmental conditions. Furthermore, morphological markers are less appropriate to be used as markers as they are limited in number to cover total genome regions of a plant species (Nadeem et al. 2018).
Nowadays, molecular markers are crucial tools for the elucidation of genetic diversity and characterization of plant genotypes with a high level of reproducibility and accuracy (Parita et al. 2018). Molecular phylogenetic studies would enable plant breeders to discriminate among genotypes by estimating the extent of variation within and between species which provides a base for appropriate breeding approaches. Well established molecular marker-based techniques such as random amplified polymorphic DNAs (RAPDs) (Faseela and Joseph 2007; Ray and Roy 2008; Popa et al. 2010; Snezana et al. 2012; Anjali et al. 2013), restriction fragment length polymorphisms (RFLPs) (Park et al. 2014), simple sequence repeats (SSRs) (Mallory et al. 2008; Suresh et al. 2014; Wang and Park 2013), amplified fragment length polymorphisms (AFLPs) (Chandi et al. 2013), inter-simple sequence repeats (ISSRs) (Nolan et al. 2010; Singh et al. 2013; Raut et al. 2014; Stefunova et al. 2014), and single nucleotide polymorphisms (SNPs) (Jimenez et al. 2013) have been utilized in genetic diversity studies in Amaranthus species. These molecular markers vary in their level of polymorphism, genomic abundance, reproducibility, locus specificity, dominance or co-dominance, technical desires, and economic investment (Zietkiewicz et al. 1994; Omondi et al. 2016).
In the last decade, ISSR markers have been successfully utilized for diversity studies. These markers have high reproducibility, which reveal many informative bands in a single amplification, therefore being one of the most commonly used markers in intra-specific diversity analysis in Amaranthus (Nolan et al. 2010; Singh et al. 2013; Raut et al. 2014; Stefunova et al. 2014). Hence, the objective of the research presented in this paper was to assess the genetic diversity of 19 genotypes from four different species of Amaranthus of India using ISSR markers to utilize the information generated for breeding and crop improvement.
Materials and methods
Genomic DNA extraction
A set of diverse 19 genotypes of amaranth (Table 1) procured from the Center for Crop Improvement, S. D. Agricultural University, Sardarkrushinagar, Gujarat, India, was used for the genetic diversity analysis in the present study. The genomic DNA of all the 19 genotypes of grain amaranth was isolated from tender fresh leaves of each genotype by using CTAB (cetyl trimethyl ammonium bromide) extraction method described by Doyle and Doyle (1990) with modifications (Dharajiya et al. 2017). All the genomic DNA samples were dissolved in 1X TE (Tris-EDTA) buffer and were stored at − 20 °C for further use. The quality of genomic DNA extracted from 19 genotypes was checked by agarose gel electrophoresis using 0.8% agarose gel (Primrose and Twyman 2013). The quality and quantity were checked by using nano-drop spectrophotometer (BioSpectrometer, Eppendorf, Germany). The ratio of A260/A280 was calculated for each sample and further used to select samples with good quality of DNA. The quantity was directly measured as nanogram/microliter, and based on the quantification data, a portion of DNA samples was diluted with an appropriate quantity of 1X TE buffer to yield a working concentration of 50 ng/μl and stored at − 20 °C for further molecular analysis.
PCR amplification and electrophoresis
PCR reaction was carried out in a volume of 15 μl containing 50 ng/μl template DNA, forward and reverse primers (concentration of 20 pmole/μl), 10X PCR buffer, 10 mM of dNTPs, 2.5 mM MgCl2, and 1.0 U of Taq DNA polymerase. A total of 11 inter simple sequence repeat (ISSR) primers were utilized to estimate molecular variability of genotypes under study (Table 2). Amplification was performed in a thermal cycler (Eppendorf, Germany). The PCR amplification was performed as follows: initial denaturation (at 94 °C for 4:00 min) followed by 35 cycles of denaturation (at 94 °C for 1:00 min), annealing (at melting temperature (°C) for 1:00 min), extension (at 72 °C for 1:30 min) and a final extension (at 72 °C for 6:00 min) followed by cooling at 4 °C. The amplification products along with the standard DNA marker were separated by electrophoresis on 1.5% agarose gel and photographed under UV light using FluorChem FC 2 gel documentation system (Alpha Innotech Corporation, USA).
Scoring and analysis of data
Data on the presence (1) or absence (0) of the bands of PCR-amplified DNA fragments were scored as binary data. Matrices were generated for binary data using Jaccard’s similarity co-efficient (Jaccard 1908) and cluster analysis using the unweighted pair group method with arithmetic mean (UPGMA). Principal component analysis (PCA) was performed with a bootstrap frequency of n = 500 in software PAST version 3.18 (Hammer et al. 2001). The software program AlphaEaseFC version 6.0.0 was used to determine the molecular weight of bands separated on the gel. The polymorphism information content (PIC) value was calculated on the basis of allele frequency by the given formula (Prevost and Wilkinson 1999). The polymorphism percentage was calculated by the following formula. Polymorphism (%) = (number of polymorphic bands/total number of bands) × 100.
Results
ISSR analysis
In the present study, genomic DNA samples of 19 grain amaranth genotypes were subjected to amplification by ISSR primers to access genetic diversity. All the 11 ISSR primers used in the study were found polymorphic, and results obtained using these 11 primers have been presented here in Table 2. The data collected from 11 ISSR primers produced a total of 114 DNA fragments, among which 98 fragments were polymorphic whereas 16 fragments were monomorphic. Average bands per primer were 10.36 in the present study. The amplification profiles obtained for grain amaranth genotypes with ISSR primers ISSR-09, ISSR-25, and ISSR-26 are shown in Fig. 1.
Banding pattern of primers a ISSR 09, b ISSR 25, c ISSR 26 among grain amaranth genotypes (1: A. edulis; 2: IC-381195; 3: IC-381135; 4: Deesa local; 5: EC-519549; 6: IC-1733; 7: Annapurna; 8: Durga; 9: BGA-2; 10: GA-1; 11: GA-2; 12: GA-3; 13: PRA-1; 14: PRA-2; 15: PRA-3; 16: PRA-4; 17: RMA-7; 18: Suvarna; 19: VL-44; L: 100 bp ladder; arrow indicates unique band)
The mean number of polymorphic bands per primer was 8.91. Overall, the size of PCR-amplified DNA fragments varied from 200 to 3702 bp. The highest number of amplified bands (13) was exhibited by primer ISSR 0, whereas the lowest number of amplified bands (5) was exhibited by primer ISSR 05. The highest polymorphism (100%) was exhibited by three primers ISSR 03, ISSR 05, and ISSR 22, while the lowest polymorphism (75.00%) was evinced with three primers, viz., ISSR 09, ISSR 25, and ISSR 26. The average percent polymorphism among Amaranthus genotypes detected by the ISSR primers in the present investigation was 87.15%. The results indicated that ISSR primers used in the present study gave good polymorphism percent, which enable them to be used for the assessment of genetic diversity.
Most of the polymorphic primers (10 out of 11) had a PIC value above 0.8. Among all the 11 polymorphic primers, 10 primers, viz., ISSR 25, ISSR 26, ISSR 22, ISSR 03, ISSR 21, ISSR 09, ISSR 10, ISSR 06, ISSR 15, and ISSR 16, had a very high PIC value above 0.8. Hence, these ten polymorphic primers having high PIC value can effectively be utilized for the assessment of genetic diversity in grain amaranth. The average PIC value of all the 11 ISSR primers was 0.853, which indicates good selection of primers in the present study for the assessment of genetic diversity.
Some primers produced genotype specific, unique bands, viz., ISSR 26 produced EC-519549 genotype-specific band of 3164 bp, ISSR 03 produced IC-381135 genotype-specific band of 530 bp, ISSR 09 produced Amaranthus edulis-specific band of 995 bp, ISSR 10 produced Amaranthus edulis-specific band of 900 bp, ISSR 05 produced Deesa local-specific band of 320 bp, ISSR 06 produced Suvarna-specific band of 3140 bp, ISSR 16 produced VL-44-specific band of 2232 bp, and ISSR 21 produced Suvarna and VL-44-specific bands of 1573 bp and 190 bp, respectively (Table 2). These unique band-producing primers can be used for the identification of genotypes.
Development of dendrogram
Jaccard’s co-efficients were used to compare a set of variables and to generate a similarity matrix. Jaccard co-efficients for all genotypes are shown in (Table 3). Similarity indices were estimated on the basis of 11 ISSR primers ranging from 0.356 (between A edulis and Suvarna) to 0.885 (between PRA-2 and PRA-4), indicating the presence of genetic diversity among the genotypes of grain amaranth.
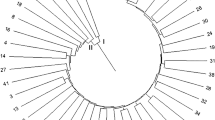
The dendrogram was prepared based on UPGMA by using Jaccard’s similarity co-efficients through PAST (version 3.18) (Fig. 2). The dendrogram (Jaccard’s distance, paired-group) clustered with the data generated by all primers and their amplicons grouped the 19 grain amaranth genotypes into two major clusters, i.e., cluster A and cluster B as shown in Fig. 2. According to the dendrogram, cluster A and cluster B had 49% similarity cutoff value on the dendrogram. The dendrogram showed a cophenetic correlation co-efficient of 0.855.
Cluster A contained two genotypes, namely EC-519549 and A. edulis. Cluster B comprised a total of 17 genotypes. The cluster B was further divided into two sub-clusters, namely B1 and B2. The similarity between sub-clusters B1 and B2 was 50%. The sub-cluster B1 contained 15 genotypes and was divided into two other sub-clusters, viz., B1:1 and B1:2. According to the dendrogram, sub-cluster B1:1 and sub-cluster B1:2 had 59% similarities. The sub-clusters B1:1 and B1:2 contain nine and six genotypes, respectively. Thus, the sub-cluster B1:1 was the largest sub-cluster having nine genotypes, viz., Deesa Local, Annapurna, GA-2, PRA-3, PRA-2, PRA-4, RMA-7, GA-1, and GA-3. The sub-cluster B1:2 contained six genotypes, viz., IC-1733, BGA-2, Durga, PRA-1, Suvarna, and VL-44. The sub-cluster B2 contained two genotypes, namely IC-381195 and IC-381135. The results indicated that genotypes PRA-2 and PRA-4 had highest similarity whereas A. edulis and Suvarna were found to be the most diverse genotypes. The clustering of grain amaranth genotypes into two groups showed reasonable variability, which may be exploited for selecting parents for breeding purposes. The present study indicated that the ISSR marker is an easy and simple technique for genetic diversity assessment of grain amaranth genotypes which can be employed for the improvement of amaranth.
2D plot of PCA
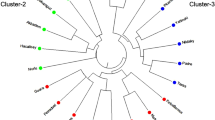
In the 2D plot of principle component analysis (PCA) derived from the ISSR genotyping data (plotted in the coordinate system given by the two most important components), it can be observed that major three groups/clusters were formed on the PCA plot (Fig. 3). The PCA revealed the first three most informative principal components with Eigenvalues of 2.88, 2.56, and 1.65, respectively, which together accounted for 48.03% of the total genetic variation. The results of the PCA were comparable to those obtained with cluster analysis.
The groups formed on PCA plot resembled with the results of the dendrogram although some genotypes have been diverted on the PCA plot. On PCA plot, three groups have been formed and other three genotypes, namely IC-1733, EC-519549, and A. edulis, scattered on the plot and do not fall under any of the group. Group I contained four genotypes, viz., VL-44, Durga, Suvarna, and PRA-1, which were placed between − 3.10 and − 1.50 on the X-axis. Group II contained, a total of nine genotypes, namely PRA-2, Annapurna, PRA-3, Deesa local, GA-1, GA-2, GA-3, RMA-7, and PRA-4, which were placed between 0.75 to 2.10 on X-axis. Group III contained two genotypes, viz., IC-381135 and IC-381195. The grouping of the genotypes in the PCA corresponds with the cluster analysis with some minor differences. From the above discussion, it may be concluded that ISSR analysis revealed substantial polymorphism in grain amaranth. The technique may be used to obtain reasonably precise information on the genetic relationship among grain amaranth genotypes. Such information may be useful for selecting the diverse parents and monitoring the genetic diversity periodically in the breeder’s working collection of grain amaranth.
Discussion
ISSR analysis
In the present investigation, average bands per primer were 10.36. Raut et al. (2014) and Oduwaye et al. (2014) reported 4.41 and 6.57 average bands per ISSR primers in amaranth genotypes, respectively, which are very lower than the results of the present study. However, Ray and Roy (2007) and Žiarovská et al. (2013) found 11.36 and 16.00 bands per primer, respectively, which is higher than the findings of the present investigation. Overall, primers used in the present study gave the good amplification and can be used in the diversity analysis in grain amaranth.
The average percent polymorphism among Amaranthus genotypes detected by the ISSR primers in the present investigation was 87.15%, which is higher than the findings of the previous reports, viz., 86.88% (Raut et al. 2014) and 80.4% (Xu and Sun 2001), whereas it is lower than the findings of few previous reports, viz., 93% (Oduwaye et al., 2014) and 98.4% (Ray and Roy, 2007). Hence, it is concluded that the primers used in the present study possessed good power to differentiate the genotypes used in the study. The results indicated that ISSR primers used in the present study gave good polymorphism percent, which enable them to be used for the assessment of genetic diversity.
The average PIC value of all the 11 ISSR primers was 0.853, which indicates good selection of primers in the present study for the assessment of genetic diversity. Most of the previous studies were lacking of PIC calculation in diversity analysis of grain Amaranthus by ISSR; however, very low PIC values (0.48) was found in one of the past studies (Oduwaye et al. 2014). As the primers used in the present study possessed very high PIC values, they have a high promising efficiency to be used in the genetic diversity analysis in grain amaranth.
Development of dendrogram
The dendrogram was prepared based on UPGMA by using Jaccard’s similarity co-efficients. The dendrogram grouped the 19 grain amaranth genotypes into two major clusters, i.e., cluster A and cluster B. The diverse genotypes A. edulis and EC-519549 (exotic collection) formed a separate cluster (cluster A). A. edulis and EC-519549 clustering in the same cluster might be due to the similarity in regions of genome which have been amplified by the primers used in the present study. The genotypes of the same species were grouped in the same major cluster (cluster B). Stefunova et al. (2014) used ISSR approach to analyze intra- and inter-specific variability of A. caudatus, A. hypochondriacus, and A. cruentus accessions. They reported that most of the accessions of the same species were grouped in the same cluster except some accessions having different origin. In the present study, the genotypes of the same species were grouped in the same major cluster. The dendrogram showing clustering in 28 varieties of grain amaranth (A. hypochondriacus) by ISSR marker was developed by Raut et al. (2014). Singh et al. (2013) constructed dendrogram using the UPGMA method, depicting the classification of Amaranthus accessions based on ISSR marker. There are very few reports available for the inter-specific diversity analysis in grain amaranth using ISSR marker (Nolan et al. 2010; Stefunova et al. 2014; Štefúnová et al. 2015). Hence, this study can help in the molecular characterization and diversity analysis of Amaranthus species at large scale to help in the breeding program for the development of grain amaranth.
2D plot of PCA
The PCA revealed the first three most informative principal components with Eigenvalues of 2.88, 2.56, and 1.65, respectively, which together accounted for 48.03% of the total genetic variation. The first three components can be used for the PCA analysis as the value is slightly less than 50%. Moreover, it is reported that PCA in combination with cluster analysis is a useful tool to extract maximum information from molecular marker data, if the first two or three components explain > 25% of the original variation (Rajwade et al. 2010). The results of the PCA were comparable to those obtained with cluster analysis.
The grouping of the genotypes in the PCA corresponded with the cluster analysis with some minor differences. Similarly, minor discrepancies were also reported by Oduwaye et al. (2014) in an ISSR analysis. It is noteworthy that, in the past, Ray and Roy (2007) used principal component analysis (PCA) to support cluster analysis for ISSR primers. In the recent studies of Kapuria et al. 2019 and Chaudhari et al. 2019, PCA analysis has been used for the diversity analysis using RAPD and SSR markers, respectively, which supported the cluster analysis.
The necessity for the improvement of Amaranthus in these days breeding programs is realized globally. Amaranth, as a promising crop, will need a good knowledge about germplasm collections for the genetic improvement. However, still only limited evidence is available on intra- and inter-specific genetic diversity and relationships within grain amaranth (Amaranthus spp.) germplasm collections exist and results of the present study report a new understanding into it.
Conclusion
The results of the current study indicate that ISSR markers used in the study appeared to be worthy for the molecular assay for fingerprinting and evaluating the genetic relationship among genotypes of grain amaranth as they showed highly promising polymorphism and PIC values. These markers can be exploited for molecular breeding for the improvement of grain amaranth.
Availability of data and materials
All data on which abstracted of the study have been drawn are presented in the main manuscript.
Abbreviations
- CTAB:
-
Cetyl trimethyl ammonium bromide
- DNA:
-
Deoxyribonucleic acid
- ISSR:
-
Inter simple sequence repeat
- PCA:
-
Principal component analysis
- PCR:
-
Polymerase chain reaction
- PIC:
-
Polymorphism information content
- RAPD:
-
Random amplified polymorphic DNA
- SSR:
-
Simple sequence repeat
- UPGMA:
-
Unweighted pair group method with arithmetic mean
References
Anjali K, Joshi A, Maloo SR, Sharma R (2013) Assessment of the morphological and molecular diversity in Amaranthus spp. Afr J Agric Res 8(19):2307–2311
Chandi A, Milla-Lewis SR, Jordan DL, York AC, Burton JD, Zuleta MC, Whitaker JR, Culpepper AS (2013) Use of AFLP markers to assess genetic diversity in Palmer amaranth (Amaranthus palmeri) populations from North Carolina and Georgia. Weed Sci 61(1):136–145
Chaudhari BA, Patel MP, Dharajiya DT, Tiwari KK (2019) Assessment of genetic diversity in castor (Ricinus communis L.) using microsatellite markers. Biosci Biotechnol Res Asia 16(1):61–69
Dharajiya DT, Khadia SM, Pagi NK, Khatrani TJ, Jasani HV, Khunt AD, Ravindrababu Y (2017) Modified method of high quality genomic DNA extraction from mungbean [Vigna radiata (L.) Wilczek] suitable for PCR based amplification. Indian J Sci Technol 10(20):1–7
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Faseela KV, Joseph S (2007) Molecular characterization of amaranth landraces and assessment of interspecific relationships among Amaranthus spp. (L.) using RAPD markers. Indian J Genet Plant Breed 67(1):12–17
Grant WF (1959) Cytogenetic studies in Amaranthus. III. Chromosome numbers and phylogenetic aspects. Can J Genet Cytol 1(4):313–328
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: palaeontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Jaccard P (1908) Nouvelles recherches sur la distribution fl orale. Bull Soc Vaud Sci Nat 44:223–270
Janssen F, Pauly A, Rombouts I, Jansens KJ, Deleu LJ, Delcour JA (2017) Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): a food science and technology perspective. Compr Rev Food Sci Food Saf 16(1):39–58
Jimenez FR, Maughan PJ, Alvarez A, Kietlinski KD, Smith SM, Pratt DB, Elzinga DB, Jellen EN (2013) Assessment of genetic diversity in Peruvian amaranth (Amaranthus caudatus and A. hybridus) germplasm using single nucleotide polymorphism markers. Crop Sci 53(2):532–541
Kapuria M, Dharajiya D, Pachchigar K, Chauhan RM (2019) Molecular characterization and genetic diversity of Indian potato (Solanum tuberosum L.) germplasms using microsatellite and RAPD markers. Biosci Biotechnol Res Commun 12(1):80–89
Lee JR, Hong GY, Dixit A, Chung JW, Ma KH, Lee JH, Kang HK, Cho YH, Park YJ (2008) Characterization of microsatellite loci developed for Amaranthus hypochondriacus and their cross-amplifications in wild species. Conserv Genet 9(1):243–246
Mallory MA, Hall RV, McNabb AR, Pratt DB, Jellen EN, Maughan PJ (2008) Development and characterization of microsatellite markers for the grain amaranths. Crop Sci 48(3):1098–1106
Nadeem MA, Nawaz MA, Shahid MQ, Doğan Y, Comertpay G, Yıldız M, Hatipoğlu R, Ahmad F, Alsaleh A, Labhane N, Özkan H (2018) DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnol Biotechnol Equip 32(2):261–285
Nolan C, Noyes A, Bennett A, Hunter R, Hunter KL (2010) Inter simple sequence repeats (ISSR) reveal genetic variation among mid-Atlantic populations of threatened Amaranthus pumilus and phylogenetic relationships. Castanea 75(4):506–516
Oduwaye O, Baránek M, Cechová J, Raddová J (2014) Reliability and comparison of the polymorphism revealed in amaranth by amplified fragment length polymorphism (AFLPs) and inters simple sequence repeats (ISSRs). Afr J Plant Breed Crop Sci 6(4):48–56
Omondi EO, Debener T, Linde M, Abukutsa-Onyango M, Dinssa FF, Winkelmann T (2016) Molecular markers for genetic diversity studies in African leafy vegetables. Adv Biosci Biotechnol 7(3):188–197
Pagi N, Prajapati N, Pachchigar K, Dharajiya D, Solanki SD, Soni N, Patel P (2017) GGE biplot analysis for yield performance of grain amaranth genotypes across different environments in western India. J Exp Biol Agric Sci 5(3):368–376
Parita B, Kumar SN, Darshan D, Karen P (2018) Elucidation of genetic diversity among ashwagandha [Withania somnifera (L.) Dunal] genotypes using EST-SSR markers. Res J Biotechnol 13(10):52–59
Park YJ, Nishikawa T, Matsushima K, Minami M, Nemoto K (2014) A rapid and reliable PCR-restriction fragment length polymorphism (RFLP) marker for the identification of Amaranthus cruentus species. Breed Sci 64(4):422–426
Popa G, Cornea CP, Ciuca M, Babeanu N, Popa O, Marin D (2010) Studies on genetic diversity in Amaranthus species using the RAPD markers. Analele Universitatii din Oradea-Fascicula Biologie 17(2):280–285
Prevost A, Wilkinson MJ (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98(1):107–112
Primrose SB, Twyman R (2013) Principles of gene manipulation and genomics. Wiley, New York
Rajwade AV, Arora RS, Kadoo NY, Harsulkar AM, Ghorpade PB, Gupta VS (2010) Relatedness of Indian flax genotypes (Linum usitatissimum L.): an inter-simple sequence repeat (ISSR) primer assay. Mol Biotechnol 45(2):161–170
Rastogi A, Shukla S (2013) Amaranth: a new millennium crop of nutraceutical values. Crit Rev Food Sci Nutr 53(2):109–125
Raut VR, Dodake SS, Chimote VP (2014) Evaluation of genetic diversity in grain amaranth (Amaranthus hypochondriacus) at molecular level using ISSR markers. Indian J Agric Biochem 27(1):60–65
Ray T, Roy SC (2007) Phylogenetic relationships between members of Amaranthaceae and Chenopodiaceae of lower gangetic plains using RAPD and ISSR markers. Bangl J Bot 36(1):21–28.
Ray T, Roy SC (2008) Genetic diversity of Amaranthus species from the Indo-Gangetic plains revealed by RAPD analysis leading to the development of ecotype-specific SCAR marker. J Hered 100(3):338–347
Singh B, Pandey S, Kumar J (2013) A comparative study of inter simple sequence repeat (ISSR), random amplified polymorphic DNA (RAPD) and simple sequence repeat (SSR) loci in assessing genetic diversity in Amaranthus. Indian J Genet Plant Breed 73:411–418
Snezana DM, Marija K, Danijela R, Milena S, Lidija S (2012) Assessment of genetic relatedness of the two Amaranthus retroflexus populations by protein and random amplified polymorphic DNA (RAPD) markers. Afr J Biotechnol 11(29):7331–7337
Stefunova V, Bezo M, Labajová M, Senková S (2014) Genetic analysis of three Amaranth species using ISSR markers. Emir J Food Agric 26(1):35–44
Štefúnová V, Bežo M, Žiarovská J, Ražná K (2015) Detection of the genetic variability of Amaranthus by RAPD and ISSR markers. Pak J Bot 47(4):1293–1301
Suresh S, Chung JW, Cho GT, Sung JS, Park JH, Gwag JG, Baek HJ (2014) Analysis of molecular genetic diversity and population structure in Amaranthus germplasm using SSR markers. Plant Biosyst 148(4):635–644
Wang XQ, Park YJ (2013) Comparison of genetic diversity among amaranth accessions from south and Southeast Asia using SSR markers. Korean J Med Crop Sci 21(3):220–228
Xu F, Sun M (2001) Comparative analysis of phylogenetic relationships of grain amaranths and their wild relatives (Amaranthus; Amaranthaceae) using internal transcribed spacer, amplified fragment length polymorphism and double-primer fluorescent inter simple sequence repeat markers. Mol Phylogenet Evol 21(3):372–387
Žiarovská J, Ražná V, Labajová M (2013) Using of inter microsatellite polymorphism to evaluate gamma-irradiated Amaranth mutants. Emir J Food Agric 25(9):673–681
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR) anchored polymerase chain reaction amplification. Genomics 20(2):176–183
Acknowledgements
All the authors are grateful to the authorities of SDAU, Sardarkrushinagar, Gujarat, India, for providing requirements to conduct the research work.
Funding
This research work is not funded financially by any agency.
Author information
Authors and Affiliations
Contributions
MJG and DTD designed the research work and performed the experiments for extraction of DNA, PCR, electrophoresis, and scoring. SDS and NNP guided in the selection of genotypes and growing of plantlets for the DNA extraction. KKT helped in the analysis of the data. DTD analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that the work is ethically approved and consented to participate.
Consent for publication
The authors declare that the work has been consented for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gelotar, M.J., Dharajiya, D.T., Solanki, S.D. et al. Genetic diversity analysis and molecular characterization of grain amaranth genotypes using inter simple sequence repeat (ISSR) markers. Bull Natl Res Cent 43, 103 (2019). https://doi.org/10.1186/s42269-019-0146-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0146-2