Abstract
Background
The treatment of diverse diseases using plant-derived products is actively encouraged. In the past few years, cannabidiol (CBD) has emerged as a potent cannabis-derived drug capable of managing various debilitating neurological infections, diseases, and their associated complications. CBD has demonstrated anti-inflammatory and curative effects in neuropathological conditions, and it exhibits therapeutic, apoptotic, anxiolytic, and neuroprotective properties. However, more information on the reactions and ability of CBD to alleviate brain-related disorders and the neuroinflammation that accompanies them is needed.
Main body
This narrative review deliberates on the therapeutic and remedial prospects of CBD with an emphasis on neurological and neuropsychiatric disorders. An extensive literature search followed several scoping searches on available online databases such as PubMed, Web of Science, and Scopus with the main keywords: CBD, pro-inflammatory cytokines, and cannabinoids. After a purposive screening of the retrieved papers, 170 (41%) of the articles (published in English) aligned with the objective of this study and retained for inclusion.
Conclusion
CBD is an antagonist against pro-inflammatory cytokines and the cytokine storm associated with neurological infections/disorders. CBD regulates adenosine/oxidative stress and aids the downregulation of TNF-α, restoration of BDNF mRNA expression, and recovery of serotonin levels. Thus, CBD is involved in immune suppression and anti-inflammation. Understanding the metabolites associated with response to CBD is imperative to understand the phenotype. We propose that metabolomics will be the next scientific frontier that will reveal novel information on CBD’s therapeutic tendencies in neurological/neuropsychiatric disorders.
Similar content being viewed by others
Introduction
Plant-derived products, either alone or in combination with allopathic medicines, are increasingly being used to prevent and manage a wide range of ailments, including brain-related disorders. In recent years, cannabidiol (CBD), one of several cannabinoids obtained from Cannabis sativa, has been found to be of great medical importance. The alleged effectiveness of this chemical, along with other cannabinoids like delta-9-tetrahydrocannabinol (THC), in the treatment of several medical conditions has led to its rise in popularity. Moreover, CBD has been reported to be an antidote to certain disorders such as Parkinson’s disease, and seizures/epilepsy without exerting psychotropic effects, unlike THC, which is associated with psychoactive consequences (Fernández‐Ruiz et al. 2013; Porter et al. 2021).
Since the observations of Carlini et al. (1973) on the anticonvulsant effects of CBD, there has been a subsequent rise in the study of its properties, effects, and prospects, resulting in the growing appreciation of its therapeutic potential, as well as its anti-inflammatory and anti-oxidative properties (Aziz et al. 2022b; Cenova et al. 2022). According to Jean-Gilles et al. (2010), CBD has the ability to reduce the levels of pro-inflammatory cytokines, induce T-cell apoptosis, inhibit T-cell proliferation, and promote the migration and adhesion of immune cells. CBD also demonstrates the anti-oxidative potential of effecting redox balance through modification of the level and activity of oxidants and antioxidants, and captures free radicals or transforms them into inactive or less active forms (Atalay et al. 2019).
Moreover, the neuroprotective potential of CBD in various neurological disorders, such as ischaemic brain damage, has also been indicated (England and O'Sullivan 2021; Martínez-Orgado et al. 2021). CBD’s ability to inhibit inducible nitric oxide synthase (iNOS) protein expression and activation of nuclear factor kappa B (NF-KB) in the brain shows its neuroprotective tendencies (Bhunia et al. 2022; Ekiner et al. 2022). While extensive studies on the systemic therapeutic effects of CBD in the body have been done (Britch et al. 2021; Peng et al. 2022; White 2019), the potential of this phyto-cannabinoid on neurological/neuropsychiatric disorders requires further exploration regarding its effect on neuroinflammation that occurs as a consequence of these disorders. In addition to reviewing the therapeutic properties of CBD, and evaluating its potential as an anti-inflammatory agent, particularly in neurological/neuropsychiatric disorders, this article primarily identifies and states mechanisms through which CBD can help minimize inflammation caused by cytokine release. It also appraises the prospects of this phyto-cannabinoid in the management of brain-related disorders and discusses how the metabolomics approach (if adopted) can further CBD research.
Cannabidiol
Cannabidiol (CBD), known scientifically as 2-[(1R,6R)-3-methyl-6-(prop-1-en-2-yl)cyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol, has the chemical formula of C21H30O2 and molecular weight of 314.5 g/mol. It is a terpenophenol and bicyclic compound consisting of a phenolic and cyclohexene ring, illustrated as A and B respectively in Fig. 1a. The pentyl chain and the location of the hydroxyl group in the phenolic ring (A), as well as the methyl group of the cyclohexene ring (B), significantly influence the chemical activity of the compound. For example, the hydroxyl groups enable it to bind to glutamine, threonine, and other amino acids through a hydrogen bond (ElSohly and Slade 2005; Jones et al. 1977; Mechoulam and Hanuš, 2002).
As an approved prescription drug for seizure disorders (Meissner and Cascella 2022), CBD is available in various forms such as oils and tinctures, capsules, and edibles (Fig. 1b). Clinically, it is often recommended for use in combination with antiepileptic therapy for the reduction of convulsive seizures in Dravet syndrome and treatment-resistant drop seizures in patients with Lennox–Gastaut syndrome (Devinsky et al. 2019; Privitera et al. 2021). While attention is commonly drawn to the natural product extracted from cannabis plants, another derivative of CBD, that is artificially/chemically synthesized – from biological processes using modified yeast or other ingredients such as limonene – is also used for various purposes ranging from cosmetic to medicinal (Abame et al. 2021; Stahl and Vasudevan 2020).
Method of literature search
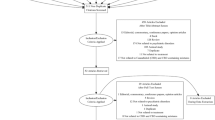
This narrative review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and reporting criteria (PRISMA). Following various scoping searches, a comprehensive literature search was conducted using available online scientific databases including Scopus, Science Direct, PubMed, Web of Science and Google Scholar. No time limit was used, and the keywords were: cannabidiol, epidiolex, cannabinoids, anti-inflammatory effects, neurological infections, neurological disorders, therapeutic effects of CBD, CBD effects on cytokines, and pro-inflammatory cytokines. Scientific papers, and commentaries written in English were also included. The initial search retrieved 414 articles, but after purposive screening and selection, only 170 of these (41%) which aligned with the objectives of this study were retained for inclusion. Respectively, Table 1 and Fig. 2 shows the screening and inclusion criteria (considered keywords) for this review.
Eligibility criteria
Studies published in English language that were eligible and included in this review are those which considered the anti-inflammatory and therapeutic potentials of CBD, pharmacological/pharmacokinetic effects of CBD, effect of CBD on neurological disorders in human or animal model (s), or studies that assessed the impact of CBD on regulation of inflammation and cytokine release (Fig. 2). Articles that passed the initial screening were given full text review. Studies that were excluded were those that did not align with the aim of this study, did not deal with the effect of CBD, used standardised cannabis extracts containing both THC and CBD, or those with no appropriate controls to evaluate the influence of CBD alone. Figure 3 illustrates the article selection process.
Data extraction
After the screening procedure, research articles that met the criteria were again reviewed and vital information was extracted including author(s), publication year, journals where the articles were published, experiments/aim of the study, target population (human or animal model), information pertaining to treatment used, interventions and dosage, route of administration and outcomes of the various included studies (Tables 2 and 3).
This study addresses the pharmacology, modes of action, absorption, metabolism, tolerance, and therapeutic qualities of cannabidiol (CBD). In addition, this phytocannabinoid's prospects and potentials in neurological disorders were examined, and the potential of CBD in treating neurological disorders was evaluated.
Pharmacology and mechanisms of action of CBD
Being a drug with many targets, CBD interacts with several signalling systems and modulates the γ-aminobutyric acid type A receptors, and 5-hydroxy tryptophan 1A receptors (5-HT1a), which are involved in neurotransmission and neuromodulation, respectively (Ghit et al. 2021; Hilaire et al. 2010; Russo et al. 2005). However, it has been observed that CBD inhibits endocannabinoid signalling (Straiker et al. 2018). The endocannabinoid system regulates many physiological activities in the human body, and it involves the endogenous cannabinoids (endocannabinoids), cannabinoid receptors, and enzymes as its main components (Lu and Mackie 2016). The endocannabinoids have been described as molecules produced by the body which are similar to cannabinoids. The two main endocannabinoids identified so far are anandamide, also known as N-arachidonoylethanolamine (AEA), and 2-arachidonoyl glycerol (2-AG), which are produced as needed and help to keep internal functions running smoothly (Rodríguez de Fonseca et al. 2005). The CB1 receptors (found mostly in the central nervous system) and the CB2 receptors (found mostly in the immune cells- peripheral nervous system) receives the endocannabinoids and signal to the endocannabinoid system to act while the fatty acid amide hydrolase (FAAH) and monoacylglycerol acid lipase (MGAL) breaks down the endocannabinoids after carrying out their function (the FAAH breaks down AEA while the MGAL breaks down 2-AG) (Basavarajappa 2007).
CBD acts as an antagonist of cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2). CB1 receptors are highly expressed in the hippocampus and modulate seizure activity, whereas CB2 receptors are localized in immune cells. This implies that CBD modulates neurotransmission, immune/inflammatory pathways, and other peripheral functions; thereby protecting against cellular damage that occurs during immune responses (Peyravian et al. 2020).
It has been established that CBD acts as a negative allosteric modulator of the CB1 receptor, and this is achieved by modifying the activity of a receptor on a distinct site from the antagonist binding site/agonists (Laprairie et al. 2015). It is important to note that allosteric modulators of CB1 receptors are capable of treating disorders of the peripheral and central nervous system while preventing the negative effects linked with antagonism of these receptors (Laprairie et al. 2015). At higher concentrations, CBD acts as an inverse agonist of G protein-coupled receptors (GPR, GPR6, GPR12, and 5-HT1a), which are phylogenetically related to CB receptors, and are novel therapeutic targets for CBD (Laun et al. 2019). CBD also binds to transient receptor potential vanilloid (TRPV1), impeding synaptosomal uptake of noradrenaline, serotonin, and dopamine (Bisogno et al. 2001; De Petrocellis et al. 2011), blocks low-voltage-activated (T-type) Ca2+ channels, acts on mitochondria Ca2+ stores and constrains the activity of FAAH and it also stimulates the action of the inhibitory glycine receptor (Massi et al. 2008).
Absorption, metabolism, and tolerability of CBD
Through inhalation, CBD is rapidly absorbed with a higher bioavailability by an average of about 35% than oral bioavailability – reported to be low (~ 6% in humans) (Davidson 2022; Lucas et al. 2018). Thus, when ingested, CBD has a lower absorption rate than when inhaled, due to first-pass hepatic metabolism (Della Rocca et al. 2022). In addition to the frequently used oral and intranasal routes, CBD can also be administered through the sublingual and rectal routes. However, the potency of these routes still needs to be established as few studies and have described the pharmacokinetics of CBD administered rectally.
When administered transdermally, significant accumulation of CBD was observed in the skin as well as in the underlying muscle; steady-state levels were reached at about 24 h and lasted for 72 h (Lodzki et al. 2003). In their study, Hosseini et al. (2021) observed that administration of CBD as a sublingual wafer (50 mg CBD) to healthy human volunteers resulted in maximum concentrations of the drug after 4 h with an estimated terminal elimination half-life of 6 h. More studies are still required on the delivery of CBD through injections; however, Fu et al. (2022) found that intramuscular injection of CBD nanocrystals resulted in a 2.0- to 2.2-fold greater concentration of CBD in fasted male Sprague–Dawley rats within 24 h, compared to oral administration.
With a biological half-life of about 18–32 h, up to 1500 mg of CBD per day can be well tolerated in the body without adverse effects, especially on the brain (Machado Bergamaschi et al. 2011; Welty et al. 2014). Through the renal and biliary systems, CBD is metabolized greatly in the liver by the isoenzymes UGT1A7, UGT1A9, UGT2B7 and cytochrome P450 enzymes (CYP2B6, CYP2C19, CYP2D6, CYP2J2, and CYP3A4), thus forming several metabolites such as the 6α- and 6β-hydroxy isomers and 7-hydroxy cannabidiol (Chayasirisobhon 2020; Jiang et al. 2011; Welty et al. 2014).
Psychotic adverse effects of CBD in humans have not been reported as it is noted to be well tolerated (Machado Bergamaschi et al. 2011). In a study to evaluate the pharmacokinetics and safety of CBD oral solution in paediatric patients with treatment-resistant epilepsy – defined as recurrent seizures despite adequate trials of three or more antiepileptic drugs and one or more prior adequate treatment courses with two or more antiepileptic drugs in combination, Wheless et al. (2019) confirmed the tolerability of CBD, as they observed how CBD was well metabolized in more than sixty human patients (age 1–17) included in the study, with few adverse effects such as somnolence, diarrhoea, and anaemia.
When used as an adjunctive treatment with anti-seizure medications, as evidenced in the study of Devinsky et al. (2018), CBD reduced the frequency of seizures in four epilepsy etiologies (Doose syndrome, Aicardi, Dup 5q and CDKL 5 deficiency disorder), with moderate side effects such as diarrhoea, fatigue, and somnolence. Similarly, the study of Kochen et al. (2023) confirms the safety and effectiveness of cannabidiol as an adjuvant in adult patients with drug-resistant focal epilepsy with adverse effects including diarrhoea, somnolence, and decreased appetite (Kochen et al. 2023). It is therefore important to emphasize that despite the effectiveness and safety of CBD, minor side effects such as those stated may occur.
Therapeutic properties of CBD
The application of CBD, singularly and in combination with other medicines in the management of various health disorders, has shown beneficial effects including antibacterial, anti-inflammatory, anti-oxidative, neuroprotective, proapoptotic, anti-proliferative, anti-migratory, and anti-invasive capabilities (Aziz et al. 2022a). That is, CBD can act against specific pathogens, block pro-inflammatory cytokines, capture and convert free radicals into passive forms, safeguard against specific neurological conditions, and prevent the invasion and migration of cancer cells (Aziz et al. 2022a; Cabrera et al. 2021).
In neuroinflammation, activated microglia can migrate toward a lesion (affected neuron) and secrete inflammatory mediators, neurotrophic factors, and reactive oxygen species (ROS) which can exert beneficial or detrimental effects on the surrounding cells (Carbonell et al. 2005; Stence et al. 2001). The activation of microglia has been linked to increased inflammatory response and could result in progressive neuron damage. Hence, persistent and overactivation of microglia has been linked to multiple neurodegenerative diseases (Kim and Joh 2006).
However, CBD has been reported to be a good modulator of microglia cell migration in inflammation. According to Martín-Moreno et al. (2011) who studied and compared the effects of CBD with those of other cannabinoids on microglial cells in Alzheimer’s disease, CBD was able to regulate microglial cell function in vitro while it also caused some beneficial effects in an in vivo model of Alzheimer’s disease. Victor et al. (2022) examined the effect of CBD on microglial inflammation activation and neurogenic response in seizures and in the absence of seizures it was reported that CBD reduced microglial migration and accumulation to the hippocampus.
Table 2 lists various studies performed on human cells, bacteria and animal models that have revealed some of the therapeutic characteristics of CBD. Table 3 describes human clinical trials and studies that were carried out on animal models to assess the potential of CBD for managing various health issues, including brain-related disorders.
Prospects of CBD in neurological disorders
In neurological disorders, an immune response-based defense is generated, which induces systemic inflammation by the continuous production of pro-inflammatory cytokines and granulocyte–macrophage colony-stimulating factor, thereby resulting in changes in cells/tissue and the possibility of organ damage (Misri et al. 2022; Seltzer et al. 2020). Signalling on the immune system involves various pathways that achieve the release of chemokines and cytokines through mitogen-activated protein kinase (MAPK), Janus kinase-signal transducer and activator of transcription (JAK-STAT), and NF-KB (Hu et al. 2021).
Owing to its action as an inverse agonist of the CB2 receptor (Thomas et al. 2007), CBD can help minimize inflammation caused by cytokine release through the following mechanisms:
-
Reduction of oxidative stress
-
Regulation of the cerebral adenosine
-
Downregulation of tumour necrosis factor (TNF-α) receptor
-
Restoration of brain-derived neurotrophic factor (BDNF)
-
Restoration of 5-hydroxytryptamine (5-HT) levels in the brain.
These five mechanisms are discussed below; Fig. 4 summarises CBD’s remedial activity in brain-related disorders in relation to these mechanisms.
Reduction of oxidative stress and inflammation
In order to mitigate the effect of pathogens and start tissue repair, excessive production of ROS by the immune system may occur, thus leading to oxidative stress (Juan et al. 2021). In brain-related disorders, the cell and tissue damage, inflammation, and decreased performance in cognitive function which results from increased ROS production and oxidative stress, leads to brain impairment over time, by initiating cell death and disrupting the blood–brain barrier (Salimi and Klein 2019).
However, CBD can activate the adenosine A2A receptors (A2A), which are distributed in the brain-striatum, nucleus accumbens, and olfactory tubercule, that could lead to anti-inflammatory activity and reduce oxidative stress (Hao et al. 2015; Magen et al. 2009; Vallée et al. 2017). A2A takes part in several pathological reactions, exhibits anti-inflammatory characteristics, and modulates inflammatory processes, thus the activation of the adenosine receptor is a major mechanism of immunosuppression in inflammation.
In the in vivo studies of Hao et al. (2015) in which the effect of CBD was examined in a mouse model of Doxorubicin-induced cardiomyopathy and that of Vallee et al. (Vallée et al. 2017) who examined the interactions of CBD and peroxisome proliferator activated receptor γ (PPARℽ) on oxidative stress and neuroinflammation, it was found that CBD is capable of reducing oxidative stress by decreasing mitochondrial dysregulation and reducing the expression of ROS-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase isoforms. This indicates that the reduction of mitochondrial dysfunction (which causes detrimental effects on the cells and plays a major role in the severity of several pathological conditions such as neurogenerative diseases) and the decrease in the expression of NADPH results in modulation of ROS production thereby regulating and reducing inflammation.
Vallée et al. (2017) also emphasized the suppression of pro-inflammatory cytokines by the activation of peroxisome proliferator-activated receptors which regulates inflammation. In the in vivo study of Pan et al. 2009, that was targeted at investigating the effects of CBD in a mouse model of Cisplatin-induced neuropathy (in which Cisplatin caused an increase in the generation of ROS, expression of NOX4 and NOX1, apoptosis, formation of nitrotyrosine, tumour necrosis factor α and interleukin-1 β in the kidneys of mice and impaired renal function), it was observed that CBD caused a decrease in oxidative stress and inflammation, thus resulting in the attenuation of Cisplatin-induced nephrotoxicity and improved renal function.
Regulation of the cerebral adenosine system
Adenosine is found in the extracellular fluid of most bodily tissues, including the brain, and reacts to pathogen invasion, stress, and damage. It occurs in normal cells in concentrations ranging from 1 to 10 mmol/L (Zimmerman et al. 2011); however, these concentrations rise quickly in response to cellular damage, and an excessive/chronic overproduction of adenosine disrupts the adenosine system, resulting in immune imbalances and immunosuppression. At high concentrations, adenosine becomes hazardous and toxic to the immune system, resulting in dysfunction and autoimmune disorders which adversely affect the brain (Borea et al. 2017).
Adequate concentrations of adenosine in the brain have significant effects on its health and development, and a person’s mood; hence, an adenosine imbalance may lead to detrimental effects on brain tissues (Figler et al. 2011; Zhang et al. 2011; Zhang and Xia 2012). According to Borea et al. (2017), as well as Feoktistov and Biaggioni (2011), excess production of adenosine has been associated with certain neurodegenerative disorders such as Parkinson’s disease. This is because excessive adenosine generation causes overexpression of the A2B receptors, thereby increasing inflammation because A2B most often plays an opposite role to that of A2A. In high concentrations, adenosine initiates the production of inflammatory cytokines such as transforming growth factor beta (TGFβ), tumour necrosis factor alpha (TNF-α), and interferon-gamma (IFN-γ) (Sauer et al. 2017).
CBD is able to regulate the cerebral adenosine system by impeding the uptake of adenosine through obstruction of the equilibrative nucleoside transporter ENT1 (Pandolfo et al. 2011). ENT1 is linked with the synaptic uptake of adenosine; thus, inhibition of ENT1 causes an increase in extracellular adenosine, thereby resulting in a decrease in neuronal hyperexcitability and neurotransmission (Pandolfo et al. 2011). The study by Carrier et al. (2006) on the effect of CBD on the ENT1 revealed that CBD reduced the uptake of adenosine in microglial cells and RAW 264.7 macrophages in murine models. Carrier et al. showed that CBD enhances adenosine signalling by inhibiting the uptake of adenosine and providing a non-cannabinoid receptor mechanism through which it can reduce inflammation.
However, Carrier et al. (2006) reported that while binding to the ENT transporter, CBD can also activate the A2A receptor. Moreover, blocking the uptake of adenosine by the microglial cells may impact the neurons which are neighbouring cells with the microglial cells in the brain parenchymal, thus causing a disruption in the activities and functions (such as cerebral blood circulation, learning) of these.
Downregulation of TNF-α receptor
Tumour necrosis factor alpha (TNF-α) is a member of the 19 TNF superfamily that is secreted in the brain in response to pathogen invasions, such as bacterial and viral infections, as well as brain injuries. TNF-α is a cytokine used by the immune system for cell signalling, as it alerts other immune cells in the brain as part of an inflammatory response to infections or foreign bodies. TNF-α is also a major cytokine in neuroinflammation, which activates NF-KB, thereby causing glial activation and production of proinflammatory cytokines (Jung et al. 2019). Various studies report that CBD plays a role in downregulating TNF production.
In the study conducted by Mammana et al. (2019) on the efficacy of CBD and cannabigerol (CBG) in negating neuroinflammation using motoneuron-like cell line, it was observed that when used on its own, CBD reduced the concentration of TNF-α levels. Likewise, when used in combination with CBG, they both exerted anti-inflammatory effects on TNF-α. Aswad et al. (2022) investigated the effects of high concentrations of THC and CBD on cytokine production in immune cells both in vivo and in vitro; it was found that CBD caused significant reductions in pro-inflammatory cytokines in human T cells and neutrophils in vitro.
Also, the in vivo studies carried out by Aswad et al. (2022) using an inflamed mouse model revealed decrease in TNF-α and interleukin-1β (IL-1β) by a high concentration of CBD even though an increase in anti-inflammatory cytokine interleukin 10 (IL-10) was observed. According to Peyravian et al. (2020), CBD impedes important activators of the Janus kinase/signal transducer, transcription signalling pathway, and the nucleotide-binding oligomerization domain-like receptor signalling pathway which consequently reduces pro-inflammatory cytokine production. The downregulation of TNF-α indicates a reduction of brain damage. However, the downregulation of TNF-α by CBD does not guarantee the efficacy of CBD’s therapeutic ability as well as its safety and toxicity in chronic immune conditions. It is important to note that varying dosages of CBD influences its immunomodulatory properties, hence the need to understand the pharmacokinetics of CBD and the doses needed for modulation of the immune system.
Restoration of BDNF expression
The brain-derived neurotrophic factor (BDNF) is one of the most active proteins, called neurotrophins, which help to stimulate and control the growth of new neurons from neural stem cells (neurogenesis) (Autry and Monteggia 2012). BDNF sustains nerve cells by aiding their growth, differentiation and maintenance (Soloey-Nilsen et al. 2022). BDNF also activates the tyrosine kinase receptors which recruit substrates such as phosphoinositide 3-kinases (PI3K) and the phospholipase c gamma (PLC-γ) in promoting neuron survival and neuroplasticity (Lin and Huang 2020). Being a crucial molecule in the development of neurons, BDNF performs vital roles in memory formation (spatial, long-term and emotional, particularly fear), which are linked to learning abilities; hence, BDNF is much implicated in psychiatric disorders (Autry and Monteggia 2012).
Decreased levels of BDNF are detrimental towards normal brain functioning as it plays a part in reducing the expression of synaptic proteins (which aids early neuronal development and regulation of neurotransmitter release), thereby leading to loss of synaptic connections and consequently the loss of neuronal ability to survive and adapt to environmental changes, and hence degeneration (Mariga et al. 2017). Reduction of BDNF also alters the ability of the nervous system to reorganize its structure and functions in response to intrinsic/extrinsic stimuli (Soloey-Nilsen et al. 2022). Apart from neuronal loss, reduced levels of BDNF have been linked with certain neurodegenerative and neuropsychiatric disorders such as Parkinson’s disease, Huntington’s disease, Alzheimer’s disease and mood disorders (Bathina and Das 2015). According to Lima Giacobbo et al. (2019), reduction in BDNF levels usually occurs as a result of long-term exposure to inflammation/neuroinflammation due to the negative effect of cytokines on BDNF-related signalling pathways.
CBD has been identified to be effective in the restoration of BDNF. From their studies, Mariga et al. (2017) and Sales et al. (2019) described the ability of CBD to stimulate and promote neurogenesis, cause drastic and lasting antidepressant-like effects through increased BDNF signalling, and formation of synapses in the prefrontal cortex, which is vital for the overall architecture of brain connections. These two studies also confirm that administration of CBD can increase the expression of the synaptic protein in the prefrontal cortex.
Winstone et al. (2022 doi.org/10.1089/can.2021.0025) administered 3 mg/kg of CBD to adolescent mice to evaluate the effect on BDNF, tropomyosin receptor kinase B (TrkB) and other synaptic markers. They observed that CBD significantly increased the expression of BDNF II, VI and IX, thereby causing growth stimulation as well as differentiation of synapses and neurons (neurogenesis). However, Winstone et al. 2022., reported a significant reduction in CB1 receptor protein in the ventral dentate gyrus (which regulates hormones and emotion), thus, the reduction in CB1 receptor may result in endocannabinoid dysregulation (Matias et al. 2006).
Moreover, differences were observed in the response of male and female mouse to CBD administration with the restoration BDNFII, VI and IX in females while CBD caused the restoration of only BDNF VI in males. Also, according to the report of Sales et al. (Sales et al. 2019), maintenance of a sustained elevated level of BDNF needs to be investigated.
Restoration of brain 5-hydroxytryptamine levels
5-Hydroxytryptamine (5-HT), commonly referred to as serotonin, plays a significant role in modulating the release of several neurotransmitters such as epinephrine/norepinephrine, dopamine, and glutamate (Vaseghi et al. 2022). Prolonged periods of stress, faulty metabolism, and others factors that deplete the level of serotonin, have been linked with neurological disorders such as depression and anxiety (Kanova and Kohout 2021). In addition, production of proinflammatory cytokines such as IFN-ℽ and TNF-α results into tryptophan depletion by activating the quinolinic and kynurenine acid pathways, which makes tryptophan less available for the synthesis of serotonin (Richard 2020). In response to inflammation, following platelet activation, 5-HT is released for the regulation of all immune cells, mostly in their defence against infections, thus preventing excessive cytokine (chemokine) production and consequently cell damage and necrosis (Kanova and Kohout 2021). Serotonin does not only help in the defence of the body against infection/inflammation, but also hinders oxidative damage (Muñoz-Castañeda et al. 2006).
Linge et al. (2016) confirmed the efficacy of CBD in enhancing serotonin and glutamate levels in the ventromedial prefrontal cortex of mice in vivo, depending on the treatment duration (longer – two weeks – treatment with lower dose (10 mg/kg) yielded better results vs high dose (50 mg/kg) over one week). Following administration of CBD (100 mg/kg), Abame et al. (2021) observed a significant increase in 5-HT and noradrenaline levels in the hippocampus of C57BL 6 J mice. According to Jenny et al. 2010, CBD inhibits cytokine-induced tryptophan degeneration, and because tryptophan is a major compound in the synthesis of serotonin, increase in the bioavailability of tryptophan increases the levels of serotonin. However, chronic administration of CBD has been associated with other unwanted consequences such as anxiogenic effects as shown in the study of ElBatsh et al. (2012).
Future of cannabidiol in treatment of neurological disorders/infections
Various anti-inflammatory drugs (such as dexamethasone) have been linked with adverse effects, ranging from moderate to severe, such as gastrointestinal side effects, allergic reactions and risks of cardiovascular conditions (van Bergen et al. 2022). However, CBD subdues inflammation with mild or negligible side effects, that do not pose a serious threat to the health of its users (Wheless et al. 2019). CBD has demonstrated safety and efficacy in the treatment of inflammation, making it a potentially more effective antidote with mild side effects that won't endanger or worsen users' health. Furthermore, CBD has further medicinal potential in addition to its anti-inflammatory qualities.
It has been shown that CBD is well tolerated, especially in its pure form, with less risk of cardiovascular and cerebrovascular effects (Harirforoosh et al. 2013). Based on its medicinal/therapeutic properties, CBD could proffer an unprecedented approach and unlock novel mechanisms for the treatment of brain-related disorders. CBD’s ability to suppress cytokines makes it a potentially useful agent in controlling neuroinflammation that may occur in brain infections, such as various forms of meningitis, thus mitigating the degree of neurological damage.
Owing to the campaign for the use of natural products in eradicating infections and their associated sequelae, as well as the curative effects of CBD, this phyto-cannabinoid would not only serve to assuage brain-related disorders but also serve as a natural panacea in inhibiting inflammation in illnesses in lieu of the traditional anti-inflammatory drugs. Considering the limitations of the non-steroidal anti-inflammatory drugs, it is necessary to regard the potential of CBD for use, on its own or as an adjunctive therapy, in the treatment of brain-related infections and its associated inflammation. The inherent polypharmaceutical properties of cannabis and CBD offer distinct advantages over the current single-target pharmaceutical model and portend to revolutionize neurological treatment into a new reality of effective interventional and even preventative treatment (Russo 2018).
However, the appropriate dosage of CBD that can penetrate the brain needs to be identified. This can be achieved through metabolomics (the study of the set of metabolites existing inside a biological system), which is a holistic approach to understanding various biological processes at the system level. This approach has not really been explored up to this point in cannabinoid research and will help to unveil the adequate concentrations of CBD and its metabolites that can penetrate the brain (especially when studied in animal models). The recent advancement of metabolomic techniques based on high-throughput analytical systems offers new ways to simultaneously detect, identify, and quantify a variety of chemical substances. In addition, there is need for more metabolomics studies on CBD to investigate the impact of this compound on neurological illnesses.
Applying metabolomics to the in vitro or in vivo investigation of CBD's effect(s) in different medical/health conditions, particularly by using animal models or cell cultures and clinical trials, will yield a wealth of data, uncovering and assisting in the identification of distinct chemical fingerprints that are the byproducts of physiological processes that take place in the cells. A direct functional assessment of the physiological condition of cells and tissues is provided by these chemical fingerprints that are left behind.
Metabolomics allows for the elucidation of the metabolite set that exists within a biological matrix, revealing complex and new phenotypic data. Therefore, the application of metabolomics will offer detailed information on the interaction and consequence of CBD in brain-related illnesses, proving the effectiveness of this compound in neurological conditions. Metabolomics will shed light and provide details on:
-
The safety of CBD when used as a treatment for neurological conditions.
-
The metabolic pathways influenced by CBD, and the phenotypic response.
-
The influence of CBD on cytokine production and inflammatory biomarkers in brain-related disorders.
Therefore, any contraindications to using CBD for the treatment of neurological illnesses can be detected by studying how the drug reacts with the body.
Metabolite analysis provides access to a vast amount of untapped knowledge and studies in this regard can be used to highlight a variety of metabolic data. Therefore, applying metabolomics in CBD research for treating neurological illnesses will yield novel information at the metabolite (phenotypic) level.
Conclusion
As reported in many studies, the reformative ability of CBD in neurological disorders is not only limited to the regulation of adenosine/oxidative stress but also the downregulation of TNF-α, restoration of BDNF mRNA expression and the recovery of serotonin levels, thus making it an important agent to consider in the production of immune suppressants and anti-inflammatory products in the future.
The ability of CBD to reduce oxidative stress indicates that in infections/neurological disorders, its administration would help in immune regulation by activating the adenosine receptors. Also, the rapid rise in cerebral adenosine, which disrupts the adenosine system and causes immune imbalances, can be managed by administration of CBD.
Despite all the remedial effects and potentials of CBD, the optimum dose of consumption has yet to be established. In addition, there are few studies that have evaluated the effect of CBD on chemokines and cytokine which occur from continuous systemic inflammations in response to certain forms of neurological infections, such as meningitis. Hence, additional studies with larger populations are recommended in this regard as this would not only provide sufficient and adequate findings but also assist in the application of experimental findings in clinical settings.
Availability of data and materials
Not applicable.
Abbreviations
- 2-AG:
-
2-Arachidonoyl glycerol
- 5-HT:
-
5-Hydroxytryptamine
- 5-HT1A:
-
5-Hydroxy tryptophan 1A receptors
- A2A :
-
Adenosine A2A receptors
- AEA:
-
N-Arachidonoylethanolamine
- BDNF:
-
Brain-derived neurotrophic factor
- CB1:
-
Cannabinoid receptor 1
- CB2:
-
Cannabinoid receptor 1
- CBD:
-
Cannabidiol
- CBG:
-
Cannabigerol
- CB:
-
Cannabinoid receptors
- CYP2B6, CYP2C19, CYP2D6, CYP2J2:
-
Cytochrome P450 enzymes
- D-AMPH:
-
D-amphetamine
- ENT:
-
Equilibrative nucleoside transporter
- FAAH:
-
Fatty acid amide hydrolase
- FJB:
-
Fluoro-JadeB
- GPR:
-
G protein-coupled receptors
- HI:
-
Hypoxic-ischaemic
- iNOS:
-
Inducible nitric oxide synthase
- ICAM:
-
Intracellular adhesion molecule 1
- IFN-γ:
-
Interferon-gamma
- IL:
-
Interleukin
- IVHL:
-
Ipsilateral hemisphere volume loss
- JAK-STAT:
-
Janus kinase-signal transducer and activator of transcription
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MGAL:
-
Monoacylglycerol acid lipase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-KB:
-
Nuclear factor kappa B
- NOX4:
-
NADPH oxidase 4
- NOX1:
-
NADPH oxidase 1
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- PB:
-
Polymyxin B
- PI3K:
-
Phosphoinositide 3-kinases
- PLC-γ:
-
Phospholipase c gamma
- PPARℽ:
-
Peroxisome proliferator-activated receptor gamma
- RAW 264.7:
-
Monocyte/macrophage cell line
- RBD:
-
Rapid eye movement sleep behaviour disorder
- ROS:
-
Reactive oxygen species
- THC:
-
Delta-9-tetrahydrocannabinol
- TGFβ:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
- TrkB:
-
Tropomyosin receptor kinase B
- TRPV1:
-
Transient receptor potential vanilloid
- UGT1A7:
-
UDP glucuronosyltransferase 1 family, polypeptide A7
- UGT1A9:
-
UDP glucuronosyltransferase 1 family, polypeptide A9
- UGT2B7:
-
UDP Glucuronosyltransferase Family 2 Member B7
- UV:
-
Ultraviolet
- UVB:
-
Ultraviolet B
- XO:
-
Xanthine oxidase
References
Abame MA, He Y, Wu S, Xie Z, Zhang J, Gong X, Wu C, Shen J. Chronic administration of synthetic cannabidiol induces antidepressant effects involving modulation of serotonin and noradrenaline levels in the hippocampus. Neurosci Lett. 2021;744:135594.
Abichabki N, Zacharias LV, Moreira NC, Bellissimo-Rodrigues F, Moreira FL, Benzi JR, Ogasawara T, Ferreira JC, Ribeiro CM, Pavan FR. Potential cannabidiol (CBD) repurposing as antibacterial and promising therapy of CBD plus polymyxin B (PB) against PB-resistant gram-negative bacilli. Sci Reports. 2022;12:1–15.
Abichabki, N., Zacharias, L.V., Moreira, N.C., Bellissimo-Rodrigues, F., Moreira, F.L., Benzi, J.R., Ogasawara, T.M., Ferreira, J.C., Pereira, L.R., Braga, G.Ú. Cannabidiol (CBD) repurposing as antibacterial: Promising therapy of CBD plus polymyxin B against superbugs. bioRxiv.2021 https://doi.org/10.1101/2021.04.12.439341.
Alvarez FJ, Lafuente H, Carmen Rey-Santano M, Mielgo VE, Gastiasoro E, Rueda M, Pertwee RG, Castillo AI, Romero J, Martinez-Orgado J. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Ped Res. 2008;64:653–8.
Anil SM, Shalev N, Vinayaka AC, Nadarajan S, Namdar D, Belausov E, Shoval I, Mani KA, Mechrez G, Koltai H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci Reports. 2021;11:1–14.
Aswad M, Hamza H, Pechkovsky A, Zikrach A, Popov T, Zohar Y, Shahar E, Louria-Hayon I. High-CBD extract (CBD-X) downregulates cytokine storm systemically and locally in inflamed lungs. Front Immunol. 2022;13:2252.
Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Anti. 2019;9:21.
Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–58.
Aziz A-I, Nguyen LC, Oumeslakht L, Bensussan A, Ben Mkaddem S. Cannabinoids as immune system modulators: cannabidiol potential therapeutic approaches and limitations. Cannabis and Cannabinoid Research. 2022;8:254.
Aziz A-I, Nguyen LC, Oumeslakht L, Bensussan A, Ben Mkaddem S. Cannabinoids as immune system modulators: cannabidiol potential therapeutic approaches and limitations. Cannabis Cannabinoid Res. 2022;8(2):254–69. https://doi.org/10.1089/can.2022.0133.
Bartschi JG, Greenwood L-M, Montgomery A, Dortants L, Weston-Green K, Huang X-F, Pai N, Potter J, Schira MM, Croft R. Cannabidiol as a treatment for neurobiological, behavioral, and psychological symptoms in early-stage dementia: a double-blind, placebo-controlled clinical trial protocol. Cannabis Cannabinoid Res. 2023;8:348–59.
Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237–46.
Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164–78.
Berger M, Li E, Amminger GP. Treatment of social anxiety disorder and attenuated psychotic symptoms with cannabidiol. BMJ Case Reports CP. 2020;13:e235307.
Bhunia S, Kolishetti N, Arias AY, Vashist A, Nair M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front Pharmacol. 2022;13:989717.
Biernacki M, Brzóska MM, Markowska A, Gałażyn-Sidorczuk M, Cylwik B, Gęgotek A, Skrzydlewska E. Oxidative stress and its consequences in the blood of rats irradiated with UV: protective effect of cannabidiol. Anti. 2021;10:821.
Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52.
Blaskovich MA, Kavanagh AM, Elliott AG, Zhang B, Ramu S, Amado M, Lowe GJ, Hinton AO, Pham DMT, Zuegg J. The antimicrobial potential of cannabidiol. Commun Biol. 2021;4:1–18.
Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pathological overproduction: the bad side of adenosine. Br J Pharmacol. 2017;174:1945–60.
Britch SC, Babalonis S, Walsh SL. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology. 2021;238:9–28.
Cabrera CLR, Keir-Rudman S, Horniman N, Clarkson N, Page C. The anti-inflammatory effects of cannabidiol and cannabigerol alone, and in combination. Pulm Pharmacol Ther. 2021;69:102047.
Carbonell WS, Murase S-I, Horwitz AF, Mandell JW. Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci. 2005;25:7040–7.
Carlini E, Leite J, Tannhauser M, Berardi A. Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J Pharm Pharmacol. 1973;25:664–5.
Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci. 2006;103:7895–900.
Cassol-Jr OJ, Comim CM, Silva BR, Hermani FV, Constantino LS, Felisberto F, Petronilho F, Hallak JEC, De Martinis BS, Zuardi AW. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010;1348:128–38.
Cenova A, Nestorovska M, Crcarevska MS, Mihailova L, Shalabalija D, Dodov MG. Evaluation of antioxidant properties of herbal mixture and CBD oil for the treatment of chronic wound. Macedonian Pharm Bull. 2022;68:557–8.
Chagas MH, Eckeli A, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira E, Camilo M, Bergamaschi M, Schenck C, Hallak JEC. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Phar Ther. 2014;39:564–6.
Chayasirisobhon S. Mechanisms of action and Pharmacokinetics of Cannabis. The Permanente Journal. 2020;25:1–3.
Chen KA, Farrar M, Cardamone M, Gill D, Smith R, Cowell CT, Truong L, Lawson JA. Cannabidiol for treating drug-resistant epilepsy in children: the New South Wales experience. Med J Aust. 2018;209:217–21.
Chesworth R, Cheng D, Staub C, Karl T. Effect of long-term cannabidiol on learning and anxiety in a female Alzheimer’s disease mouse model. Front Pharmacol. 2022;13:931384.
Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav. 1991;40:701–8.
Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, Simões MV, Bhattacharyya S, Fusar-Poli P, Atakan Z. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharm. 2011;25:121–30.
Cuñetti L, Manzo L, Peyraube R, Arnaiz J, Curi L, Orihuela S. Chronic pain treatment with cannabidiol in kidney transplant patients in Uruguay. Transplant Proc. 2018;50(2):461–4.
da Silva VK, de Freitas BS, Garcia RCL, Monteiro RT, Hallak JE, Zuardi AW, Crippa JAS, Schröder N. Antiapoptotic effects of cannabidiol in an experimental model of cognitive decline induced by brain iron overload. Transl Psychiatry. 2018;8:1–8.
Davidson, E., 2022 https://clinicaltrials.gov/ct2/show/NCT03877991. Bioequivalence Assessment of Cannabidiol (CBD) Administrated in Oral Formulations.
de Almeida CM, Brito MM, Bosaipo NB, Pimentel AV, Tumas V, Zuardi AW, Crippa JA, Hallak JE, Eckeli AL. Cannabidiol for rapid eye movement sleep behavior disorder. Mov Disord. 2021;36:1711–5.
De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–94.
Della Rocca G, Paoletti F, Conti MB, Galarini R, Chiaradia E, Sforna M, Dall’Aglio C, Polisca A, Di Salvo A. Pharmacokinetics of cannabidiol following single oral and oral transmucosal administration in dogs. Front Vet Sci. 2022;9:1104152.
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. New Engl J Med. 2017;376:2011–20.
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–8.
Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, Roberts C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia. 2019;60:294–302.
Devinsky O, Verducci C, Thiele EA, Laux LC, Patel AD, Filloux F, Szaflarski JP, Wilfong A, Clark GD, Park YD. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–7.
di Giacomo V, Chiavaroli A, Orlando G, Cataldi A, Rapino M, Di Valerio V, Leone S, Brunetti L, Menghini L, Recinella L. Neuroprotective and neuromodulatory effects induced by cannabidiol and cannabigerol in rat Hypo-E22 cells and isolated hypothalamus. Anti. 2020;9:71.
Ekiner SA, Gęgotek A, Skrzydlewska E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol. 2022;57:102489.
ElBatsh MM, Assareh N, Marsden CA, Kendall DA. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology. 2012;221:239–47.
Elms L, Shannon S, Hughes S, Lewis N. Cannabidiol in the treatment of post-traumatic stress disorder: a case series. J Altern Complement Med. 2019;25:392–7.
ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–48.
England T, O’Sullivan S. Protective effects of Cannabidivarin and Cannabigerol on cells of the blood-brain barrier under ischemic conditions. Cannabis Cannabinoid Res. 2021;6:315–26.
Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, Stone JM, Reichenberg A, Brenneisen R, Holt D. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19–27.
Fagherazzi EV, Garcia VA, Maurmann N, Bervanger T, Halmenschlager LH, Busato SB, Hallak JE, Zuardi AW, Crippa JA, Schröder N. Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology. 2012;219:1133–40.
Feoktistov I, Biaggioni I. Role of adenosine A2B receptors in inflammation. Adv Pharmacol. 2011;61:115–44.
Fernández-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, Martínez-Orgado J. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–33.
Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60:669–79.
Fu X, Xu S, Li Z, Chen K, Fan H, Wang Y, Xie Z, Kou L, Zhang S. Enhanced intramuscular bioavailability of Cannabidiol using nanocrystals: formulation, in vitro appraisal, and pharmacokinetics. AAPS PharmSciTech. 2022;23:85.
García-Arencibia M, González S, de Lago E, Ramos JA, Mechoulam R, Fernández-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–70.
Ghit A, Assal D, Al-Shami AS, Hussein DEE. GABAA receptors: structure, function, pharmacology, and related disorders. J Genet Engine Biotechnol. 2021;19:1–15.
Gildea L, Ayariga JA, Ajayi OS, Xu J, Villafane R, Samuel-Foo M. Cannabis sativa CBD extract shows promising antibacterial activity against Salmonella typhimurium and S. Newington. Molecules. 2022;27:2669.
Giuliano C. Neuroprotective and symptomatic effects of Cannabidiol in an animal model of Parkinson’s disease. Int J Mol Sci. 2021;22:8920.
Giuliano C, Francavilla M, Ongari G, Petese A, Ghezzi C, Rossini N, Blandini F, Cerri S. Neuroprotective and symptomatic effects of cannabidiol in an animal model of Parkinson’s disease. Int J Mol Sci. 2021;22:8920.
Gu Z, Singh S, Niyogi RG, Lamont GJ, Wang H, Lamont RJ, Scott DA. Marijuana-derived cannabinoids trigger a CB2/PI3K axis of suppression of the innate response to oral pathogens. Front Immunol. 2019;10:2288.
Hammell D, Zhang L, Ma F, Abshire S, McIlwrath S, Stinchcomb A, Westlund K. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936–48.
Hampson A, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−) Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci. 1998;95:8268–73.
Han X, Song X, Song D, Xie G, Guo H, Wu N, Li J. Comparison between cannabidiol and sertraline for the modulation of post-traumatic stress disorder-like behaviors and fear memory in mice. Psychopharmacology. 2022;239:1605–20.
Hao E, Mukhopadhyay P, Cao Z, Erdélyi K, Holovac E, Liaudet L, Lee W-S, Haskó G, Mechoulam R, Pacher P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol Med. 2015;21:38–45.
Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16:821–47.
Heineman JT, Forster GL, Stephens KL, Cottler PS, Timko MP, DeGeorge BR Jr. A randomized controlled trial of topical cannabidiol for the treatment of thumb basal joint arthritis. J Hand Surg. 2022;47:611–20.
Hermush V, Ore L, Stern N, Mizrahi N, Fried M, Krivoshey M, Staghon E, Lederman VE, Bar-Lev Schleider L. Effects of rich cannabidiol oil on behavioral disturbances in patients with dementia: a placebo controlled randomized clinical trial. Front Med. 2022;9:951889.
Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol. 2010;174:76–88.
Hosseini A, McLachlan AJ, Lickliter JD. A phase I trial of the safety, tolerability and pharmacokinetics of cannabidiol administered as single-dose oil solution and single and multiple doses of a sublingual wafer in healthy volunteers. Br J Clin Pharmacol. 2021;87:2070–7.
Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402.
Hunter D, Oldfield G, Tich N, Messenheimer J, Sebree T. Synthetic transdermal cannabidiol for the treatment of knee pain due to osteoarthritis. OsCar. 2018;26:S26.
Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM, Salsitz E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176:911–22.
Jean‐Gilles L, Braitch M, Latif ML, Aram J, Fahey AJ, Edwards LJ, et al. Effects of pro‐inflammatory cytokines on cannabinoid CB 1 and CB 2 receptors in immune cells. Acta Physiologica. 2015;214(1):63–74.
Jenny M, Schröcksnadel S, Überall F, Fuchs D. The potential role of cannabinoids in modulating serotonergic signaling by their influence on tryptophan metabolism. Pharmaceuticals. 2010;3(8):2647–60.
Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89:165–70.
Jones PG, Falvello L, Kennard O, Sheldrick G, Mechoulam R. Cannabidiol. Acta Crystallogr Sect b: Struct Crystallogr Cryst Chem. 1977;33:3211–4.
Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021;22:4642.
Jung YJ, Tweedie D, Scerba MT, Greig NH. Neuroinflammation as a factor of neurodegenerative disease: thalidomide analogs as treatments. Front Cell Dev Biol. 2019;7:313.
Kanova M, Kohout P. Serotonin—Its synthesis and roles in the healthy and the critically ill. Int J Mol Sci. 2021;22:4837.
Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci. 2017;114:11229–34.
Khaksar S, Bigdeli M, Samiee A, Shirazi-Zand Z. Antioxidant and anti-apoptotic effects of cannabidiol in model of ischemic stroke in rats. Brain Res Bull. 2022;180:118–30.
Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–47.
Kochen S, Villanueva M, Bayarres L, Daza-Restrepo A, Martinez SG, Oddo S. Cannabidiol as an adjuvant treatment in adults with drug-resistant focal epilepsy. Epilepsy Behav. 2023;144:109210.
Kosgodage US, Matewele P, Awamaria B, Kraev I, Warde P, Mastroianni G, Nunn AV, Guy GW, Bell JD, Inal JM. Cannabidiol is a novel modulator of bacterial membrane vesicles. Front Cell Infect Microbiol. 2019;9:324.
Kozela E, Juknat A, Kaushansky N, Rimmerman N, Ben-Nun A, Vogel Z. Cannabinoids decrease the Th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol. 2013;8:1265–76.
Laprairie R, Bagher A, Kelly M, Denovan-Wright E. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805.
Laun AS, Shrader SH, Brown KJ, Song Z-H. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin. 2019;40:300–8.
Lees R, Hines LA, Hindocha C, Baio G, Shaban ND, Stothart G, Mofeez A, Morgan CJ, Curran HV, Freeman TP. Effect of four-week cannabidiol treatment on cognitive function: secondary outcomes from a randomised clinical trial for the treatment of cannabis use disorder. Psychopharmacology. 2023;240:337–46.
Leweke F, Piomelli D, Pahlisch F, Muhl D, Gerth C, Hoyer C, Klosterkötter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94–e94.
Lima Giacobbo B, Doorduin J, Klein HC, Dierckx RA, Bromberg E, de Vries EF. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol. 2019;56:3295–312.
Lin C-C, Huang T-L. Brain-derived neurotrophic factor and mental disorders. Biomed J. 2020;43:134–42.
Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimarães FS, Crippa JA. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Brazilian J Psychiatry. 2018;41:9–14.
Linge R, Jiménez-Sánchez L, Campa L, Pilar-Cuéllar F, Vidal R, Pazos A, Adell A, Díaz Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26.
Liput DJ, Hammell DC, Stinchcomb AL, Nixon K. Transdermal delivery of cannabidiol attenuates binge alcohol-induced neurodegeneration in a rodent model of an alcohol use disorder. Pharmacol Biochem Behav. 2013;111:120–7.
Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E. Cannabidiol—transdermal delivery and anti-inflammatory effect in a murine model. J Control Release. 2003;93:377–87.
Lowin T, Tingting R, Zurmahr J, Classen T, Schneider M, Pongratz G. Cannabidiol (CBD): A killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020;11:1–11.
Lu H-C, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiat. 2016;79:516–25.
Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477–82.
Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, Crippa AS. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Safety. 2011;6:237–49.
Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol. 2009;51:528–34.
Mammana S, Cavalli E, Gugliandolo A, Silvestro S, Pollastro F, Bramanti P, Mazzon E. Could the combination of two non-psychotropic cannabinoids counteract neuroinflammation? Effectiveness of cannabidiol associated with cannabigerol. Medicina. 2019;55:747.
Mariga A, Mitre M, Chao MV. Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol Dis. 2017;97:73–9.
Martín-Moreno AM, Reigada D, Ramírez BG, Mechoulam R, Innamorato N, Cuadrado A, de Ceballos ML. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol. 2011;79:964–73.
Martin EL, Strickland JC, Schlienz NJ, Munson J, Jackson H, Bonn-Miller MO, Vandrey R. Antidepressant and anxiolytic effects of medicinal cannabis use in an observational trial. Front Psych. 2021;12:1554.
Martínez-Orgado J, Villa M, Del Pozo A. Cannabidiol for the treatment of neonatal hypoxic-ischemic brain injury. Front Pharmacol. 2021;11:584533.
Massi P, Valenti M, Vaccani A, Gasperi V, Perletti G, Marras E, Fezza F, Maccarrone M, Parolaro D. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem. 2008;104:1091–100.
Matias I, Gonthier M-P, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R. Regulation, function, and dysregulation of endocannabinoids in models of adipose and β-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–80.
McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, Almanza C, Pakdel A, Lee J, Limbad C. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2011;129:37–47.
McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225–31.
Mechoulam R, Hanuš L. Cannabidiol: an overview of some chemical and pharmacological aspects. Part I: chemical aspects. Chem Phys Lipid. 2002;121:35–43.
Meissner, H., Cascella, M., 2022 https://www.ncbi.nlm.nih.gov/books/NBK556048/
Misri S, Kaul K, Mishra S, Charan M, Verma AK, Barr MP, Ahirwar DK, Ganju RK. Cannabidiol inhibits tumorigenesis in cisplatin-resistant non-small cell lung cancer via TRPV2. Cancers (basel). 2022;14:1181.
Mohammed N, Ceprian M, Jimenez L, Ruth Pazos M, Martínez-Orgado J. Neuroprotective effects of cannabidiol in hypoxic ischemic insult. The therapeutic window in newborn mice. CNS Neurol Disord-Drug Targets. 2017;16:102–8 (Formerly Current Drug Targets-CNS & Neurological Disorders).
Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38:2433–6.
Muhammad F, Liu Y, Wang N, Zhao L, Zhou Y, Yang H, Li H. Neuroprotective effects of cannabidiol on dopaminergic neurodegeneration and α-synuclein accumulation in C. elegans models of Parkinson’s disease. Neurotoxicology. 2022;93:128–39.
Muñoz-Castañeda JR, Montilla P, Padillo FJ, Bujalance I, Muñoz MC, Muntané J, Túnez I. Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiol Exp. 2006;66:1–6.
Murillo-Rodríguez E, Millán-Aldaco D, Palomero-Rivero M, Mechoulam R, Drucker-Colín R. Cannabidiol, a constituent of Cannabis sativa, modulates sleep in rats. FEBS Lett. 2006;580:4337–45.
Oláh A, Tóth BI, Borbíró I, Sugawara K, Szöllõsi AG, Czifra G, Pál B, Ambrus L, Kloepper J, Camera E. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Investig. 2014;124:3713–24.
Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, Haskó G, Pacher P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–14.
Pandolfo P, Silveirinha V, dos Santos-Rodrigues A, Venance L, Ledent C, Takahashi RN, Cunha RA, Köfalvi A. Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur J Pharmacol. 2011;655:38–45.
Patra PH, Barker-Haliski M, White HS, Whalley BJ, Glyn S, Sandhu H, Jones N, Bazelot M, Williams CM, McNeish AJ. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2019;60:303–14.
Peng J, Fan M, An C, Ni F, Huang W, Luo J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin Pharmacol Toxicol. 2022;130:439–56.
Peres FF, Diana MC, Levin R, Suiama MA, Almeida V, Vendramini AM, Santos CM, Zuardi AW, Hallak JE, Crippa JA. Cannabidiol administered during peri-adolescence prevents behavioral abnormalities in an animal model of schizophrenia. Front Pharmacol. 2018;9:901.
Peyravian N, Deo S, Daunert S, Jimenez JJ. Cannabidiol as a novel therapeutic for immune modulation. ImmunoTargets Ther. 2020a;9:131.
Pokorski I, Clement N, Phung N, Weltman M, Fu S, Copeland J. Cannabidiol in the management of in-patient cannabis withdrawal: clinical case series. Future Neurol. 2017;12:133–40.
Porcari GS, Fu C, Doll ED, Carter EG, Carson RP. Efficacy of artisanal preparations of cannabidiol for the treatment of epilepsy: Practical experiences in a tertiary medical center. Epilepsy Behav. 2018;80:240–6.
Porter B, Marie BS, Milavetz G, Herr K. Cannabidiol (CBD) use by older adults for acute and chronic pain. J Gerontol Nurs. 2021;47:6–15.
Privitera M, Bhathal H, Wong M, Cross JH, Wirrell E, Marsh ED, Mazurkiewicz-Beldzinska M, Villanueva V, Checketts D, Knappertz V. Time to onset of cannabidiol (CBD) treatment effect in Lennox-Gastaut syndrome: Analysis from two randomized controlled trials. Epilepsia. 2021;62:1130–40.
Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B, Becker L. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–25.
Ramer R, Merkord J, Rohde H, Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010;79:955–66.
Richard, R., 2020. A Healthy Inflammatory Response Depends on Neuroendocrine Balance. https://sanescohealth.com/blog/inflammatory-response-and-neuroendocrine-balance/#:~:text=Proinflammatory%20cytokines%20such%20as%20interleukin,to%20a%20reduction%20in%20serotonin.
Rieder CR. Cannabidiol in Parkinson’s disease. Bra J Psychiatry. 2020;42:126–7.
Rizkallah E, Mongeau-Pérusse V, Lamanuzzi L, Stip E, Juteau L-C, Brissette S, Bruneau J, Dubreucq S, Jutras-Aswad D. Cannabidiol effects on cognition in individuals with cocaine use disorder: exploratory results from a randomized controlled trial. Pharmacol Biochem Behav. 2022;216:173376.
Rodríguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol. 2005;40:2–14.
Run M, Yuan-hui S, Na X. Antioxidant effect of cannabidiol in mice with liver fibrosis. Chin J Pub Health. 2022;38:181–5.
Russo C, Lavorgna M, Nugnes R, Orlo E, Isidori M. Comparative assessment of antimicrobial, antiradical and cytotoxic activities of cannabidiol and its propyl analogue cannabidivarin. Sci Rep. 2021;11:22494.
Russo EB. Cannabis therapeutics and the future of neurology. Front Integr Neurosci. 2018;12:51.
Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–43.
Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai A, Colombo A, Parolaro D, Massi P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159:97–105.
Sagredo O, Ramos JA, Decio A, Mechoulam R, Fernández-Ruiz J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur J Neurosci. 2007;26:843–51.
Sales AJ, Fogaça MV, Sartim AG, Pereira VS, Wegener G, Guimarães FS, Joca SR. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol Neurobiol. 2019;56:1070–81.
Salimi H, Klein RS. Disruption of the Blood-Brain Barrier During Neuroinflammatory and Neuroinfectious Diseases. In: Mitoma H, Manto M, editors. Neuroimmune Diseases. Contemporary Clinical Neuroscience. 2019. p. 195–234.
Sauer AV, Hernandez RJ, Fumagalli F, Bianchi V, Poliani PL, Dallatomasina C, Riboni E, Politi LS, Tabucchi A, Carlucci F. Alterations in the brain adenosine metabolism cause behavioral and neurological impairment in ADA-deficient mice and patients. Sci Rep. 2017;7:1–13.
Schiavon AP, Soares LM, Bonato JM, Milani H, Guimarães FS, Weffort de Oliveira RM. Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox Res. 2014;26:307–16.
Seltzer ES, Watters AK, MacKenzie D Jr, Granat LM, Zhang D. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers (basel). 2020;12:3203.
Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in anxiety and sleep: a large case series. Permanente J. 2019;23:18.
Shannon S, Opila-Lehman J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm J. 2016;20:16.
Shoval G, Shbiro L, Hershkovitz L, Hazut N, Zalsman G, Mechoulam R, Weller A. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology. 2016;73:123–9.
Soloey-Nilsen H, Nygaard-Odeh K, Kristiansen MG, Brekke OL, Mollnes TE, Reitan SK, Oiesvold T. Association between brain-derived neurotropic factor (BDNF), high-sensitivity C-reactive protein (hs-CRP) and psychiatric symptoms in medicated and unmedicated patients. BMC Psychiatry. 2022;22:1–7.
Stahl V, Vasudevan K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: a preliminary observation. Cureus. 2020;12:e6809.
Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–66.
Straiker A, Dvorakova M, Zimmowitch A, Mackie K. Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Mol Pharmacol. 2018;94:743–8.
Szaflarski JP, Bebin EM, Comi AM, Patel AD, Joshi C, Checketts D, Beal JC, Laux LC, De Boer LM, Wong MH. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: expanded access program results. Epilepsia. 2018;59:1540–8.
Thomas A, Baillie G, Phillips A, Razdan R, Ross RA, Pertwee R. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–23.
Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. AcBBS. 2017;49:853–66.
Valvassori SS, Elias G, de Souza B, Petronilho F, Dal-Pizzol F, Kapczinski F, Trzesniak C, Tumas V, Dursun S, Nisihara Chagas MH. Effects of cannabidiol on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychopharm. 2011;25:274–80.
van Bergen E, Monnikhof M, Lafeber F, Schutgens R, Mastbergen S, van Vulpen L. The fear for adverse bleeding and cardiovascular events in hemophilia patients using (non-) selective non-steroidal anti-inflammatory drugs: a systematic review reporting on safety. Blood Rev. 2022;56:100987.
Vaseghi S, Arjmandi-Rad S, Eskandari M, Ebrahimnejad M, Kholghi G, Zarrindast M-R. Modulating role of serotonergic signaling in sleep and memory. Pharmacol Rep. 2022;74:1–26.
Victor TR, Hage Z, Tsirka SE. Prophylactic administration of cannabidiol reduces microglial inflammatory response to kainate-induced seizures and neurogenesis. Neuroscience. 2022;500:1–11.
Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–9.
Welty TE, Luebke A, Gidal BE. Cannabidiol: promise and pitfalls: Cannabidiol: promise and pitfalls. Epilepsy Curr. 2014;14:250–2.
Wheless JW, Dlugos D, Miller I, Oh DA, Parikh N, Phillips S, Renfroe JB, Roberts CM, Saeed I, Sparagana SP. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs. 2019;33:593–604.
White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J Clin Pharm. 2019;59:923–34.
Winstone, J., Shafique, H., Clemmer, M.E., Mackie, K., Wager-Miller, J. Effects of Tetrahydrocannabinol and Cannabidiol on Brain-Derived Neurotrophic Factor and Tropomyosin Receptor Kinase B Expression in the Adolescent Hippocampus. Cannabis and Cannabinoid Research. 2022 https://doi.org/10.1089/can.2021.0025
Wójcik P, Gęgotek A, Žarković N, Skrzydlewska E. Disease-dependent antiapoptotic effects of cannabidiol for keratinocytes observed upon UV irradiation. Int J Mol Sci. 2021;22:9956.
Zanelati T, Biojone C, Moreira F, Guimarães FS, Joca SRL. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol. 2010;159:122–8.
Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med. 2011;17:79–86.
Zhang Y, Xia Y. Adenosine signaling in normal and sickle erythrocytes and beyond. Microb Infect. 2012;14:863–73.
Zhao J, Gao X, Zhao L, Wang Y, Zhang J, Liu S. Effects of Cannabidiol on Parkinson’s disease in a transgenic mouse model by gut-brain metabolic analysis. Evid-Based Complement Altern Med. 2022;2022:1525113.
Zieba J, Sinclair D, Sebree T, Bonn-Miller M, Gutterman D, Siegel S, Karl T. Cannabidiol (CBD) reduces anxiety-related behavior in mice via an FMRP-independent mechanism. Pharmacol Biochem Behav. 2019;181:93–100.
Zimmerman JJ, von Saint André-von Arnim A, McLaughlin J. Cellular respiration. Ped Crit Care. 2011;4:1058–72.
Zuardi AW, Cosme R, Graeff FG, Guimarães F. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharm. 1993;7:82–8.
Acknowledgements
The authors acknowledge the North-West University for the enabled environment provided for the research.
Funding
Open access funding provided by North-West University. No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
SM and OPO conceived and conceptualized the study, which was critiqued by SM and YL. OPO wrote the original draft and all authors agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors consent.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omotayo, O.P., Lemmer, Y. & Mason, S. A narrative review of the therapeutic and remedial prospects of cannabidiol with emphasis on neurological and neuropsychiatric disorders. J Cannabis Res 6, 14 (2024). https://doi.org/10.1186/s42238-024-00222-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42238-024-00222-2