Abstract
COVID-19 has left mankind desperately seeking how to manage dramatically rising infection rates associated with severe disease progressions. COVID-19 courses range from mild symptoms up to multiple organ failure and death, triggered by excessively high serum cytokine levels (IL 1β, IL 6, TNF α, IL 8). The vagally driven cholinergic anti-inflammatory pathway (CAP) stops the action of nuclear factor κB (NF-κB), the transcriptional factor of pro-inflammatory cytokines. Thus, well-balanced cytokine release depends on adequate vagal signaling. Coronaviruses replicate using NF-κB transcriptional factor as well. By degrading the cytoplasmatic inhibitor of NF-κB subunits (IκB), coronaviruses induce unrestricted NF-κB expression accelerating both, virus replication and cytokine transcription.
We hypothesize that CAP detriment due to depressed vagal tone critically determines the severity of COVID-19.
Similar content being viewed by others
SARS-CoV-2 has set the world on fire
The severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) shows broad genetic similarities with other coronaviruses (SARS-CoV, SARS-CoV-RaTG13) (Palayew et al. 2020). However, its affinity to cellular angiotensin converting enzyme 2 (ACE 2) receptors is 10-fold higher than that of SARS-CoV (Wang et al. 2020a). This explains the high contagiousness, which led to a pandemic within a few months after its appearance in Wuhan in the People’s Republic of China. The explosive spread of COVID-19, caused by SARS-CoV-2, forced the World Health Organization (WHO) to declare a Public Health Emergency of International Concern (PHEIC) on January 30th (Wang et al. 2020a). Moreover, the consequences of this major threat to health care systems and the accompanying economic and social lockdown in most of the countries are incalculable. This underlines the urgent need for therapeutic and preventive solutions to combat COVID-19.
In our clinical practice, the symptoms of SARS-CoV-2 infection presented along a wide range; from mild disease courses with influenza-like symptoms up to acute respiratory distress syndrome (ARDS) (see Glossary), and death. Despite ARDS we saw patients with liver damage, severe intestinal dysfunction, rhabdomyolysis, acute renal failure, and coagulopathy with subsequent hemorrhage or embolism. In the critically-ill patients, we saw excessively high pro-inflammatory cytokine serum levels (interleukin 1β - IL 1β (see Glossary), interleukin 6 – IL 6 (see Glossary), tumor necrosis factor α - TNF α (see Glossary), interleukin 8 – IL 8 (see Glossary)) and the extent of their hyperexpression was of high prognostic relevance. Concerning this, many authors speak about a hyperinflammatory syndrome or a cytokine storm (Mehta et al. 2020; Feldmann et al. 2020; Moore and June 2020).
Which course this potentially lethal infectious disease takes, is mainly dependent on where the overwhelming inflammatory cascade stops itself or can be therapeutically interrupted.
Elderly patients and patients with cardiovascular, respiratory, or metabolic disorders, as well as patients with cancer are most likely to have a severe course of COVID-19 (Jordan et al. 2020) (see Table 1). The question then arises: Which immunological commonality do these conditions share? And if there is commonality: Is this common immunological defect capable of causing those hyperinflammatory COVID-19 courses?
All these predisposing medical conditions have an imbalance of the autonomic nervous system (ANS) in common (Jarczok et al. 2019; Dalise et al. 2020). This is to the detriment of autonomic parasympathetic compartment and can be interpreted as an illness-adaptive sympathetic overexcitation to maintain homeostasis (Goldberger et al. 2019). But vagal signaling essentially controls cytokine expression and release by blocking nuclear factor κB (NF-κB) (see Table 2), the transcriptional factor (TF) of pro-inflammatory cytokines (Borovikova et al. 2000; Tracey 2007). Moreover, like cytokine transcription, the replication of coronavirus is promoted by NF-κB (Poppe et al. 2017). For its self-replication, the virus hijacks the NF-κB pathway (Poppe et al. 2017) neutralizing NF-κB subunit inhibitors. Under physiological conditions, this transcriptional pathway is restricted by vagal signaling. Thus, despite unrestricted virus replication, the virus-driven, uncontrolled acceleration of the NF-κB action leads to excessive cytokine transcription, inducing the cytokine storm (Sallenave and Guillot 2020).
Because the pro-inflammatory NF-κB-pathway plays a pivotal role in several SARS-CoV-2 associated pathomechanisms and its control underlies mainly vagal signaling capacity, the modulation of impaired vagus nerve activity due to pre-existing conditions seems to be worth exploring further in COVID-19 patients.
Cellular invasion of SARS-CoV-2, immune response and resulting tissue damage
Cellular invasion
For coronaviruses two preconditions must be given to allow cellular invasion. First, the viral spike- or S-protein (see Glossary) must couple to the angiotensin converting enzyme 2 (ACE2) receptor at the surface of susceptible cells (Hoffmann et al. 2020). Second, the S-protein must be proteolytically primed by the transmembrane protease serine subtype 2 (TMPRSS2) beforehand (Hoffmann et al. 2020). TRMPRSS2 is mostly found on the epithelial cells in the respiratory tract, allowing cellular fusion between coronavirus and cell (Hoffmann et al. 2020). It cleaves the viral S-protein into S1 and S2 subunits. This cleavage enhances cellular adherence of the virus and promotes its endosome-independent cell entry. Due to this tissue tropism, SARS-CoV-2 yields high replication rates in the cells of the upper airways and pulmonary inflammation sites (Hui et al. 2020).
It was shown that especially epithelial cells of upper and lower airways such as monocytes, macrophages, and dendritic cells are capable of releasing cytokines along coronavirus infections (Dienz et al. 2012). SARS-CoV-2 RNA presents as a pathogen associated molecular pattern (PAMP) (see Table 3) in those cells and is recognized and captured by pattern recognition receptors (PRR’s) (see Glossary) (i.e. endosomal Toll-like receptors TLR3, TLR7, TLR8 and TLR9) inducing NF-κB activation with subsequent downstream release of NF-κB dependent cytokines (Poppe et al. 2017; Cervantes et al. 2012; Dosch et al. 2009; Moreno-Eutimio et al. 2020). Despite accelerating the inflammatory NF-κB pathway, PAMP recognition and damage-associated molecular patterns (DAMPs) (see Table 3) lead to inflammasome assembly. Inflammasomes are multimeric protein complexes composed of cytoplasmic sensors together with apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC or PYCARD) (see Glossary), and pro-caspase-1 (Lara et al. 2020). Receptor protein activation attracts ASC and caspase-1 to assemble an inflammasome (i.e. NLRP3)(see Glossary). This assembly induces self-cleavage and caspase-1 activation. Activated caspase-1 causes the release of IL 1β, IL 18 (see Glossary), and other inflammatory factors. Additionally, it leads to pyroptotic cell death, which promotes clearance of pathogens and damaged cells.
IL1β is a principal mediator of systemic inflammation. It induces the expression of a large amount of pro-inflammatory genes and acts on numerous target organs (Jéru and Amselem 2011). Furthermore it is supposed to cause the additional release of pro-inflammatory cytokines involving IL 6, IL 8 and TNF α (Lucherini et al. 2018). Since IL 8 drives chemotactic migration of additional inflammatory cells, it promotes the perpetuation of the inflammatory process.
Tissue damage and systemic cytokine distribution
The cytokines of the IL 1 family cause fever and inflammatory fibrotic conversion of pulmonary stroma. Histologic studies of pulmonary tissue from COVID-19 patients revealed diffuse alveolar damage due to infiltration of mononuclear cells and macrophages, as well as diffuse thickening of the alveolar wall forming a hyaline membrane due to virus related cytokine liberation (Xu et al. 2020). Despite perivascular T-cell infiltration, the vasculature of lungs from patients with COVID-19 also showed severe endothelial injury. This was associated with the presence of intracellular virus and disrupted cell membranes. Moreover, histologic analysis of pulmonary vessels in patients with COVID-19 revealed widespread thrombosis with microangiopathy (Ackermann et al. 2020). Taken together, the described mechanisms represent the dramatic pulmonary tissue damage with subsequent critical respiratory dysfunction that is seen in severe cases of COVID-19 (Conti 2020). Moreover, the self-enhancing cytokine pathways stand for the systemic flood of pro-inflammatory cytokines called cytokine storm (Mehta et al. 2020). At the cardiac level, cytokine storm related interleukins (IL 6, IL 1, TNFα) are known to be involved in inhibiting cardiomyocytic membrane channels (hERG-K+ channels/Ca + channels) (Lazzerini et al. 2020a) which take response for myocardial repolarization. Cytokine related impact on these channels is called inflammatory cardiac channelopathy (Lazzerini et al. 2020a). This in turn increases the risk of QT-prolongation related ventricular arrhythmias, significantly contributing to multi-organ dysfunction (Lazzerini et al. 2020a). The mechanisms of direct infection-related cardiomyocytic damage demonstrated by significantly elevated troponin-T levels remain speculative (Lazzerini et al. 2020a).
The cause of the dysfunctions in several other extrapulmonary organs consisting of tissues with few or no ACE 2 receptors (Fu et al. 2020) mentioned above remains unclear. It could be attributed to a direct virus attack or, more likely, a result of the locally or systemically impaired tissue oxygen supply due to infection associated coagulopathy which is in the majority of cases a coagulation predominant-type coagulopathy with embolism and ischemia resulting in tissue damage (Zuo et al. 2020).
COVID-19 - autonomic imbalance, hyperinflammation, and viral replication
The autonomic imbalance
With regard to the patient groups with an increased risk of a more severe progression of the disease, it can be seen that, all medical conditions predisposing for severe COVID-19 courses have a disturbed balance of the autonomic nervous system (ANS), with underrepresentation of the vagal branch in common (Jarczok et al. 2019; Dalise et al. 2020). This is due to the necessity that any impaired organ function must be compensated by additional performance from undisturbed organs to maintain homeostasis. This requirement is described as extrinsic autonomic dysfunction and is conducted via sympathetic overexcitation (Goldberger et al. 2019). In contrast to the extrinsic autonomic dysfunction, there is the intrinsic autonomic dysfunction describing a primary damage to the fibers of the autonomic nervous system. The leading cause for intrinsic autonomic dysfunction is diabetes mellitus (Goldberger et al. 2019). Histological evaluations in diabetic subjects have shown loss of and/or damage to myelinated vagus nerve axons. These findings provide evidence of the autonomic nervous system damage caused by diabetes, which is most likely a result of the hyperglycemia. (Duchen 1980). Thus, dispositional augmented sympathetic tone, and impaired parasympathetic autonomic signaling respectively, are part of all the disease patterns related to severe COVID-19 courses.
Despite the aforementioned conditions with concurrent autonomic balance disorders, several authors indicate that genetic differences (e.g. gender related) are associated with variations in the vagal tone (Evans et al. 2001) and the acute autonomic responses (e.g. to myocardial infarction) (Huikuri et al. 1996).
The cholinergic anti-inflammatory pathway and hyperinflammation
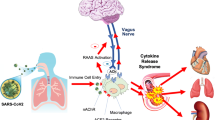
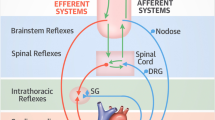
Borovikova (Borovikova et al. 2000) and Tracey (Tracey 2002) first described the cholinergic anti-inflammatory pathway (CAP). CAP controls cytokine release via damage-associated afferent vagal signaling (Borovikova et al. 2000; Tracey 2007; Tracey 2002) onto the dorsal vagal complex (DVC). DVC stands for a group of vagal neurons (area postrema - AP, ambiguous nucleus - NA, solitary nucleus - NTS, dorsal motor nucleus of the vagus - DMV) in the brainstem (Travagli et al. 2006). These central parasympathetic structures are responsible for receiving and processing that vagal afferent signaling (NTS). Muscarinic (M1) agonist network communication to certain neural groups modulates the signal to create a somatotopic efferent vagal impulse (DMV) (Tracey 2002). This impulse leads to acetylcholine (ACh) secretion at the site of injury (Tracey 2007; Tracey 2002). ACh couples to α7 subunit-characterized nicotinic ACh receptors (α7nAChR’s), in turn stopping the action of nuclear factor κB (NF-κB), the transcriptional factor (TF) (Tracey 2007). Thus, it prevents further transcription and downstream release of NF-κB-dependent pro-inflammatory cytokines (Borovikova et al. 2000; Tracey 2007; Tracey 2002). Equilibration between pro- and anti-inflammatory processes is thereby achieved and provides innate immunity without damaging host tissue (Tracey 2007). (Fig. 1). The spleen, as the principal source of pro-inflammatory cytokines, amplifies local cytokine liberation up to systemic cytokine storm and its involvement as main target of the CAP via catecholaminergic splenic nerve fibers is essential for CAP functionality (Huston et al. 2006).
The cholinergic anti-inflammatory pathway (CAP) and its impairment in the case of SARS-2-CoV infection. Cellular virus invasion leads despite local liberation of pro-inflammatory cytokines (i.e. IL 1β, IL 6, IL 8, TNF α) to afferent vagal signaling. In central vagal structures (solitary nucleus (NTS), dorsal motor nucleus of the vagus (DMV)) this signal is after receiving (NTS) and interactional communication to different central instances via a M1 agonist responsive cholinergic brain network transformed into an appropriate efferent vagal impulse (DMV), which is liberating acetylcholine (ACh) at the spot of injury. Thus, cytokine distribution is controlled by somatotopically vagal secreted acetylcholine (ACh) coupling to α7-nicotinergic-acetylcholine receptors (α7AChR´s), this way blocking the cytokine transcriptional factor nuclear factor kappa B (nf-κB). This mechanism is dependent on appropriate parasympathetic (vagal) representation within the autonomic nervous system (ANS). Depressed vagal tone leads to functional impairment of CAP with subsequent excessive cytokine distribution (cytokine storm) followed by tissue injury, pulmonal dysfunction, ARDS and immune paralysis, whereas in the case of ANS balance, controlled cytokine distribution is generating tissue repair and virus elimination
Since controlled cytokine release is imperatively linked to a well-balanced autonomic nervous system (ANS) (Tracey 2002), a controlled pro-inflammatory cytokinergic response to the SARS-CoV-2 invasion appears unlikely in patients with the aforementioned vagus-compromising, pre-existing conditions.
The coronavirus hijacks NF-κB transcriptional pathway
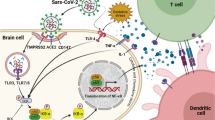
Coronaviruses use the NF-κB pathway for their replication (Poppe et al. 2017). Under unimpaired conditions cytoplasmatic pre-existing subunits of NF-κB (p65, p50, or p52, c-Rel, RelA or RelB) (see Table 4) are bound to inhibitor proteins (IκB) (see Table 4), preventing their passage through the nuclear membrane (Poppe et al. 2017). They are set free along the NF-κB pathway. This release of the subunits is executed via proteolytical degradation of IκB. When a coronavirus invades the cytoplasm, this process is initiated by viral-activated IκB kinase complex (IKK) (see Table 4) followed by proteasome-derived (see Table 4) proteolytic degradation of IκB. In turn, it brings about the nuclear translocation of the NF-κB subunits to form mature NF-κB heterodimers inducing transcription of NF-κB dependent proteins. Thereby both, NF-κB driven virus replication and cytokine syntheses, are accelerated (Poppe et al. 2017). Since CAP controls NF-κB action by ACh coupled to α7nAChR (Tracey 2007), inadequate vagal representation is the cause of both unrestricted virus replication and uncontrolled cytokine release along the viral-hijacked NF-κB pathway (Poppe et al. 2017). (Fig. 2, Key Figure).
(Key Figure) The cellular virus invasion (SARS-CoV-2) with viral hijacking and amplification of the nuclear factor kappa B (NF-κB) pathway for its replication with additional excessive release of pro-inflammatory cytokines (cytokine storm). In the normal cell cycle, subunits (p65, p50 or p52, c-Rel, RelA or RelB) of the transcriptional factor of numerous pro-inflammatory cytokines (NF-κB) are bound by inhibitor proteins of NF-κB subunits (IκB) in the cytoplasm, which leads to regulation of NF-κB production. After cellular virus invasion (SARS-CoV-2) via the ACE 2 receptor, the virus amplifies the activity of the IκB kinase complex (IKK) catalyzing the proteolytic degradation of IκB by the proteasome. This in turn leaves free NF-κB subunits to translocate unrestricted to the nucleus, where they form NF-κB. NF-κB binds to nuclear DNA in order to enhance virus replication as well as the synthesis and release of pro-inflammatory cytokines (i.e. IL 6, IL 8, TNF α). Vagally secreted acetylcholine (ACh) binds to α7 nicotinic acetylcholine receptors (α7nAchRs) at the cellular surface, blocking the NF-κB action and controlling both, virus replication and release of pro-inflammatory cytokines. Afferent vagal nerve stimulation leads to rebalanced vagal representation in a hypersympathetic, imbalanced autonomic nervous system (ANS), which can be seen in several medical conditions, and during stress in general, but also along the course of critical illness such as severe forms of COVID-19 itself
Once the cytokine storm has started causing critical illness, depression of the vagal compartment of the ANS becomes a prognostic factor describing the severity of critical illness (Arbo et al. 2020; Pong et al. 2019). This perpetuates the hyperinflammatory syndrome and makes a therapeutic breakthrough a seemingly futile venture.
Concluding remarks and future perspectives
Our observations, together with previous investigations, led us to hypothesize, that individuals with unimpaired ANS conditions, respond to SARS-CoV-2 infection with a well-balanced, innate, and adaptive immune response causing mild symptoms of COVID-19. This balancing is conducted via the vagus nerve-driven CAP, limiting NF-κB action in cytokine transcription. In addition, the dynamics of viral replication seem to be inversely correlated with vagal signaling, due to the replications NF-κB dependency. Thus, endogenous parasympathetic regulatory circuits are involved in the control of viral load.
Outstanding questions • Is vagal nerve stimulation capable of stopping critical illness and cytokine storm, or is it just preventive to avoid severe COVID-19 courses? • Can vagal nerve stimulation reduce the incidence of extrapulmonary organ failure? • Which vagus nerve stimulation approach is qualified for the use in viral infections? • Since stimulation parameters are particularly important in vagus nerve stimulation approaches, which parameters are suitable under a virus-driven cytokine storm? • Are there adverse effects of vagal nerve stimulation in the treatment of COVID-19? What role does the initial virus load play? • Are there contraindications for vagal nerve stimulation in the field of infectious hyperinflammatory syndromes? |
We believe that sympatho-vagal balance and vagal nerve stimulation (VNS) should be taken into consideration when evaluating diagnostic and therapeutic approaches to severe courses of COVID-19. This can be achieved diagnostically by measuring heart rate variability (HRV) (see Glossary), which is a widely accepted, noninvasive diagnostic method for the evaluation of autonomic balance (Schwerdtfeger et al. 2020). Moreover, HRV-evaluation is cost-neutral and available for use under study- and clinical conditions (Schwerdtfeger et al. 2020; Gevirtz 2015). Thus, efforts should be made to observe correlation between ANS balance compromising disorders and severity of SARS-CoV-2 infection courses. In numerous disease patterns related to uncontrolled cytokine release, VNS has been established to be part of the therapeutic approach (Beekwilder and Beems 2010; Milev et al. 2016; Koopman et al. 2016; Merrill et al. 2006; Bonaz et al. 2016; Bonaz et al. 2013; Albert et al. 2015; Berry et al. 2013; Critchley et al. 2007; Guarini et al. 2003; Hoshide and Jandial 2018; Neren et al. 2016). Moreover, VNS has been shown to inhibit NF-κB action (Guarini et al. 2003). In a preclinical study, VNS was proven to prevent ARDS development, after scorpion poison (mesobuthus tamulus) had been administered (Akella and Deshpande 2015).
Due to its propensity to bind not only to ACE 2 receptors but most likely to nAChR’s as well, SARS-CoV-2 is hypothesized to be capable inducing a primary or secondary neuroinfection (Changeux et al. 2020; Steardo et al. 2020). These infections differ on one hand symptomatically and prognostically from the courses with primary invasion of the respiratory tract cells and on the other hand suggest the high effectiveness of nicotine as a therapeutic agent competing with SARS-CoV-2 for nAChR’s thus, ameliorating COVID-19 courses (Changeux et al. 2020). The presumed nAChR assisted cell invasion is as scientifically interesting as is the opportunity for the virus to compromise CAP action by blocking of nAChR’s. This so-called `nicotine hypothesis` (Changeux et al. 2020) and our currently introduced hypothesis are to be considered together.
As for peripheral circulating and central administered Angiotensin II (AII), shows direct vagus nerve depressing effects (Vaile et al. 1998) and VNS vice versa could be proven to be cardioprotective to chronic heart failure subjects (Li et al. 2004) both, ANS balance and the renin-angiotensin system (RAAS) show a broad interconnection. This and the described anti-inflammatory potential of ACE antagonistic agents (Di Raimondo et al. 2012) shift the controversy concerning the potentially harmful versus the potentially beneficial effects of angiotensin-converting–enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) clearly into the beneficial column and the preventive withholding of these drugs to prevent severe COVID-19 courses appears to be no longer worth discussing (Speth 2020).
The cardiac proarrhythmic pathognomonic and therapeutic dilemma mentioned above is complicated by the fact that drugs commonly used in severe COVID-19 cases (chloroquine/hydroxychloroquine, lopinavir/ritonavir, protease inhibitors, macrolides, fluoroquinolones) have a QT prolonging potential themselves. Especially the use of the IL 6 monoclonal antibody (tocilizumab), which seemingly reduces mortality in COVID-19, is limited by its hERG-K+ channel blocking and thus pro-arrhythmic characteristics (Lazzerini et al. 2020b).
Therefore, we believe that VNS could be one therapeutic key to avoid or to attenuate severe COVID-19 courses. There are several methods described for VNS (Johnston and Webster 2009). We know direct electrical efferent and afferent VNS. Moreover, several transcutaneous VNS (Baig et al. 2019) approaches are also in use (Johnston and Webster 2009). Recently, Staats et al. (Staats et al. 2020) reported distinct symptom relief (fatigue, low appetite, and coughing bouts such as dyspnea and chest pressure/tightness) in two SARS-CoV-2 positive tested patients under the usage of noninvasive vagal nerve stimulation (nVNS). Bonaz et al. (Bonaz et al. 2020) suggest to target CAP in the treatment of COVID-19 patients using VNS and utilize its proven cytokine controlling, anti-inflammatory potential. Moreover, several pharmacological agents (i.e. CNI-1493 (see Glossary) (Oke and Tracey 2007), nicotine, GTS-21 (see Glossary) (Wang et al. 2020b), or ghrelin (Bansal et al. 2012)) are known to enhance vagal signaling (Johnston and Webster 2009). We should mention, that several authors investigating biofeedback methods to increase the vagal tone, found a respiratory frequency of 0.1 Hz to be the resonance frequency to amplify vagal signaling, measured by increasing HRV (Schwerdtfeger et al. 2020; Gevirtz 2015). Further, they were able to show significant amelioration via biofeedback in illness patterns related to sympatho-vagal imbalance or rather elevated serum cytokine levels (Gevirtz 2015; Gevirtz et al. 1996). Because of their wide availability and negligible costs, biofeedback mechanisms should be explored for their potential in restoring ANS balance and therefore preventing severe COVID-19 courses.
Such an exploration becomes urgent, especially when looking at the COVID-19 avalanche coming towards countries with little potential to manage this catastrophe in a sufficient manner.
Many other viruses (PRSSV (see Glossary) (Wang et al. 2013), RSV (see Glossary) (Masaki et al. 2011), HIV-1C (see Glossary) (Dave et al. 2020; Hiscott et al. 2001), HBV (see Glossary) (Hiscott et al. 2001; Liu et al. 2020), HCV (Hiscott et al. 2001), HIV (Pahl 1999), EBV (see Glossary) (Hiscott et al. 2001)) have been proven to use the described NF-κB pathway for replication. This offers a wide area of therapeutic applications for vagal nerve stimulation.
Even if the described hypotheses reflect just a part of the complex pathophysiological processes in viral infections, they might provide direction guiding input for further basic research to combat COVID-19. Nevertheless, some questions concerning VNS in COVID-19 have got to be answered (see outstanding questions). The question if VNS is able to control the cytokine storm, when critical illness has taken already place or if it is capable more likely to avoid such severe courses of COVID-19, needs to be addressed, as well as the question for the effectiveness of VNS in extrapulmonary disease manifestations. Since different approaches of VNS are described (i.e. afferent or efferent electrical VNS, transcutaneous or bio-feedback VNS), these approaches should be investigated with regard to their differences in terms of effectiveness, security and possible contraindications, or adverse effects in critical ill subjects before any clinical use begins. Nonetheless, concerning the high dynamics of the COVID-19 associated pathologic processes, the question for the stimulative patterns in the case of using electrical (transcutaneous or direct) VNS, seems to be crucially important.
Availability of data and materials
Not applicable.
References
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;NEJMoa2015432.
Akella A, Deshpande SB. Vagal efferent stimulation protects against Mesobuthus tamulus venom-induced acute respiratory distress syndrome in rats. Toxicon. 2015;108:189–201.
Albert U, Maina G, Aguglia A, Vitalucci A, Bogetto F, Fronda C. Vagus nerve stimulation for treatment-resistant mood disorders: a long-term naturalistic study. BMC Psychiatry. 2015;15:64.
Arbo JE, Lessing JK, Ford WJH, Clark S, Finkelsztein E, Schenck EJ, Heart rate variability measures for prediction of severity of illness and poor outcome in ED patients with sepsis. Am J Emerg Med. 2020;S0735675720300127.
Baig SS, Falidas K, Laud PJ, Snowdon N, Farooq MU, Ali A. Transcutaneous Auricular Vagus Nerve Stimulation with Upper Limb Repetitive Task Practice May Improve Sensory Recovery in Chronic Stroke. J Stroke Cerebrovasc Dis. 2019;28(12):104348.
Bansal V, Ryu SY, Lopez N, Allexan S, Krzyzaniak M, Eliceiri B. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation. 2012;35(1):214–20.
Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27(2):130–8.
Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med Devices (Auckl). 2013;6:17–35.
Bonaz B, Picq C, Sinniger V, Mayol JF, Clarençon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25(3):208–21.
Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motility. 2016;28(6):948–53.
Bonaz B, Sinniger V, Pellissier S. Targeting the cholinergic anti-inflammatory pathway with vagus nerve stimulation in patients with Covid-19? Bioelectron Med. 2020;6(1):15.
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 25. 2000;405(6785):458–462.
Cervantes JL, Weinerman B, Basole C, Salazar JC. TLR8: the forgotten relative revindicated. Cell Mol Immunol. 2012;9(6):434–8.
Changeux J-P, Amoura Z, Rey FA, Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Comptes Rendus Biologies 2020;343(1):33–39.
Conti P. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):1.
Critchley HD, Lewis PA, Orth M, Josephs O, Deichmann R, Trimble MR. Vagus nerve stimulation for treatment-resistant depression: behavioural and neural effects on encoding negative material. Psychosomatic Med. 2007;69(1):17.
Dalise AM, Prestano R, Fasano R, Gambardella A, Barbieri M, Rizzo MR. Autonomic Nervous System and Cognitive Impairment in Older Patients: Evidence From Long-Term Heart Rate Variability in Real-Life Setting. Front Aging Neurosci. 2020;12:40.
Dave RS, Ali H, Sil S, Knight LA, Pandey K, Madduri LSV. NF-κB Duplications in the Promoter-Variant HIV-1C LTR Impact Inflammation Without Altering Viral Replication in the Context of Simian Human Immunodeficiency Viruses and Opioid-Exposure. Front Immunol. 2020;11:95.
Di Raimondo D, Tuttolomondo A, Butta C, Miceli S, Licata G, Pinto A. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. CPD. 8. 2012;18(28):4385–4413.
Dienz O, Rud JG, Eaton SM, Lanthier PA, Burg E, Drew A. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5(3):258–66.
Dosch SF, Mahajan SD, Collins AR. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142(1–2):19–27.
Duchen LW. Pathology of Autonomic Neuropathy in Diabetes Mellitus. Ann Intern Med.1980;92(2_Part_2):301.
Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Applied Physiol. 2001;91(6):2611–8.
Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;S0140673620308588.
Fu J, Zhou B, Zhang L, Balaji KS, Wei C, Liu X. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep. 2020;47(6):4383–92.
Gevirtz R. Integrating heart rate variability biofeedback into mindfulness-based therapies. Biofeedback. 2015;43(3):129–32.
Gevirtz RN, Hubbard DR, Harpin RE. Psychophysiologic treatment of chronic lower back pain. Prof Psychol Res Pract. 1996;27(6):561–6.
Goldberger JJ, Arora R, Buckley U, Shivkumar K. Autonomic nervous System dysfunction. J Am Coll Cardiol. 2019;73(10):1189–206.
Guarini S, Altavilla D, Cainazzo M-M, Giuliani D, Bigiani A, Marini H. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107(8):1189–94.
Hiscott J, Kwon H, Génin P. Hostile takeovers: viral appropriation of the NF-kB pathway. J Clin Invest. 2001;107(2):143–51.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8.
Hoshide R, Jandial R. Regaining consciousness: the effect of vagal nerve stimulation on a patient in a permanent vegetative state. Neurosurgery. 2018;82(3):N29–30.
Hui KP, Cheung M-C, Perera RA, Ng K-C, Bui CB, Ho JC, Tropism of the Novel Coronavirus SARS-CoV-2 in Human Respiratory Tract: An Analysis in Ex Vivo and In Vitro Cultures. SSRN Journal. 2020 [zitiert 18. April 2020]; Verfügbar unter: https://www.ssrn.com/abstract=3552870.
Huikuri HV, Pikkuja¨MSA¨ SM, Airaksinen KEJ, Ika¨heimo MJ, Rantala AO, Kauma H, Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation. 1996;94(2):122–125.
Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203(7):1623–8.
Jarczok MN, Koenig J, Wittling A, Fischer JE, Thayer JF. First Evaluation of an Index of Low Vagally-Mediated Heart Rate Variability as a Marker of Health Risks in Human Adults: Proof of Concept. JCM 2019;8(11):1940.
Jéru I, Amselem S. Inflammasome et interleukine 1. La Revue de Médecine Interne. 2011;32(4):218–24.
Johnston GR, Webster NR. Cytokines and the immunomodulatory function of the vagus nerve. Br J Anaesth. 2009;102(4):453–62.
Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;m1198.
Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci. 2016;113(29):8284–9.
Lara PC, Macías-Verde D, Burgos-Burgos J. Age-induced NLRP3 Inflammasome over-activation increases lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis. 2020;11(4):756.
Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, arrhythmic risk, and inflammation: mind the gap! Circulation. 2020a;142(1):7–9.
Lazzerini PE, Laghi-Pasini F, Acampa M, Boutjdir M, Leopoldo Capecchi P. IL-6 (interleukin 6) blockade and heart rate corrected QT interval prolongation in COVID-19. Circ: arrhythmia and electrophysiology [internet]. September 2020b [zitiert 12. Oktober 2020];13(9). Verfügbar unter: https://www.ahajournals.org/doi/10.1161/CIRCEP.120.008791.
Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109(1):120–4.
Liu L, Zhu J, Yang J, Li X, Yuan J, Wu J, GP73 facilitates hepatitis B virus replication by repressing the NF-κB signaling pathway. J Med Virol. 2020;jmv.25718.
Lucherini OM, Rigante D, Sota J, Fabiani C, Obici L, Cattalini M, Updated overview of molecular pathways involved in the most common monogenic autoinflammatory diseases. Clin Exp Rheumatol. 2018;36 Suppl 110(1):3–9.
Masaki T, Kojima T, Okabayashi T, Ogasawara N, Ohkuni T, Obata K, A nuclear factor-κB signaling pathway via protein kinase C δ regulates replication of respiratory syncytial virus in polarized normal human nasal epithelial cells. Mostov KE, Herausgeber. MBoC. 2011;22(13):2144–2156.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;S0140673620306280.
Merrill CA, Jonsson MAG, Minthon L, Ejnell H, C-son Silander H, Blennow K. Vagus nerve stimulation in patients with Alzheimer’s disease: additional follow-up results of a pilot study through 1 year. J Clin Psychiatry. 2006;67(8):1171–8.
Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the Management of Adults with major depressive disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61(9):561–75.
Moore BJB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;eabb8925.
Moreno-Eutimio MA, López-Macías C, Pastelin-Palacios R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020;22(4–5):226–9.
Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA. Vagus nerve stimulation and other Neuromodulation methods for treatment of traumatic brain injury. Neurocrit Care. 2016;24(2):308–19.
Oke SL, Tracey KJ. From CNI-1493 to the immunological homunculus: physiology of the inflammatory reflex. J Leukocyte Biol. 2007;83(3):512–7.
Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18(49):6853–66.
Palayew A, Norgaard O, Safreed-Harmon K, Andersen TH, Rasmussen LN, Lazarus JV. Pandemic publishing poses a new COVID-19 challenge. Nat Hum Behav. 2020;4(7):666–9.
Pong JZ, Fook-Chong S, Koh ZX, Samsudin MI, Tagami T, Chiew CJ, Combining Heart Rate Variability with Disease Severity Score Variables for Mortality Risk Stratification in Septic Patients Presenting at the Emergency Department. IJERPH. 16. 2019;16(10):1725.
Poppe M, Wittig S, Jurida L, Bartkuhn M, Wilhelm J, Müller H, The NF-κB-dependent and -independent transcriptome and chromatin landscapes of human coronavirus 229E-infected cells. Enjuanes L, Herausgeber. PLoS Pathog. 2017;13(3):e1006286.
Sallenave J-M, Guillot L. Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets? Front Immunol. 2020;11:1229.
Schwerdtfeger AR, Schwarz G, Pfurtscheller K, Thayer JF, Jarczok MN, Pfurtscheller G. Heart rate variability (HRV): from brain death to resonance breathing at 6 breaths per minute. Clin Neurophysiol. 2020;131(3):676–93.
Speth RC. Keep taking your ACE inhibitors and ARBs during the COVID 19 pandemic. J Travel Medicine. 2020;27(3):taaa045.
Staats P, Giannakopoulos G, Blake J, Liebler E, Levy RM. The Use of Non-invasive Vagus Nerve Stimulation to Treat Respiratory Symptoms Associated With COVID −19: A Theoretical Hypothesis and Early Clinical Experience. Neuromodulation. 2020;ner.13172.
Steardo L, Steardo L, Zorec R, Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020 [zitiert 12. Oktober 2020];229(3). Verfügbar unter: https://onlinelibrary.wiley.com/doi/abs/10.1111/apha.13473.
Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–9.
Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Investigation. 2007;117(2):289–96.
Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68(1):279–305.
Vaile JC, Fletcher J, Littler WA, Coote JH, Townend JN. Angiotensin II modulates cardiovascular autonomic control in the absence of baroreflex loading. Heart. 1998;80(2):127–33.
Wang D, Cao L, Xu Z, Fang L, Zhong Y, Chen Q, MiR-125b Reduces Porcine Reproductive and Respiratory Syndrome Virus Replication by Negatively Regulating the NF-κB Pathway. Zhao S, Herausgeber. PLoS ONE. 2013;8(2):e55838.
Wang H, Cai D, Chen Z, Wang Y. GTS-21 Promotes α7 nAChR to Alleviate Intestinal Ischemia-Reperfusion-Induced Apoptosis and Inflammation of Enterocytes. Med Sci Monit 2020b;26:e921618.
Wang L, Wang Y, Ye D, Liu Q. A review of the 2019 Novel coronavirus (COVID-19) based on current evidence. Int J Antimicrobial Agents März 2020a;105948.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Zuo M, Huang Y, Ma W, Xue Z, Zhang J, Gong Y, Expert Recommendations for Tracheal Intubation in Critically ill Patients with Noval Coronavirus Disease 2019. CMSJ. 2020;0(0):0.
Acknowledgements
We thank Mr. Frank Jabin for his persistent and accurate work creating the images. Thanks also to Mr. Creed Weiler helping us to find the right words in adequate English language.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Marco Leitzke drafted the manuscript and has been investigating for several years in the field of modulation of autonomic balance disorders with vagal nerve stimulation. Stefan Schimpf revised the manuscript has been investigating several years in the field of modulation of autonomic balance disorders with vagal nerve stimulation. Marco Leitzke, Jan-Jacob Meyer and Dragan Stefanovic had been working together on the intensive care unit at the Helios Klinik Leisnig, making the described observations and discussing them, which led to the submitted hypothesis. Jan-Jakob Meyer and Dragan Stefanovic revised the manuscript. Peter Schönknecht shared his experience and observations on COVID-19 at the hospital sächsisches Krankenhaus Arnsdorf and revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Marco Leitzke, Stefan Schimpf are inventors on one patent application (DE 10 2010 055 792.7 Number) related to gastric electrical stimulation, but do not receive any proceeds related to this patent, so that no financial or non-financial competing interests are given. Peter Schönknecht, Dragan Stefanovic and Jan-Jakob Meyer report no financial and no non-financial conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- ARDS
-
Acute respiratory distress syndrome (ARDS) describes a respiratory failure characterized by rapid onset of widespread inflammation in the lungs. 1
- ASC
-
Apoptosis-associated speck-like protein containing a CARD, also named PYCARD, is mainly stored in the nucleus of monocytes and macrophages. It acts as a pivotal adaptor protein in activation of the inflammasome. 4
- CNI-1493
-
CNI-1493 is a chemical inducing activation of central vagal neurons. 10
- EBV
-
The Epstein–Barr virus (EBV), a herpesvirus, is one of the most common viruses in humans. 11
- GTS-21
-
GTS-2 (DMXBA) acts as a partial agonist on neural nicotinic acetylcholine receptors. 10
- HBV
-
Hepatitis B virus (HBV), is a DNA virus causing hepatitis B. 11
- HCV
-
The hepatitis C virus causes hepatitis C and some cancers, such as hepatocellular carcinoma. 11
- HIV
-
The human immunodeficiency viruses causes the acquired immunodeficiency syndrome (AIDS). 11
- HIV-1C
-
HIV-1C, a HIV subtype predominant in southern Africa 11, 14
- HRV
-
Heart rate variability measures the physiologically inconsistent gaps between each heartbeat and is used as an index to evaluate the balance of the autonomic nervous system. 10, 11, 13
- IL 18
-
Interleukin-18 is a proinflammatory cytokine produced by many cell types and a factor that induces interferon-γ (IFN-γ) production. 4
- IL 1β
-
interleukin 1β is an important mediator of the inflammatory response, and is involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis. 1, 2, 4, 7
- IL 6
-
Interleukin 6 is an important mediator of fever and of the acute phase response and is secreted by macrophages in response to specific microbial molecules, referred to as pathogen-associated molecular patterns (PAMPs) 1, 2, 4, 7, 9
- IL 8
-
Interleukin 8 is a chemokine secreted by macrophages and other cells such as epithelial cells, airway smooth muscle cells, or endothelial cells. IL-8 acts as a chemokine, attracting immune cells to immigrate. 1, 2, 4, 7, 9
- NLRP3
-
The NLRP3 (also known as NALP3 and cryopyrin) inflammasome is the best characterized inflammasome. It comprises the NLR (Nod-like-receptor) protein NLRP3, the adapter ASC and pro-caspase-1. 4
- PRR
-
Pattern recognition receptors detect molecules specific for pathogens. They are expressed by dendritic cells, macrophages, monocytes, neutrophils and epithelial cells 4
- PRSSV
-
Porcine reproductive and respiratory syndrome virus 11
- RSV
-
Respiratory syncytial virus 11
- S-protein
-
The spike protein is part of the viral envelope and interacts with its complement host cell receptor, which is central in determining the tissue tropism, infectivity, and species range of the virus. 3
- TNF α
-
Tumor necrosis factor alpha is a cytokine promoting systemic inflammation and inducing the acute phase reaction. It is produced mainly by activated macrophages, but can be produced by many other cell types. 1, 2, 4, 7, 9
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leitzke, M., Stefanovic, D., Meyer, JJ. et al. Autonomic balance determines the severity of COVID-19 courses. Bioelectron Med 6, 22 (2020). https://doi.org/10.1186/s42234-020-00058-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42234-020-00058-0