Abstract
The use of cannabidiol (CBD) for therapeutic purposes is receiving considerable attention, with speculation that CBD can be useful in a wide range of conditions. Only one product, a purified form of plant-derived CBD in solution (Epidiolex), is approved for the treatment of seizures in patients with Lennox-Gastaut syndrome, Dravet syndrome, or tuberous sclerosis complex. Appraisal of the therapeutic evidence base for CBD is complicated by the fact that CBD products sometimes have additional phytochemicals (like tetrahydrocannabinol (THC)) present, which can make the identification of the active pharmaceutical ingredient (API) in positive studies difficult. The aim of the present review is to critically review clinical studies using purified CBD products only, in order to establish the upcoming indications for which purified CBD might be beneficial. The areas in which there is the most clinical evidence to support the use of CBD are in the treatment of anxiety (positive data in 7 uncontrolled studies and 17 randomised controlled trials (RCTs)), psychosis and schizophrenia (positive data in 1 uncontrolled study and 8 RCTs), PTSD (positive data in 2 uncontrolled studies and 4 RCTs) and substance abuse (positive data in 2 uncontrolled studies and 3 RCTs). Seven uncontrolled studies support the use of CBD to improve sleep quality, but this has only been verified in one small RCT. Limited evidence supports the use of CBD for the treatment of Parkinson’s (3 positive uncontrolled studies and 2 positive RCTs), autism (3 positive RCTs), smoking cessation (2 positive RCTs), graft-versus-host disease and intestinal permeability (1 positive RCT each). Current RCT evidence does not support the use of purified oral CBD in pain (at least as an acute analgesic) or for the treatment of COVID symptoms, cancer, Huntington’s or type 2 diabetes. In conclusion, published clinical evidence does support the use of purified CBD in multiple indications beyond epilepsy. However, the evidence base is limited by the number of trials only investigating the acute effects of CBD, testing CBD in healthy volunteers, or in very small patient numbers. Large confirmatory phase 3 trials are required in all indications.
Similar content being viewed by others
Background
The use, acceptance and legality of cannabinoid-based medicines and the cannabinoid cannabidiol (CBD) have grown considerably in the last 10 years. CBD is in the unusual position where it is available as both a health supplement and as a licensed medicine. As a health supplement, it is estimated that ~ 10% of populations are using CBD [1]. In the UK, the recommended daily allowance of CBD is 70 mg/day in these types of products [2]. Anecdote and survey data suggest that people take CBD for anxiety, stress, depression, sleep, gastrointestinal problems and pain [3,4,5]. Some of the reputation of CBD’s therapeutic utility across such a broad range of conditions is based on scientific evidence, albeit still at a preclinical level.
Clinically, a purified oral solution of CBD (Epidiolex) is approved for the management of seizures in patients with Lennox-Gastaut syndrome, Dravet syndrome or tuberous sclerosis complex on the basis of multiple positive, phase 3 randomised controlled trials (RCTs) [6]. Patients typically take a dose of between 20 and 25 mg/kg/day, with an average seizure reduction of ~ 50% [7] and a mean serum concentration of ~ 125 ng/ml [8].
CBD products come in many forms; they can be purified synthetically or by plant extraction processes (usually at least ~ 98% pure CBD), broad spectrum distillates (including many of the other phytocannabinoids and phytochemicals) or full spectrum (including all the phytochemicals from the cannabis plant from which the CBD was extracted). Spectrum products are sometimes also called enriched or artisan products. CBD products can also come in defined ratios with Δ9tetrahydrocannabinol (THC), for example, 20:1 CBD to THC products. In any of the non-purified CBD products that have therapeutic utility, it is difficult to tell whether the benefit of the product arises from CBD as the active pharmaceutic ingredient (API), or as a consequence of the other chemicals present, or as a consequence of any interaction (pharmacodynamic or pharmacokinetic) between the compounds (referred to as the entourage hypothesis).
The aim of this review is to establish what indications there are clinical evidence for the efficacy of purified CBD products only, without any complication of additional APIs or drug interactions. This is critical in the understanding of the CBD molecule. We have not considered epilepsy, where multiple reviews already exist, and the efficacy of purified CBD is well established [9, 10]. We addressed the aim of the study by appraising the published literature of clinical studies that have examined the therapeutic benefit of purified CBD in various conditions. A total of 99 clinical studies testing purified CBD were identified through systematic searching of engines and hand searching; 29 were uncontrolled/observational studies, and 70 were randomised controlled trials (RCTs). These studies were categorised by disease indication and are discussed below in the order of the weight of evidence/number of studies per condition. A summary of the patient cohorts in which CBD has clinical evidence of significant efficacy through controlled studies is presented in Table 1 and Fig. 1.
Purified CBD as an anxiolytic
The greatest number of studies that have investigated purified CBD have been in the area of reducing anxiety. There is strong preclinical evidence to support CBDs anxiolytic effects in multiple animal models, mediated by a number of mechanisms including 5-HT1, 5-HT3, CB1, GPR3 and GPR6, GABA and PPARγ (for reviews, see [50, 51]).
Six uncontrolled clinical studies have reported on the efficacy of CBD in anxiety. Four case reports document that CBD was successful at reducing anxiety symptoms in teenagers/young adults with substance abuse, social anxiety and depression [52], social anxiety and psychotic symptoms [53], cannabis withdrawal syndrome [54] and Crohn’s disease with social phobia [55]. Similarly, two case series report that CBD reduced symptoms of anxiety in several hundred patients prescribed CBD via clinics [56, 57]. Most recently, Berger and colleagues report that 12 weeks treatment with purified CBD (up to 800 mg/day) led to a significant reduction in anxiety and depression in an open label study of 30 patients with anxiety disorders [58].
Since the 1970s, researchers have been investigating the ability of CBD to reduce anxiety and stress in RCTs in healthy (mostly male) volunteers. Early studies showed that acute doses of CBD (60 mg (n = 40) or 1 mg/kg (n = 8)) could reduce the anxiety produced by THC administration [39, 40]. A series of studies went on to show that a single oral administration of CBD (300 mg) is able to reduce the acute anxiety caused by public speaking in a total of 157 volunteers (some of which received placebo or other CBD doses) [41,42,43]. Functional brain imaging studies in healthy (mostly male) volunteers showed that CBD (600 mg) attenuated the blood oxygenation signal in the amygdala and the anterior and posterior cingulate cortex while subjects (n = 15) were processing intensely fearful faces [59] and reduced prefrontal-subcortical neural connectivity when shown fearful faces (n = 15) [46], brain region imaging findings that could be translated into anxiolytic effects. Similarly, a single dose of 400 mg reduced anxiety in healthy males (n = 10) associated with neuroimaging changes in the limbic and paralimbic brain areas [45]. A single inhalation of CBD (32 mg, n = 48) enhanced the consolidation of extinction learning (that is, helps people to forget fearful memories), which may underpin the anxiolytic effects of CBD [44]. Acute (n = 9) [47] and 7 days (n = 26) [49] oral treatment with CBD (600 mg) also reduces resting blood pressure and the blood pressure response to mental or physical stress in healthy males. However, the three most recent studies in healthy volunteers did not report anxiolytic effects of CBD. Stanley and colleagues found that a single oral dose CBD (150, 300 or 600 mg) did not affect self-reported anxiety induced by an experimentally induced examination scenario in 32 college students (although the vehicle group had higher levels of anxiety across timepoints) [60]. It is interesting that this study was mainly in female participants, and recent preclinical data suggest that the anxiolytic effects of CBD are more pronounced in males rats and vary greatly across the female rat hormonal cycle [61]. A single oral of CBD (600 mg) also did not affect emotional processing or self-reported stress (to mental arithmetic) in healthy participants (12 male and 12 female) in a neuroimaging study [62]. And finally, a single dose of oral CBD (150, 300 or 600 mg across 61 healthy adults) did not alter the self-reported fear, panic or tachycardia induced by a 10% carbon dioxide-enriched air breathing challenge [63]. It is possible that CBD modifies some stressful/anxiogenic situations and not others, and it is worth noting that neither the examination scenario or mental arithmetic induced large increases in anxiety in the most recent studies.
Using more relevant patient cohorts, nine RCTs have assessed the anxiolytic effects of CBD, of which three were negative, and 6 were positive. Looking first at the negative studies, a single dose of CBD (600 mg) did not affect persecutory ideation and anxiety, cortisol or blood pressure in patients with high paranoid traits [64]. Ten days treatment with CBD (300 mg) did not improve indicators of anxiety, depression or sleep in 31 patients with crack cocaine dependence [65]. Weekly administration of CBD (300 mg, one per week for 8 weeks) in conjunction with a therapy session, did not enhance the therapy responses in 80 patients with social anxiety disorder and panic disorder with agoraphobia [66]. However, positive effects of CBD were observed in other patient cohorts. In treatment-naïve patients with generalised social anxiety disorder (SAD, n = 10), a single dose of CBD (400 mg) reduced subjective anxiety associated with changes in blood flow in limbic and paralimbic areas [25]. Similarly, a single dose of CBD (600 mg) reduced anxiety and cognitive impairment in 36 treatment-naïve SAD patients induced by public speaking [26]. Another RCT showed that 4 weeks treatment with CBD (300 mg) reduced negative evaluation and anxiety scores in teenagers with SAD (n = 40) [27]. In patients with heroin use disorder (n = 42), 3 days treatment with CBD (400 or 800 mg) reduced craving and anxiety, cortisol and tachycardia induced by the presentation of drug cues, which persisted 7 days after the final CBD exposure [34]. Oral CBD (600 mg for 1 week, n = 58) also reduced the anxiety induced by public speaking in patients at high risk for psychosis [18] and in 24 patients with Parkinson’s disease (one 300 mg dose [22]).
In conclusion, in healthy volunteers, there is conclusive evidence that a single dose of CBD (300–600 mg) can reduce blood pressure and anxiety associated with changes in brain activity and connectivity shown by neuroimaging. Several studies suggest CBD is effective in SAD, with mixed results on anxiety induced by substance abuse, and in anxiety comorbid to other conditions. There are a number of ongoing trials investigating CBD in SAD (NCT05571592, NCT03549819) and in anxiety associated with paediatric epilepsy (NCT05324449), advanced breast cancer (NCT04482244), anorexia nervosa (NCT04878627), bipolar disorder (NCT05457465) and Alzheimer’s disease (NCT04075435), which will add to this growing evidence base.
Purified CBD in psychiatric conditions (psychosis, schizophrenia and bipolar disorder)
Many preclinical studies have demonstrated the role of pure CBD in ameliorating the symptoms of psychosis, and in models of schizophrenia (such as reducing stereotypy, hyperlocomotion, normalisation of social withdrawal and cognitive improvements) involving CB1 and CB2, 5-HT1A receptor and neurogenesis factors [67,68,69].
Zuardi and colleagues first reported in 1995 in a patient that oral CBD (up to 1500 mg/day) led to improvements in Brief Psychiatric Rating Scale (BPRS) symptoms and a diazepam dose reduction in a 19-year-old female, which worsened with discontinuation of CBD [70]. In another case series, a pattern of symptom improvement was observed with oral CBD in one patient (from 40 mg/day to a maximum dose of 1280 mg/day), with symptoms worsening after discontinuation [71]. However, two patients in the same series did not demonstrate any change in symptoms of psychosis with the same dosing regimen [71]. Two case reports assessing CBD (oral dosing from 600 mg/day to 1200 mg/day for 24 days) in patients with mania associated with bipolar disorder did not report any symptom improvement during monotherapy phases (although one patient demonstrated some improvement while on concurrent olanzapine and CBD) [72].
Several RCTs have looked at repeated CBD treatment in patients with schizophrenia. When administered as an adjunctive therapy, 1000 mg oral CBD per day reduced psychotic symptoms over a period of 6 weeks (n = 88), as assessed by the Positive and Negative Syndrome Scale (PANSS) and by clinical assessment [13]. Oral CBD (200 mg/day, increased to a maximum of 800 mg/day for 4 weeks) alleviated psychotic symptoms (assessed by PANSS and the Brief Psychiatric Rating Scale) after 14 days and increased serum levels of the endocannabinoid, anandamide (n = 42 [12]).
Evidence of CBD improving central nervous system (CNS) function or reducing CNS dysfunction in patients with schizophrenia or psychosis, or in patients at high risk of psychosis, has also been obtained through RCTs. Specific benefits include improvements from baseline with CBD in visual memory, visuomotor coordination and processing speed (200 mg/day, increased to 800 mg/day, over 6 weeks, n = 42 [11]) and reduced cortisol and psychological responses to social stress (600 mg/day, oral capsules for 8 days, n = 32 [18]). However, a single dose oral CBD capsule (300 mg or 600 mg) did not affect performance on the Stroop Color Word Test in patients with schizophrenia (n = 28) [73].
A series of functional neuroimaging studies have investigated the effects of a single 600 mg dose of oral CBD. CBD attenuates dysfunctions in mediotemporal and prefrontal brain regions and hippocampal-striatal functional connectivity in patients with psychosis (n = 34 [74]) and improves the attenuation of brain activation associated with reward processing in patients at risk for psychosis (n = 52 [17]). CBD also results in changes in parahippocampal gyrus and striatum activation (n = 52) [16]) and striatum, medial temporal cortex and midbrain regions activation (n = 52) [15] in untreated psychosis at-risk subjects.
In conclusion, although the data were often drawn from smaller numbers of patients, some clinical evidence exists to support the use of CBD as an adjunctive treatment in patients with schizophrenia or at high risk for developing psychosis. This is an active area of research with many registered trials ongoing investigating CBD for treatment of early psychosis (NCT04411225), psychosis comorbid to cannabis use (NCT04105231) and as a novel treatment in schizophrenia (NCT04700930, NCT02088060, NCT02926859, NCT00916201) that will continue to add to the evidence base.
Purified CBD as a sleep aid
Anecdotal and survey data suggest that people take CBD to help improve sleep quality [3,4,5], and drowsiness and sleepiness are often reported as side effects of CBD medication [75]. There is a limited amount of preclinical research in this area, but a small number of studies indicate that cannabinoids modulate sleep-wake cycles [76, 77].
A number of published case reports or uncontrolled clinical trials cite improvements in sleep quality with CBD administration in patients, although only one of these was specifically in patients with sleep disorders. In an open-label study in 23 patients with epilepsy, Epidiolex (up to 50 mg/kg/day) lead to an improvement in sleep architecture recorded by electroencephalogram in 85% of e cases [78]. Another open-label study in 28 epilepsy patients found a 33% improvement in sleep duration recorded by the children’s sleep habit questionnaire after 12 months treatment with CBD [79]. Increased sleep quality has also been noted in a case report of a child with PTSD (25 mg at bedtime for 5 months [80]), in an adult with cannabis addiction (24 then 18 mg for 5 month [81]) and in 4 patients with Parkinson’s disease (75 or 300 mg/day for 6 weeks [82]). An audit [56] and a case series [57] similarly found 12% and 67% of CBD prescribed patients reported an improvement in sleep.
Three RCTs have investigated the effect of CBD with measures of sleep as primary endpoints. A study in healthy volunteers (n = 26) found no effect of a single dose of CBD (300 mg) in polysomnography results [83]. In a follow on to the positive effects of CBD reported in a case series in Parkinson’s, de Almeida and colleagues investigated 14 weeks treatment with CBD (75 to 300 mg/day) in 33 Parkinson’s patients with REM sleep behaviour disorder. There was no significant difference in the primary outcomes (mean nights of REM disorder/week and clinical global impression scale), but CBD was associated with an improvement in sleep satisfaction in the CBD group [23]. The only positive RCT is an early study showing that participants (n = 15) with sleep issues reported having slept better after receiving a single dose of 160 mg CBD compared to placebo [31].
In conclusion, although anecdote and observational studies suggest CBD improves sleep (usually comorbid to another condition), there is little RCT evidence to support this. It is not clear whether improvements in sleep are secondary to improvements in other symptoms (for example pain or anxiety), or whether there is a direct effect of CBD on sleep. Currently registered trials in this space are assessing changes in sleep disturbances comorbid to multiple sclerosis (NCT05269628) and HIV (NCT05097651).
Purified CBD as an analgesic
There are dozens of papers investigating the analgesic effects of CBD in numerous rodent models of neuropathic and inflammatory pain, and this topic is well reviewed [84]. Preclinical data also shows that CBD can enhance the analgesic effects of opioids and attenuate opioid tolerance [85, 86]. Anecdotally, one of the primary reasons that people use CBD as a health supplement is for the relief of pain and joint pain [3,4,5].
In a prospective, non-randomised, open-label trial in chemotherapy-induced neuropathy (n = 54 patients), 8 days treatment (over the chemotherapy cycle) with 300 mg/day purified CBD led to a reduction in nerve damage (measured by vibrometry) and patient-reported cold sensitivity and swallowing/throat discomfort compared to a historical control [87].
Since 2020, seven RCTs have been published testing the analgesic properties of CBD: four testing the acute effects of CBD and three testing repeated dosing (2 and 12 weeks administration).
Looking first at the acute administration of CBD, no analgesic effects of a CBD solution (200, 400 or 800 mg) were observed in healthy volunteers (n = 17) who underwent a cold pressor test [88]. Another study in healthy volunteers (n = 20) also found no effect of CBD (800 mg in an oil based solution) on pain induced by intradermal electrical stimulation [89]. A single dose of CBD (150 mg in oil) also did not affect perceived soreness in untrained men (n = 13) after isokinetic exercises (exercise-induced muscle damage) [90]. Similarly, in patients with acute, non-traumatic low back pain (n = 100), CBD (400 mg in medium chain triglyceride oil) administration did not reduce pain scores or oxycodone use [91].
By contrast to the negative acute CBD pain studies, 4 weeks topical treatment with CBD in patients with symptomatic neuropathy (n = 29, mostly diabetic neuropathy) led to a significant reduction in intense pain, sharp pain, cold and itchy sensations in the CBD group when compared to the placebo group [33]. The product patients were using contained 250 mg of CBD per 3 fl. oz container and was advised to be applied up to 4 times per day to symptomatic areas. Another recent trial with a topical CBD product found 2 weeks treatment with topical CBD (6.2 mg/mL CBD with shea butter twice daily) resulted in improvements from baseline among patient-reported outcome measures compared to the control arm in patients (n = 18) with symptomatic thumb basal joint arthritis [32]. However, in patients with hand osteoarthritis or psoriatic arthritis (n = 129), oral treatment with CBD (20–30 mg/day) for 12 weeks did not affect pain intensity, sleep quality, depression or anxiety [92], perhaps reflecting too low a dose to be effective, or the difference between systemic and local administration of CBD.
In conclusion, the available clinical evidence does not support the acute analgesic effects of purified CBD. Two of three RCTs support the analgesic effects of topical CBD treatment to affected areas in neuropathy and arthritis. There is no evidence to date that oral CBD is an effective analgesic, although only one RCT has tested this, and used a low dose of CBD. However, this continues to be a strong area of research and there are more than 30 registered active trials investigating the use of CBD in chronic pain, endometriosis, musculoskeletal pain, back pain, dental pain, post-operative pain and opioid sparing. The data generated through these clinical trials should provide the necessary evidence as to whether CBD is an effective analgesic, and in which pain conditions.
Purified CBD in movement disorders (Parkinson’s disease and Huntington’s disease)
The neuroprotective effects of CBD in multiple neurodegenerative conditions including the movement disorders Parkinson’s (PD) and Huntington’s (HD) diseases have been well studied preclinically, with CBD’s benefits mediated by its antioxidant and anti-inflammatory effects, and through direct or indirect activation of CB1, CB2, PPARγ and 5HT1A [93].
Small, uncontrolled pilot studies in patients with Parkinson’s have demonstrated improvements in dystonia over 6 weeks with oral CBD capsules (100–600 mg/day) [94] and in psychosis symptoms (Brief Psychiatric Rating Scale and the Parkinson Psychosis Questionnaire) over 4 weeks with oral CBD (n = 6, starting at 150 mg/day [95]). A small (n = 4), uncontrolled study by Chagas and colleagues also showed improvements in REM sleep behaviour disorder (RBD) in patients with Parkinson’s disease over 6 weeks (with individualised dosing in the 75–300 mg/day range) [82].
In contrast with the findings of the uncontrolled studies, two larger RCTs with 33 patients each examined the symptoms associated with Parkinsons, and did not report any differences in the frequency of nights with RBD symptoms [23] or severity of associated restless leg syndrome [96] with oral CBD doses of 75–300 mg over 14 weeks. In an RCT that included 24 patients, symptoms associated with anxiety, including anxiogenic tremor, were improved with a single 300 mg dose of CBD in a simulated public speaking test in patients with Parkinson’s disease (de Faria et al., 2020). Quality-of-life (PDQ-39) has also been shown to improve with the use of CBD (capsules, CBD 74 mg/day or 300 mg/day) daily over 6 weeks (n = 21) [24].
The effect of CBD on Huntington’s disease has only been examined in one RCT (n = 15), which did not show any difference in chorea severity between groups when CBD capsules were used at a dose of 10 mg/kg/day over 6 weeks [97].
In conclusion, although inconclusive, Parkinson’s patients may experience improvements in anxiety-related symptoms and overall quality-of-life. There is no evidence to support the use of CBD in Huntington’s disease. There are no registered trials investigating the potential of CBD in these indications, but the existing evidence would suggest this is worth pursuing.
Purified CBD in the treatment of posttraumatic stress disorder (PTSD)
Preclinical evidence shows that pure CBD reduces symptoms in animal models of PTSD [67] and can confer additional benefits in combination with medications like sertraline [98], by similar mechanisms of action as have been identified for anxiety and psychosis,.
Three uncontrolled clinical studies have been published on the use of pure CBD in the treatment of PTSD. A case series in eleven patients found that oral CBD (mean total dose of 49 mg/day; 4 subjects received CBD via oral capsule only, 1 subject received CBD as an oral liquid spray and 6 patients received CBD either by capsules or spray) administered for 8 weeks decreased PTSD symptom severity as assessed by the PTSD Checklist for DSM-5 (PCL-5) [99]. A case report of a 10-year-old female survivor of acute sexual violence also showed that 5 months of CBD oil (25 mg orally at bedtime and 6–12 mg sublingual spray as required during the day) decreased anxiety and improved sleep quality and quantity [100]. Preclinical data suggests that CBD may prevent the development of PTSD [101]. This “prophylactic” use of CBD in PTSD has only been tested in one patient, a 15-year-old female survivor of acute sexual violence, but 7 days oral (capsule) dosing with 300 mg CBD did not prevent the onset of PTSD in this situation [102].
Several RCTs have examined the effects of a single dose of CBD in PTSD patients or healthy volunteers. Among 48 healthy volunteers, Das et al. (2013) examined the use of a single inhaled CBD dose (32 mg) and demonstrated that CBD attenuates fearful responses experienced during recall and reinstatement, suggesting the potentiation of consolidation of extinction memory, which is a key feature of PTSD. Bolsoni and colleagues reported that a single 300 mg CBD dose improved Visual and Analogical Mood Scale cognitive impairment factor scores for up to 1 week after administration in patients with PTSD (n = 33) [20]. In a separate report, the same investigators found that a single 300 mg CBD dose reduced subjective and physiological changes associated with traumatic recall in patients (n = 33) with a history of nonsexual trauma, but not in patients with sexual trauma [19].
Crippa and colleagues examined the effects of prolonged CBD treatment (150 mg twice daily for 28 days) in frontline health care professionals (n = 118) working with patients with COVID-19 (without pre-existing PTSD), on the basis that the risk of PTSD, emotional distress, depression and anxiety has been shown to be increased in that context. They found that, although PTSD symptoms did not differ between the treatment and controls arms, CBD did alleviate symptoms of anxiety, depression and emotional exhaustion [21]. A follow-up study found that the anxiolytic effects of CBD in frontline workers were maintained up to one month after discontinuation of treatment [103].
In conclusion, clinical evidence exists to support the use of single doses of CBD to reduce symptoms of PTSD. This is an area that continues to be researched in adults in PTSD (NCT05269459, NCT04197102), as an adjunctive to prolonged exposure therapy in PTSD (NCT03518801, NCT05132699), and in PTSD comorbid to traumatic brain injury (NCT04550377).
Purified CBD for the management of substance abuse
There is preclinical evidence to show that CBD has anti-addictive properties and can reduce drug seeking behaviour for multiple substances including alcohol, opioids, cocaine and methamphetamine [104]. This has been investigated clinically against various substance abuse disorders, which are discussed below by drug.
In cannabis use disorder, one case report documents that oral CBD (300 mg/day, incrementing to 600 mg/day over 10 days) improved withdrawal, anxiety and dissociative symptoms seen with cannabis withdrawal syndrome [54]. A small uncontrolled study (n = 9) showed that inhaled CBD (mean 215.8 mg/day, with or without nicotine) administered via electronic cigarette led to a reduction in the overall consumption of cannabis by half over a period of 12 weeks in 30% of patients [105]. One RCT in cannabis use disorder has also found that CBD (400 or 800 mg per day over 4 weeks) can reduce urinary THC-COOH to creatinine concentrations, and increase cannabis abstinence more than placebo (n = 34) [36].
The evidence for the use of CBD in patients with cocaine use disorder is mixed. Although an RCT (n = 78) by Mongeau-Pérusse and colleagues did not show any difference in craving scores in response to drug cues with 800 mg/day oral CBD solution during 10-day detoxification and 12 weeks of subsequent follow-up [106], a nested substudy in 48 of the patients found that there was a reduction in inflammatory cytokines (IL-6, VEGF), monocytes and natural killer cells after CBD treatment, suggesting an anti-inflammatory response to CBD in these patients [35]. In patients with crack-cocaine dependence (n = 31), 10 days treatment with oral CBD (300 mg/day) did not affect measures of craving, anxiety, depression and sleep alterations [65].
One study has been carried out in patients with heroin use disorder (n = 42) which found that oral CBD (400 mg or 800 mg per day) over 3 days reduced craving and anxiety and improved heart rate and salivary cortisol responses in patients who were exposed to drug cues while abstinent [34].
In conclusion, evidence from a small number of trials shows that oral or inhaled CBD may help with abstinence in patients with cannabis or heroin abuse disorders and may reduce the inflammatory response seen when withdrawing from cocaine use. This indication continues to be well researched, with active trials with CBD in cannabis use disorder (NCT04105231), alcohol use disorder (NCT04873453, NCT05159830, NCT05387148), heroin use disorder (NCT04567784) and particularly in opioid addiction/sparing (NCT04308148, NCT03813095, NCT05299944, NCT03787628, NCT04982029, NCT04587791, NCT05076370).
Purified CBD in gastrointestinal disorders
Preclinical evidence suggests that pure CBD could be useful in the treatment of a range of gastrointestinal disorders including the suppression of nausea and vomiting, intestinal hypermotility, intestinal inflammation, visceral pain, intestinal hyperpermeability and colon cancer [107, 108], with CB1, CB2, TRPV1, PPARα and 5HT1A implicated as the mechanisms of action.
The clinical evidence for CBD in gastrointestinal conditions comes from four RCTs, with mixed evidence of efficacy. Naftali and colleagues examined the effects of 8 weeks treatment with low-dose CBD (20 mg/day in an olive oil solution) in patients (n = 19) with Crohn’s disease. No difference was seen in the Crohn’s disease activity index between CBD and placebo groups at the end of treatment [109]. In healthy male volunteers (n = 30), a single dose of CBD (600 mg by capsule) significantly reduced the acute increase in intestinal permeability induced by aspirin [48]. Most recently, 4 weeks treatment with Epidiolex (20 mg/kg/day) did not improve any measured symptoms in patients (n = 48) with functional dyspepsia [110]. A chewing gum formulation of CBD (50 mg per gum for 3 weeks using ~ 6 gums per week) also had no impact on pain or quality of life scores in patients (n = 32) with irritable bowel syndrome (IBS) [111].
In conclusion, clinical data is currently lacking to support the use of purified CBD in gastrointestinal disorders. It is likely that the doses used in the IBD and IBS studies were too low, and therefore, further studies at clinically relevant doses are required to conclusively answer whether pure CBD could be beneficial in inflammatory bowel conditions, although there does not appear to be any active trials in this area.
Purified CBD in autism
While there is limited preclinical research on the effects of CBD in autism spectrum disorder (ASD), there is growing real world evidence that CBD can be useful in the management of a number of symptoms of ASD, albeit usually using a CBD-dominant spectrum product containing THC and other phytochemicals [112, 113].
Focusing on studies where just purified CBD has been administered to autistic patients, there have been three RCTs. Two studies examined the effects of a single dose of CBD (600 mg in solution) on brain function in adult males with autism (n = 34) using functional magnetic resonance imaging and magnetic resonance spectroscopy. Both studies showed that 2 h after taking CBD, there are significant changes in neural connectivity and neurotransmitter (glutamate- gamma-aminobutyric acid (GABA)) signalling in areas of the brain implicated in ASD [28, 29]. While ASD symptoms were not measured in these studies, they demonstrate that pure CBD causes acute changes in brain activity in key areas of the brain controlling movement, language, social and visual processing in a relevant population.
A recent (n = 8) pilot study found that 8 weeks treatment with CBD (up-titrated to 20 mg/kg/day in two divided doses, with a maximum dose of 500 mg twice/day in an oil solution) improved the Aberrant Behaviour Checklist (ABC) score in children (age 8–16 years) with intellectual disability with severe behavioural problems [30]. Specific symptoms improved included irritability, social withdrawal, stereotypic behaviour and hyperactivity. While this was a feasibility study (well tolerated) and not powered to assess efficacy, it suggests that CBD may positively modify ASM symptoms as well as modifying brain function.
In conclusion, emerging evidence is promising that pure CBD can ameliorate symptoms associated with ASD. There are multiple active registered clinical trials (NCT03900923, NCT04520685, NCT04745026 and NCT04821856) that are investigating the effects of pure CBD in ASD (mostly in the form of Epidiolex) that will deliver data over the coming years in larger patient numbers.
Purified CBD for the management of blood pressure
There is a strong preclinical evidence base that CBD has multiple effects on the cardiovascular system and has utility in the treatment of conditions including vascular dysfunction associated with diabetes, hypertension (particularly in stressful situations), myocardial infarction, cardiomyopathy, myocarditis and stroke [114,115,116].
There have been a limited number of studies that have attempted to translate this evidence to the clinic. In an RCT in healthy male volunteers, Jadoon and colleagues found that people (n = 9) who had taken pure CBD (600 mg by capsule) had lower blood pressure and a blunted blood pressure response to stress [47]. In a follow-up study, the ability of CBD to blunt the blood pressure response to stress was maintained in healthy volunteers (n = 26) after 7 days dosing with CBD (600 mg/day) [49]. This study also found that treatment with CBD increased internal carotid artery diameter, reduced arterial stiffness and improved endothelial function compared to day 1 in these healthy volunteers. A published clinical trial protocol suggests the concept that CBD has positive vascular effects is being tested in an RCT that is examining the influence of 5 weeks CBD administration on 24-h blood pressure, arterial stiffness, CBD and vascular health biomarkers, inflammation, heart rate variability in individuals with mild or moderate hypertension [117].
In conclusion, preclinical and healthy volunteer data suggest there is a positive impact of pure CBD on the blood pressure response to stress and on vascular health. Ongoing research will validate whether this effect is observed in a clinically relevant population, although this is an area with limited active research.
Purified CBD in the treatment of cancer
The anti-tumoural effects of CBD have been extensively studied. A systematic review of preclinical literature identified 83 studies in human cancer cell lines showing that pure CBD inhibits cell viability and proliferation, inhibits cancer cell migration, is anti-inflammatory and reduces angiogenesis and metastasis in multiple cancer types in vitro and in vivo [118].
No RCTs with purified CBD in cancer progression in patients have been conducted. However, there are three peer-reviewed publications documenting the cases of cancer patients who have observed positive effects of CBD on cancer progression. Retrospective case reports of patients (n = 17 across two publications) with brain tumours (glioblastoma multiforme and high-grade glioma) found that daily treatment with CBD (between 100 and 600 mg/day by capsule) was associated with longer survival rates than was expected in this cohort [119, 120]. In 2018, an analysis of 119 cancer patients with solid tumours from a private clinic claimed that 92% of cases showed some kind of clinical response (reduced circulating tumour cells or a reduction in tumour size) to low-dose purified CBD (average 10 mg twice daily) [121].
In conclusion, preclinical and published case reports suggest an anti-tumoural effect of pure CBD, but this is yet to be tested through controlled clinical trials. Several registered trials are examining the utility of CBD in ameliorating symptoms of cancer including anxiety (NCT04482244) and pain (NCT04754399 and NCT05388058), but not on tumour progression.
Purified CBD in COVID
With the outbreak of COVID-19, many postulated that CBD could potentially be a therapy to reduce the acute or chronic symptoms of the virus. One RCT has since been published in this area (the CANDIDATE Study). This randomised, double-blind, placebo-controlled trial examined the use of oral CBD (300 mg/day) over 2 weeks in 91 patients with mild-to-moderate COVID-19 symptoms. There was no between-group difference in the primary outcome of time to symptom resolution, or secondary outcomes, including inflammatory markers, emotional symptom scales, viral load, smell test or side effects. Thus, current evidence does not support the use of CBD to treat non-severe COVID-19 [21].
Purified CBD for the management of smoking
The potential use of CBD for managing substance abuse has already been discussed, and some studies have also examined whether CBD could be used to reduce nicotine intake. A small (n = 24) randomised pilot study in 2013 demonstrated that inhaled CBD (400 µg doses over 1 week) reduced the consumption of cigarettes by 40% in the treatment group compared with placebo [38]. Hindocha and colleagues also showed that a single 800 mg oral dose of CBD reduced the pleasantness and salience of cigarette smoking cues compared with placebo in an RCT of 30 patients [37]. These studies support the use of, and further research into, CBD as a component of smoking cessation programs. Only one registered trial appeared to be continuing this research (NCT05445804).
Purified CBD in the treatment of type 2 diabetes
Preclinical studies have shown that pure CBD treatment can reduce pancreatic inflammation and the incidence of type 1 diabetes [122, 123], improve metabolic dysfunction (increased insulin, reduced blood glucose, lower fructosamine, lower lipid levels and total cholesterol levels ) in type 2 diabetes [124], reduce diabetic neuropathy [125] and is neuroprotective in middle aged diabetic rats [126]. However, the only RCT has examined the effects of pure CBD (100 mg twice daily for 13 weeks) in patients with type 2 diabetes did not see any positive metabolic effects of CBD [127]. At this dose, CBD failed to affect the primary endpoint (HDL cholesterol) or other markers of metabolic function (glycaemic control, lipid profile, insulin sensitivity, body weight, liver triglyceride content, adipose tissue distribution, appetite, markers of inflammation, blood pressure, markers of vascular function, and circulating endocannabinoids). Within the CBD group, there was a significant decrease in the adipokine resistin, and an increase in the gut hormone glucose-dependent insulinotropic peptide (GIP), although the significance of these changes is unknown given the lack of response against other metabolic parameters. A registered trial continues to investigate the impact of CBD on glucose tolerance in volunteers with a BMI greater than 25 m2 (NCT05285449), although using a low dose that is unlikely to show efficacy (30 mg twice per day). Given the preclinical evidence, it is likely that the dose of CBD used in the RCT was not sufficient to see beneficial effects, and further research is required to test the use of CBD in diabetes, either for direct effects on metabolic function, or for associated problems such as vascular dysfunction, cardiopathy, neuropathy or neuroprotection.
Purified CBD in graft versus host disease
Because of the anti-inflammatory and immunosuppressive effects of CBD, a single prospective trial (n = 48) examined the effect of CBD on the incidence and severity of graft-versus-host disease (GVHD) after allogeneic haematopoietic cell transplantation [128]. In this study, the majority (79%) of patients had acute leukaemia or myelodysplastic syndrome. Among patients receiving 150 mg CBD twice daily from 7 days pre-transplantation to 30 days post-transplantation, in addition to standard GVHD prophylaxis, the authors observed a reduction in grades II to IV acute GVHD compared with controls. The authors also noted that no patient developed GVHD while taking CBD. Although data are limited to a single study, CBD may have some benefits in terms of reducing GVHD for patients undergoing allogeneic hematopoietic cell transplantation. The status of several registered trials in this area is unknown (NCT01596075, NCT01385124, NCT02478424), although there is still one active trial (NCT03840512).
Purified CBD in the treatment of intraocular pressure
There is a small body of literature that has assessed the effects of cannabinoids (mainly THC) in the eye, with a limited number of studies showing benefit of CBD in models of diabetic retinopathy and corneal hyperalgesia [129]. There is only one clinical trial in this field that examined the effects of THC (5 mg) or CBD (20 and 40 mg, acute dose, sublingual) in patients with ocular hypertension or early primary open angle glaucoma [130]. Although THC reduced intraocular pressure 2 h after administration, CBD did not reduce intraocular pressure at any time, and in fact the higher dose produced a transient increase in intraocular pressure at 4 h.
Effective doses and the therapeutic ranges
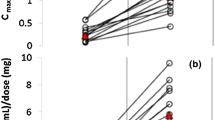
A big question in the clinical use of CBD is how much is enough? To answer this, we have looked at the randomised controlled studies with CBD, as uncontrolled studies tend to underestimate effective doses. Single doses of CBD that have a significantly different biological impact compared to placebo are in the range 160 mg to 800 mg (see Fig. 2A). In RCTs where a positive effect of repeated CBD treatment (on any endpoint) in patients has been recorded compared to placebo, the daily dosing was in the range of 300 to 1200 mg/day (see Fig. 2B). In RCTs where no impact of CBD (on any endpoint) was observed, dosing was in the range of 20 to 800 mg. While there is considerable overlap here, studies that were positive did tend to use a higher mean dose of CBD. Negative trials with CBD could be the result of insufficient dosing or could also be due to poor clinical trial design or the wrong patient populations. Thus, it is sometimes difficult to tell from negative studies whether CBD is ineffective in that particular condition or whether the doses tested were too low. This is confounded by the fact that the oral bioavailability of CBD is low, and only about ~ 10% of the drug will enter circulation [131], and by the fact that many published studies have only looked at a single dose of CBD.
The effective (green) and ineffective (orange) doses of purified CBD (mg/day) tested through randomised controlled trials (RCTs) in the acute setting A and with repeated dosing B across different indications (including healthy volunteer studies). C A summary of all doses in all contexts tested though RCTs. D Plasma or blood levels of CBD (where tested) in acute dosing or repeated doing clinical studies with purified CBD that reported positive (green) or negative (orange) outcomes. Each dot represents an RCT
Unfortunately, very few studies have measured the plasma or tissue levels of CBD as a guide to the therapeutic range of CBD, but where reported, the mean plasma levels of CBD in studies that report a significant effect of CBD on endpoints measured (in either acute or repeated dosing studies) are higher than those that do not (~ 150 nM versus 30 nM respectively, see Fig. 2D). For reference, the mean serum concentration of CBD is ~ 125 ng/ml (~ 350 nM) in patients with epilepsy [8]. CBD has dozens of molecular targets with various potency [50], mostly low, and the therapeutic target levels for CBD probably vary between conditions depending on the most important molecular targets in that particular condition.
Conclusions
Purified CBD is licensed for the treatment of some epilepsy conditions, but mounting evidence suggests that this drug could be used for the treatment of other conditions. This evidence is strongest for psychiatric conditions including anxiety, psychosis and schizophrenia, PTSD and substance abuse, which is underpinned by preclinical data implicating many of the same mechanisms of actions (for example, cannabinoid, serotonin, glycine and GABA receptors). However, this evidence is based on small, proof of concept clinical studies with low patient numbers and so considered weak in the hierarchy of evidence. The strength of evidence for CBD across multiple conditions is presented in Fig. 3. Many of these indications continue to be investigated in registered clinical studies, so the evidence base may be strengthened in the short to medium term. By contrast, there are far fewer studies that have investigated CBD in non-neurological conditions despite a wealth of preclinical evidence of CBDs benefits in conditions such as cardiometabolic disorders and cancer that warrant testing clinically. There is also little active research in this area, so limited data will be emerging in these indications. Future clinical studies should look at the efficacy of ranges of doses of CBD in larger patient numbers and in both genders, establishing target plasma levels for CBD across various indications. Future studies could then establish whether the efficacy of purified CBD can be enhanced with the addition of other phytochemicals, and how CBD affects the efficacy of concomitant treatments.
Availability of data and materials
Not applicable.
References
YouGov. report on CBD (CBD.xls (yougov.com)).
FSA CBD. recommendation (Cannabidiol (CBD) | Food Standards Agency).
Corroon J, Phillips JA. A cross-sectional study of Cannabidiol users. Cannabis Cannabinoid Res. 2018;3:152–61. https://doi.org/10.1089/can.2018.0006.
Leas EC, Hendrickson EM, Nobles AL, Todd R, Smith DM, Dredze M, et al. Self-reported cannabidiol (CBD) use for conditions with proven therapies. JAMA Netw Open. 2020;3(10):e2020977.
Moltke J, Hindocha C. Reasons for cannabidiol use: a cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. J Cannabis Res. 2021;3(1):5. https://doi.org/10.1186/S42238-021-00061-5.
Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, del Giovane C, et al. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78:1791–804. https://doi.org/10.1007/s40265-018-0992-5.
Laux LC, Bebin EM, Checketts D, Chez M, Flamini R, Marsh ED, et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or dravet syndrome: expanded access program results. Epilepsy Res. 2019;154:13–20. https://doi.org/10.1016/J.EPLEPSYRES.2019.03.015.
Cohen NT, Bahar B, Conry JA, Schreiber JM. Variability in serum concentrations and clinical response in artisanal versus pharmaceutical cannabidiol treatment of pediatric pharmacoresistant epilepsy. J Pediatr Pharmacol Ther. 2022;27:558–63. https://doi.org/10.5863/1551-6776-27.6.558.
Silvinato A, Floriano I, Bernardo WM. Use of cannabidiol in the treatment of epilepsy: Lennox-Gastaut syndrome, Dravet syndrome, and tuberous sclerosis complex. Rev Assoc Med Bras. 1992;2022(68):1345–57. https://doi.org/10.1590/1806-9282.2022D689.
Georgieva D, Langley J, Hartkopf K, Hawk L, Margolis A, Struck A, et al. Real-world, long-term evaluation of the tolerability and therapy retention of Epidiolex® (cannabidiol) in patients with refractory epilepsy. Epilepsy Behav. 2023;141:109159.
Leweke FM, Rohleder C, Gerth CW, Hellmich M, Pukrop R, Koethe D. Cannabidiol and amisulpride improve cognition in acute schizophrenia in an explorative, double-blind, active-controlled, randomized clinical trial. Front Pharmacol. 2021;12:614811.
Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225–31. https://doi.org/10.1176/appi.ajp.2017.17030325.
O’Neill A, Wilson R, Blest-Hopley G, Annibale L, Colizzi M, Brammer M, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020. https://doi.org/10.1017/S0033291719003519.
Bhattacharyya S, Wilson R, Appiah-Kusi E, O’Neill A, Brammer M, Perez J, et al. Effect of cannabidiol on medial temporal, midbrain, and Striatal Dysfunction in people at clinical high risk of psychosis: a Randomized Clinical Trial. JAMA Psychiatry. 2018;75:1107–17. https://doi.org/10.1001/jamapsychiatry.2018.2309.
Davies C, Wilson R, Appiah-Kusi E, Blest-Hopley G, Brammer M, Perez J, et al. A single dose of cannabidiol modulates medial temporal and striatal function during fear processing in people at clinical high risk for psychosis. Transl Psychiatry. 2020;10(1):311. https://doi.org/10.1038/S41398-020-0862-2.
Wilson R, Bossong MG, Appiah-Kusi E, Petros N, Brammer M, Perez J, et al. Cannabidiol attenuates insular dysfunction during motivational salience processing in subjects at clinical high risk for psychosis. Transl Psychiatry. 2019;9(1):203. https://doi.org/10.1038/S41398-019-0534-2.
Appiah-Kusi E, Petros N, Wilson R, Colizzi M, Bossong MG, Valmaggia L, et al. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology. 2020;237:1121–30. https://doi.org/10.1007/s00213-019-05442-6.
Bolsoni LM, Crippa JAS, Hallak JEC, Guimaraes FS, Zuardi AW. The anxiolytic effect of cannabidiol depends on the nature of the trauma when patients with post-traumatic stress disorder recall their trigger event. Braz J Psychiatry. 2022;44:298–307. https://doi.org/10.1590/1516-4446-2021-2317.
Bolsoni LM, Crippa JAS, Hallak JEC, Guimarães FS, Zuardi AW. Effects of cannabidiol on symptoms induced by the recall of traumatic events in patients with posttraumatic stress disorder. Psychopharmacology. 2022;239:1499–507. https://doi.org/10.1007/S00213-021-06043-Y.
Crippa JAS, Zuardi AW, Guimarães FS, Campos AC, de Lima Osório F, Loureiro SR, et al. Efficacy and safety of cannabidiol plus standard care vs standard care alone for the treatment of emotional exhaustion and burnout among frontline health care workers during the COVID-19 pandemic: a randomized clinical trial. JAMA Netw Open. 2021;4(8):e2120603.
de Faria SM, de Morais Fabrício D, Tumas V, Castro PC, Ponti MA, Hallak JEC, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a simulated public speaking test in patients with Parkinson’s disease. J Psychopharmacol. 2020;34:189–96. https://doi.org/10.1177/0269881119895536.
de Almeida CMO, Brito MMC, Bosaipo NB, Pimentel A v., Tumas V, Zuardi AW, et al. Cannabidiol for rapid eye movement sleep behavior disorder. Mov Disord. 2021;36:1711–5. https://doi.org/10.1002/MDS.28577.
Chagas MHN, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–92. https://doi.org/10.1177/0269881114550355.
Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FLS, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–30. https://doi.org/10.1177/0269881110379283.
Bergamaschi MM, Queiroz RHC, Chagas MHN, de Oliveira DCG, de Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients. Neuropsychopharmacology. 2011;36:1219–26. https://doi.org/10.1038/npp.2011.6.
Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front Psychol. 2019;10:2466. https://doi.org/10.3389/fpsyg.2019.02466.
Pretzsch CM, Freyberg J, Voinescu B, Lythgoe D, Horder J, Mendez MA, et al. Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology. 2019;44:1398–405. https://doi.org/10.1038/S41386-019-0333-8.
Pretzsch CM, Voinescu B, Mendez MA, Wichers R, Ajram L, Ivin G, et al. The effect of cannabidiol (CBD) on low-frequency activity and functional connectivity in the brain of adults with and without autism spectrum disorder (ASD). J Psychopharmacol. 2019;33:1141–8. https://doi.org/10.1177/0269881119858306.
Efron D, Freeman JL, Cranswick N, Payne JM, Mulraney M, Prakash C, et al. A pilot randomised placebo-controlled trial of cannabidiol to reduce severe behavioural problems in children and adolescents with intellectual disability. Br J Clin Pharmacol. 2021;87:436–46. https://doi.org/10.1111/bcp.14399.
Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21(S1):417S-427S. https://doi.org/10.1002/J.1552-4604.1981.TB02622.X.
Heineman JT, Forster GL, Stephens KL, Cottler PS, Timko MP, DeGeorge BR. A randomized controlled trial of topical cannabidiol for the treatment of thumb basal joint arthritis. J Hand Surg. 2022;47:611–20. https://doi.org/10.1016/j.jhsa.2022.03.002.
Xu DH, Cullen BD, Tang M, Fang Y. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol. 2019;21:390–402. https://doi.org/10.2174/1389201020666191202111534.
Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176:911–22. https://doi.org/10.1176/appi.ajp.2019.18101191.
Morissette F, Mongeau-Pérusse V, Rizkallah E, Thébault P, Lepage S, Brissette S, et al. Exploring cannabidiol effects on inflammatory markers in individuals with cocaine use disorder: a randomized controlled trial. Neuropsychopharmacology. 2021;46:2101–11. https://doi.org/10.1038/S41386-021-01098-Z.
Freeman TP, Hindocha C, Baio G, Shaban NDC, Thomas EM, Astbury D, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive bayesian trial. Lancet Psychiatry. 2020;7:865–74. https://doi.org/10.1016/S2215-0366(20)30290-X.
Hindocha C, Freeman TP, Grabski M, Stroud JB, Crudgington H, Davies AC, et al. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction (Abingdon England). 2018;113:1696. https://doi.org/10.1111/ADD.14243.
Morgan CJA, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38:2433–6. https://doi.org/10.1016/J.ADDBEH.2013.03.011.
Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76:245–50. https://doi.org/10.1007/BF00432554.
Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9 - tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–7. https://doi.org/10.1016/0014-2999(74)90129-0.
Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimarães FS, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41:9–14. https://doi.org/10.1590/1516-4446-2017-0015.
Zuardi AW, Rodrigues NP, Silva AL, Bernardo SA, Hallak JEC, Guimarães FS, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. https://doi.org/10.3389/fphar.2017.00259.
Zuardi AW, Cosme RA, Graeff FG, Guimaraes FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82–8. https://doi.org/10.1177/026988119300700112.
Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology. 2013;226:781–92. https://doi.org/10.1007/s00213-012-2955-y.
de Souza Crippa JA, Zuardi AW, Garrido GEJ, Wichert-Ana L, Guarnieri R, Ferrari L, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417–26. https://doi.org/10.1038/SJ.NPP.1300340.
Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010;13:421–32. https://doi.org/10.1017/S1461145709990617.
Jadoon KA, Tan GD, O’Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight. 2017;2(12):e93760.
Couch DG, Cook H, Ortori C, Barrett D, Lund JN, O’Sullivan SE. Palmitoylethanolamide and cannabidiol prevent inflammation-induced hyperpermeability of the human gut in vitro and in vivo-a randomized, placebo-controlled, double-blind controlled trial. Inflamm Bowel Dis. 2019;25(6):1006–18. https://doi.org/10.1093/ibd/izz017.
Sultan SR, O’Sullivan SE, England TJ. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: a randomised controlled trial. Br J Clin Pharmacol. 2020. https://doi.org/10.1111/bcp.14225.
Vitale RM, Iannotti FA, Amodeo P. The (poly)pharmacology of cannabidiol in neurological and neuropsychiatric disorders: molecular mechanisms and targets. Int J Mol Sci. 2021;22(9):4876. https://doi.org/10.3390/IJMS22094876.
O’Sullivan SE, Stevenson CW, Laviolette SR. Could cannabidiol be a treatment for coronavirus disease-19-related anxiety disorders? Cannabis Cannabinoid Res. 2021;6:7–18. https://doi.org/10.1089/CAN.2020.0102.
Laczkovics C, Kothgassner OD, Felnhofer A, Klier CM. Cannabidiol treatment in an adolescent with multiple substance abuse, social anxiety and depression. Neuropsychiatrie. 2020. https://doi.org/10.1007/s40211-020-00334-0.
Berger M, Li E, Amminger GP. Treatment of social anxiety disorder and attenuated psychotic symptoms with cannabidiol. BMJ Case Reports CP. 2020;13:e235307. https://doi.org/10.1136/BCR-2020-235307.
Crippa JAS, Hallak JEC, Machado-De-Sousa JP, Queiroz RHC, Bergamaschi M, Chagas MHN, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2013;38:162–4. https://doi.org/10.1111/JCPT.12018.
Klier CM, de Gier C, Felnhofer A, Laczkovics C, Amminger PG. A case report of cannabidiol treatment of a Crohn’s disease patient with anxiety disorder. J Clin Psychopharmacol. 2020;40:90–2. https://doi.org/10.1097/JCP.0000000000001152.
Gulbransen G, Xu W, Arroll B. Cannabidiol prescription in clinical practice: an audit on the first 400 patients in New Zealand. BJGP Open. 2020. https://doi.org/10.3399/bjgpopen20x101010. :bjgpopen20X101010.
Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18–041. https://doi.org/10.7812/TPP/18-041.
Berger M, Li E, Rice S, Davey CG, Ratheesh A, Adams S, et al. Cannabidiol for treatment-resistant anxiety disorders in young people: an open-label trial. J Clin Psychiatry. 2022;83(5):21m14130. https://doi.org/10.4088/JCP.21M14130.
Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. https://doi.org/10.1001/archgenpsychiatry.2008.519.
Stanley TB, Ferretti ML, Bonn-Miller MO, Irons JG, Double-Blind A. Randomized, placebo-controlled test of the effects of cannabidiol on experiences of test anxiety among college students. Cannabis Cannabinoid Res. 2022. https://doi.org/10.1089/CAN.2022.0062.
Fabris D, Carvalho MC, Brandão ML, Prado WA, Zuardi AW, Crippa JA, et al. Sex-dependent differences in the anxiolytic-like effect of cannabidiol in the elevated plus-maze. J Psychopharmacol. 2022;36(12):1371–83. https://doi.org/10.1177/02698811221125440.
Bloomfield MAP, Yamamori Y, Hindocha C, Jones APM, Yim JLL, Walker HR, et al. The acute effects of cannabidiol on emotional processing and anxiety: a neurocognitive imaging study. Psychopharmacology. 2022;239:1539–49. https://doi.org/10.1007/S00213-022-06070-3.
Leen-Feldner EW, Bynion TM, Eglit GML, Bonn-Miller MO, Gournay LR, Feldner MT. A double-blind, randomized, placebo-controlled test of the effects of cannabidiol on fear elicited by a 10% carbon dioxide-enriched air breathing challenge. Psychopharmacology. 2022. https://doi.org/10.1007/S00213-022-06258-7.
Hundal H, Lister R, Evans N, Antley A, Englund A, Murray RM, et al. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. J Psychopharmacol. 2018;32:276–82. https://doi.org/10.1177/0269881117737400.
de Meneses-Gaya C, Crippa JA, Hallak JE, Miguel AQ, Laranjeira R, Bressan RA, et al. Cannabidiol for the treatment of crack-cocaine craving: an exploratory double-blind study. Braz J Psychiatry. 2021;43:467–76. https://doi.org/10.1590/1516-4446-2020-1416.
Kwee CM, Baas JM, van der Flier FE, Groenink L, Duits P, Eikelenboom M, et al. Cannabidiol enhancement of exposure therapy in treatment refractory patients with social anxiety disorder and panic disorder with agoraphobia: a randomised controlled trial. Eur Neuropsychopharmacol. 2022;59:58–67. https://doi.org/10.1016/J.EURONEURO.2022.04.003.
García-Gutiérrez MS, Navarrete F, Gasparyan A, Austrich-Olivares A, Sala F, Manzanares J, Cannabidiol. A potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules. 2020;10:1–34. https://doi.org/10.3390/biom10111575.
Elsaid S, Kloiber S, le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog Mol Biol Transl Sci. 2019;167:25–75. https://doi.org/10.1016/bs.pmbts.2019.06.005.
Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9:204512531988191. https://doi.org/10.1177/2045125319881916.
Zuardi A, Morais SL, Guimarães F, Mechoulam R. Antipsychotic effect of cannabidiol. Undefined 1995.
Zuardi AW, Hallak JEC, Dursun SM, Morais SL, Sanches RF, Musty RE, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20:683–6. https://doi.org/10.1177/0269881106060967.
Zuardi AW, Crippa JAS, Dursun SM, Morais SL, Vilela JAA, Sanches RF, et al. Cannabidiol was ineffective for manic episode of bipolar affective disorder. J Psychopharmacol. 2010;24:135–7. https://doi.org/10.1177/0269881108096521.
Hallak JEC, Machado-de-Sousa JP, Crippa JAS, Sanches RF, Trzesniak C, Chaves C, et al. Performance of schizophrenic patients in the stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Braz J Psychiatry. 2010;32:56–61. https://doi.org/10.1590/S1516-44462010000100011.
O’Neill A, Wilson R, Blest-Hopley G, Annibale L, Colizzi M, Brammer M, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2021;51:596–606. https://doi.org/10.1017/S0033291719003519.
Souza JDR, Pacheco JC, Rossi GN, de-Paulo BO, Zuardi AW, Guimarães FS, et al. Adverse Effects of oral cannabidiol: an updated systematic review of randomized controlled trials (2020–2022). Pharmaceutics. 2022;14:2598. https://doi.org/10.3390/PHARMACEUTICS14122598.
Vaseghi S, Arjmandi-Rad S, Nasehi M, Zarrindast MR. Cannabinoids and sleep-wake cycle: the potential role of serotonin. Behav Brain Res. 2021;412:113440. https://doi.org/10.1016/J.BBR.2021.113440.
Lavender I, McGregor IS, Suraev A, Grunstein RR, Hoyos CM. Cannabinoids, insomnia, and other sleep disorders. Chest. 2022;162:452–65. https://doi.org/10.1016/J.CHEST.2022.04.151.
Klotz KA, Grob D, Schönberger J, Nakamura L, Metternich B, Schulze-Bonhage A, et al. Effect of cannabidiol on interictal epileptiform activity and sleep architecture in children with intractable epilepsy: a prospective open-label study. CNS Drugs. 2021;35:1207–15. https://doi.org/10.1007/S40263-021-00867-0.
Anderson CL, Evans V, Gorham L, Liu Z, Johnson CR, Carney PR. Seizure frequency, quality of life, behavior, cognition, and sleep in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsy Behav. 2021;124:108325.
Shannon S, Opila-Lehman J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm J. 2016;20:16–005. https://doi.org/10.7812/TPP/16-005.
Shannon S, Opila-Lehman J. Cannabidiol Oil for decreasing addictive use of marijuana: a case report. Integr Med (Encinitas). 2015;14:31–5.
Chagas MHN, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther. 2014;39:564–6. https://doi.org/10.1111/JCPT.12179.
Linares IMP, Guimaraes FS, Eckeli A, Crippa ACS, Zuardi AW, Souza JDS, et al. No acute effects of cannabidiol on the sleep-wake cycle of healthy subjects: a randomized, double-blind, placebo-controlled, crossover study. Front Pharmacol. 2018;9:315. https://doi.org/10.3389/FPHAR.2018.00315.
Mlost J, Bryk M, Starowicz K. Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci. 2020;21:1–22. https://doi.org/10.3390/ijms21228870.
Neelakantan H, Tallarida RJ, Reichenbach ZW, Tuma RF, Ward SJ, Walker EA. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav Pharmacol. 2015;26:304–14. https://doi.org/10.1097/FBP.0000000000000119.
Jesus CHA, Ferreira MV, Gasparin AT, Rosa ES, Genaro K, de Souza Crippa JA. Cannabidiol enhances the antinociceptive effects of morphine and attenuates opioid-induced tolerance in the chronic constriction injury model. Behav Brain Res. 2022;435:114076.
Nielsen SW, Hasselsteen SD, Dominiak HSH, Labudovic D, Reiter L, Dalton SO, et al. Oral cannabidiol for prevention of acute and transient chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2022;30:9441–51. https://doi.org/10.1007/S00520-022-07312-Y.
Arout CA, Haney M, Herrmann ES, Bedi G, Cooper ZD. A placebo-controlled investigation of the analgesic effects, abuse liability, safety and tolerability of a range of oral cannabidiol doses in healthy humans. Br J Clin Pharmacol. 2022;88:347–55. https://doi.org/10.1111/bcp.14973.
Schneider T, Zurbriggen L, Dieterle M, Mauermann E, Frei P, Mercer-Chalmers-Bender K, et al. Pain response to cannabidiol in induced acute nociceptive pain, allodynia, and hyperalgesia by using a model mimicking acute pain in healthy adults in a randomized trial (CANAB I). Pain. 2022;163:E62–71. https://doi.org/10.1097/j.pain.0000000000002310.
Cochrane-Snyman KC, Cruz C, Morales J, Coles M. The effects of cannabidiol oil on noninvasive measures of muscle damage in men. Med Sci Sports Exerc. 2021;53:1460–72. https://doi.org/10.1249/MSS.0000000000002606.
Bebee B, Taylor DM, Bourke E, Pollack K, Foster L, Ching M, et al. The CANBACK trial: a randomised, controlled clinical trial of oral cannabidiol for people presenting to the emergency department with acute low back pain. Med J Aust. 2021;214:370–5. https://doi.org/10.5694/mja2.51014.
Vela J, Dreyer L, Petersen KK, Arendt-Nielsen L, Duch KS, Kristensen S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: a randomized, double-blind, placebo-controlled trial. Pain. 2022;163:1206–14. https://doi.org/10.1097/j.pain.0000000000002466.
Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abílio VC. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9:482. https://doi.org/10.3389/FPHAR.2018.00482.
Consroe P, Sandyk R, Snider SR. Open label evaluation of cannabidiol in dystonic movement disorders. Int J Neurosci. 1986;30:277–82. https://doi.org/10.3109/00207458608985678.
Zuardi AW, Crippa JAS, Hallak JEC, Pinto JP, Chagas MHN, Rodrigues GGR, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol. 2009;23:979–83. https://doi.org/10.1177/0269881108096519.
de Almeida CMO, Brito MMC, Bosaipo NB, Pimentel AV, Sobreira-Neto MA, Tumas V, et al. The effect of cannabidiol for restless legs syndrome/Willis-Ekbom disease in Parkinson’s disease patients with REM sleep behavior disorder: a post hoc exploratory analysis of phase 2/3 clinical trial. Cannabis Cannabinoid Res. 2022. https://doi.org/10.1089/CAN.2021.0158.
Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, et al. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav. 1991;40:701–8. https://doi.org/10.1016/0091-3057(91)90386-G.
Gasparyan A, Navarrete F, Manzanares J. Cannabidiol and sertraline regulate behavioral and brain gene expression alterations in an animal model of PTSD. Front Pharmacol. 2021;12:694510.
Elms L, Shannon S, Hughes S, Lewis N. Cannabidiol in the treatment of post-traumatic stress disorder: a case series. J Altern Complement Med. 2019;25:392–7. https://doi.org/10.1089/acm.2018.0437.
Shannon S, Opila-Lehman J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm J. 2016;20:108–11. https://doi.org/10.7812/TPP/16-005.
Han X, Song X, Song D, Xie G, Guo H, Wu N, et al. Comparison between cannabidiol and sertraline for the modulation of post-traumatic stress disorder-like behaviors and fear memory in mice. Psychopharmacology. 2022;239:1605–20. https://doi.org/10.1007/S00213-022-06132-6.
Bolsoni LM, da Silva TDA, Quintana SM, de Castro M, Crippa JA, Zuardi AW. Changes in cortisol awakening response before and after development of posttraumatic stress disorder, which cannot be avoided with use of cannabidiol: a case report. Perm J. 2019;23:18.300. https://doi.org/10.7812/TPP/18.300.
Souza JDS, Zuardi AW, Guimarães FS, Osório F, de Loureiro L, Campos SR. Maintained anxiolytic effects of cannabidiol after treatment discontinuation in healthcare workers during the COVID-19 pandemic. Front Pharmacol. 2022;13:856846.
Paulus V, Billieux J, Benyamina A, Karila L. Cannabidiol in the context of substance use disorder treatment: a systematic review. Addict Behav. 2022;132:107360.
Cleirec G, Desmier E, Lacatus C, Lesgourgues S, Braun A, Peloso C, et al. Efficiency of inhaled cannabidiol in cannabis use disorder: the pilot study Cannavap. Front Psychiatry. 2022;13:899221.
Mongeau-Pérusse V, Brissette S, Bruneau J, Conrod P, Dubreucq S, Gazil G, et al. Cannabidiol as a treatment for craving and relapse in individuals with cocaine use disorder: a randomized placebo-controlled trial. Addiction (Abingdon England). 2021;116:2431–42. https://doi.org/10.1111/ADD.15417.
Martínez V, Iriondo De-Hond A, Borrelli F, Capasso R, del Castillo MD, Abalo R. Cannabidiol and other non-psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: useful nutraceuticals? Int J Mol Sci. 2020;21(9):3067. https://doi.org/10.3390/IJMS21093067.
Couch DG, Maudslay H, Doleman B, Lund JN, O’Sullivan SE. The use of cannabinoids in colitis: a systematic review and Meta-analysis. Inflamm Bowel Dis. 2018;24:680–97. https://doi.org/10.1093/IBD/IZY014.
Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci. 2017;62:1615–20. https://doi.org/10.1007/s10620-017-4540-z.
Atieh J, Maselli D, Breen-Lyles M, Torres M, Katzka D, Ryks M, et al. Cannabidiol for functional dyspepsia with normal gastric emptying: a randomized controlled trial. Am J Gastroenterol. 2022;117:1296–304. https://doi.org/10.14309/AJG.0000000000001805.
van Orten-Luiten A-CB, de Roos NM, Majait S, Witteman BJM, Witkamp RF. Effects of cannabidiol chewing gum on perceived pain and well-being of irritable bowel syndrome patients: a placebo-controlled crossover exploratory intervention study with Symptom-Driven Dosing. Cannabis Cannabinoid Res. 2022;7:436–44. https://doi.org/10.1089/CAN.2020.0087.
Pedrazzi JFC, Ferreira FR, Silva-Amaral D, Lima DA, Hallak JEC, Zuardi AW, et al. Cannabidiol for the treatment of autism spectrum disorder: hope or hype? Psychopharmacology. 2022;239:2713–34. https://doi.org/10.1007/S00213-022-06196-4.
Poleg S, Golubchik P, Offen D, Weizman A. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:90–6. https://doi.org/10.1016/J.PNPBP.2018.08.030.
Stanley CP, Hind WH, O’Sullivan SE. Is the cardiovascular system a therapeutic target for cannabidiol? Br J Clin Pharmacol. 2013;75:313–22. https://doi.org/10.1111/j.1365-2125.2012.04351.x.
Kicman A, Toczek M. The Effects of Cannabidiol, a non-intoxicating compound of cannabis, on the cardiovascular system in health and disease. Int J Mol Sci. 2020;21:1–45. https://doi.org/10.3390/IJMS21186740.
Sultan SR, Millar SA, England TJ, O’Sullivan SE. A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front Pharmacol. 2017;8:81. https://doi.org/10.3389/fphar.2017.00081.
Kumric M, Bozic J, Dujic G, Vrdoljak J, Dujic Z. Chronic effects of effective oral cannabidiol delivery on 24-h ambulatory blood pressure and vascular outcomes in treated and untreated hypertension (HYPER-H21-4): study protocol for a randomized, placebo-controlled, and crossover study. J Pers Med. 2022;12(7):1037. https://doi.org/10.3390/JPM12071037.
Pagano S, Coniglio M, Valenti C, Federici MI, Lombardo G, Cianetti S, et al. Biological effects of cannabidiol on normal human healthy cell populations: systematic review of the literature. Biomed Pharmacother. 2020;132:110728.
Dall’Stella PB, Docema MFL, Maldaun MVC, Feher O, Lancellotti CLP. Case report: clinical outcome and image response of two patients with secondary high-grade glioma treated with chemoradiation, PCV, and cannabidiol. Front Oncol. 2019;9:643. https://doi.org/10.3389/FONC.2018.00643/BIBTEX.
LIKAR R, KOESTENBERGER M, STUTSCHNIG M. Cannabidiol Μay prolong survival in patients with Glioblastoma Multiforme. Cancer Diagn Progn. 2021;1:77. https://doi.org/10.21873/CDP.10011.
Kenyon J, Liu W, Dalgleish A. Report of Objective clinical responses of cancer patients to pharmaceutical-grade synthetic cannabidiol. Anticancer Res. 2018;38:5831–5. https://doi.org/10.21873/ANTICANRES.12924.
Weiss L, Zeira M, Reich S, Slavin S, Raz I, Mechoulam R, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54:244–9. https://doi.org/10.1016/J.NEUROPHARM.2007.06.029.
Lehmann C, Fisher NB, Tugwell B, Szczesniak A, Kelly M, Zhou J. Experimental cannabidiol treatment reduces early pancreatic inflammation in type 1 diabetes. Clin Hemorheol Microcirc. 2016;64:655–62. https://doi.org/10.3233/CH-168021.
Zorzenon MRT, Santiago AN, Mori MA, Piovan S, Jansen CA, Perina Padilha ME, et al. Cannabidiol improves metabolic dysfunction in middle-aged diabetic rats submitted to a chronic cerebral hypoperfusion. Chem Biol Interact. 2019;312:108819.
Jesus CHA, Redivo DDB, Gasparin AT, Sotomaior BB, de Carvalho MC, Genaro K, et al. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res. 2019;1715:156–64. https://doi.org/10.1016/J.BRAINRES.2019.03.014.
Santiago AN, Mori MA, Guimarães FS, Milani H, Weffort de Oliveira RM. Effects of cannabidiol on diabetes outcomes and chronic cerebral hypoperfusion comorbidities in middle-aged rats. Neurotox Res. 2019;35:463–74. https://doi.org/10.1007/S12640-018-9972-5.
Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39:1777–86. https://doi.org/10.2337/dc16-0650.
Yeshurun M, Shpilberg O, Herscovici C, Shargian L, Dreyer J, Peck A, et al. Cannabidiol for the prevention of graft-versus-host-disease after allogeneic hematopoietic cell transplantation: results of a phase II study. Biol Blood Marrow Transplant. 2015;21:1770–5. https://doi.org/10.1016/J.BBMT.2015.05.018.
Wang MTM, Danesh-Meyer H. v. Cannabinoids and the eye. Surv Ophthalmol. 2021;66:327–45. https://doi.org/10.1016/J.SURVOPHTHAL.2020.07.002.
Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma. 2006;15:349–53. https://doi.org/10.1097/01.IJG.0000212260.04488.60.
Tayo B, Taylor L, Sahebkar F, Morrison GA, Phase I, Open-Label P-G. Single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet. 2019. https://doi.org/10.1007/s40262-019-00841-6.
Acknowledgements
We would like to thank Samantha Elmhurst at Living Art for the preparation of Fig. 1.
Funding
This work was funded by Fertin Pharma and Vectura Pharma.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conceptions (JH and SOS), analysis (all), and drafting the work (all), and all have approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Saoirse Elizabeth O’Sullivan is a paid consultant to Vectura Fertin Pharma, Switzerland, and a paid employee of Artelo Biosceince, USA. Sanne Skov Jensen, Gitte Nykjær Nikolajsen and Heidi Ziegler Bruun are paid employees of Fertin Pharma, Denmark. Rhenu Bhuller and Julia Hoeng are paid employees of Vectura Fertin Pharma, Switzerland.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Sullivan, S.E., Jensen, S.S., Nikolajsen, G.N. et al. The therapeutic potential of purified cannabidiol. J Cannabis Res 5, 21 (2023). https://doi.org/10.1186/s42238-023-00186-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42238-023-00186-9