Abstract
Objective
To review the influence of metabolic dysfunction of glucose after traumatic brain injury on patient mortality.
Materials and methods
We searched PubMed, Scopus, EBSCOhost, Medline, and Embase electronic databases, involving publications from 1980 to August 2017 in English and Spanish.
Results
The glucose metabolism in brain involved in brain signal conduction, neurotransmission, synaptic plasticity, and cognitive function. Insulin levels traverse the blood–brain barrier by utilizing an insulin receptor protein as a carrier, playing a pivotal role in various cognitive functions while also regulating energy metabolism. TBI causes elevated blood glucose levels. Hyperglycemia is attributed to an acute sympatho-adrenomedullary response, resulting in elevated catecholamines, increased levels of cortisol, and IL-6. Moreover, there is a potential association with hypothalamic involvement. Additionally, hyperglycemia is linked to lactic acidosis at the tissue level, ultimately contributing to higher mortality rates.
Conclusions
The monitoring and control of glucose should be an important part of multimodal monitoring in patients with moderate to severe traumatic brain injury managed in neurocritical care units. A management protocol should ensure normoglycemia and early detection and correction of glucose abnormalities since it improves patients' clinical outcomes.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is considered a public health problem by the World Health Organization (WHO), representing a significant cause of disability worldwide [1, 2] and is correlated with death and permanent disability [3]. TBI causes metabolic dysfunction in the brain, increases energy demand, and is associated with alterations in the expression of the glutamate transporter, oxidative stress, and biochemical changes in the cerebral vasculature, with insulin being a possible mediator of these processes. The cerebral vascular resistance to these changes increases vulnerability to the effects caused by brain injury [4]. Hyperglycemia after TBI is a marker of lesion severity and an independent predictor of mortality [2, 5].
Materials and methods
Type of study
A Scoping review of medical literature in which original articles and review articles were included.
Inclusion criteria
We selected publications related to traumatic brain injury that specifically address the disorders of glucose metabolism in the context of the traumatic brain injury.
Search strategy
An electronic search was conducted in the PubMed, Scopus, EBSCOhost, Medline, and Embase databases, involving all publications from 1980 to March 2023. In addition. The search was performed using the following keywords and Booleans descriptors: "((Hyperglycemia) OR insulin resistance) AND cerebral Trauma."
Selection of studies and review
Title and abstracts were reviewed, and full texts were selected based on the criteria for inclusion, which addressed the impact of cerebral glucose metabolism on traumatic brain injury.
Results
In the search carried out in the PubMed, Scopus, EBSCOhost, Medline, and Embase databases with the keywords and descriptors Booleans “((Hyperglycemia) OR insulin resistance) AND cerebral Trauma,” we obtained 245 results. Flowchart of the included and excluded articles is presented, resulting in 24 articles selected for this review (Fig. 1).
Role of insulin and glucose in the brain
The importance of glucose in the brain
The brain utilizes approximately 20% of the total energy produced by the body. The constant activation of neurons to regulate vital functions, even during sleep, results in a higher energetic expenditure than in other cells. Since the brain relies almost exclusively on glucose for its energy needs, a continuous supply of glucose is crucial for proper brain metabolic activities, vitality, signal conduction, neurotransmission, synaptic plasticity, and cognitive function. Variations in glucose levels can compromise these functions. Glucose is regulated by several hormones and physiological responses that ensure it remains within a non-harmful range. Normal blood glucose concentrations when fasting are < 6.1 mmol/L (< 110 mg/dL) and < 7.8 mmol/L (< 140 mg/dL) in postprandial glycemia or with HbA1c < 6% [6].
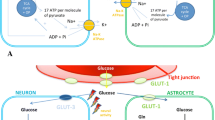
After a traumatic brain injury (TBI), glucose metabolism at the brain level is disrupted, and it has been observed that insulin has a significant impact on metabolism, particularly in the motor cortex. The glucose uptake pathway is initiated by insulin binding to its receptor, which becomes phosphorylated upon binding, recruiting insulin receptor substrate for the binding of the phosphoinositide kinase-3, activated mitogen protein kinase MAP, and protein kinase B/AKT. Phosphoinositide kinase-3, through protein kinase C, induces the translocation of the glucose transporter GLUT4 to the membrane. Phosphorylated B/AKT protein kinase inhibits glycogen kinase-3 synthase, which typically inactivates glycogen synthase [7].
Tumor necrosis factor (TNF) reduces insulin-induced phosphorylation at the receptor, insulin receptor substrate proteins, B/AKT protein kinase, glycine kinase-3 synthase, activation of GLUT4, and inadequate activation of the glucose synthase system, despite the higher energy demand after TBI. This leads to a relative deficiency of insulin, an alteration of energy metabolism, and ultimately cell death [7] (Figs. 2 and 3). The insulin receptor pathway contributes to the repair of small, myelinated fibers by enhancing mitochondrial membrane potential and ATP production, while simultaneously decreasing NADPH and hexokinase activity [7].
Role of insulin in the brain
Insulin levels in the brain are 10–100 times higher than in plasma. It crosses the blood–brain barrier using an insulin receptor protein as a transporter. Local synthesis in pyramidal (but not glial cell) neuronal brain cells of the hippocampus, prefrontal cortex, enteral cortex, olfactory bulb, hypothalamus, and amygdala is a secondary source of insulin in the CNS. A large number of insulin receptors are present in the median temporal lobe, hippocampus, and prefrontal cortex, so in addition to regulation in energy metabolism and energy reserves, it affects cognition, particularly long-term memory, and working memory. The hippocampus has the highest number of insulin receptors, glucocorticoids, and glutamate [6].
Activation of the insulin receptor signaling pathways leads to stimulation of the production of proteins involved in antioxidant neuronal defense, glucose metabolism, and antiapoptotic or neuroprotection mechanisms.
The insulin receptor pathways upgrade mitochondrial membrane potential and ATP input, and reduce NAPH and hexokinase activity, which improves adult neuronal glucose metabolism and axonal proliferation and differentiation or neuromodulation. This pathway is involved in the regeneration of small myelinated fibers (neurotropism) and the improvement of cerebral synaptic plasticity and the formation of new brain circuits essential for learning, memory formation, consolidation, storage, and retrieval.
Insulin hormone promotes the release of adrenaline and norepinephrine in the adrenergic terminals, and this inhibits the synaptic reuptake of norepinephrine, modifies catecholamine kinetics, and stimulates the neuronal uptake of serotonin. Additionally, it is capable of modulating the expression of N-methyl-D-aspartate (NMDA) receptors, increasing the neuronal flow of Ca2+, and thus reinforcing synaptic communication between neurons [6].
In the brain, the insulin degradative enzyme—IDE—degrades insulin and amyloid β protein, so an increase in peripheral insulin may induce a negative regulation response by decreasing intracerebral insulin levels, favoring the accumulation of amyloid β protein, and thus clinically worsening Alzheimer's disease [6].
Diabetes mellitus and trauma
DM is associated with long-term complications such as diabetic retinopathy, diabetic nephropathy, peripheral vascular disease, and heart disease. In the CNS, the main complications are circumscribed peripheral and autonomic neuropathies, cerebrovascular disease, epilepsy, cognitive deficits, and depression. Progressive cognitive impairment, cerebral infarction, cerebral atrophy, and neurodegeneration are explained by microstructural and macrostructural changes in DM brain injury [6].
The cause of hyperglycemia after cranial trauma is multifactorial [4, 8]. The acute sympathetic response causes an increase in serum levels of catecholamines, which cause an increase in blood glucose levels by increasing glycogenolysis in skeletal muscle and liver tissue, increasing gluconeogenesis, and inhibiting insulin secretion. An increase in the levels of cortisol and cytokines such as interleukin-6 may also contribute to hyperglycemia in these patients [8, 9]. Traumatic brain injury also induces generalized metabolic dysfunction in the brain, in which glucose requirements increase to meet the needs associated with trauma recovery resulting in a state of hyperglycolysis, and insulin resistance similar to the patient with diabetes mellitus type 2 (DMT2) [3, 4]. This energy crisis is associated with increased susceptibility to the harmful effects of brain injury. Proposed mechanisms for this phenomenon include alteration of glutamate transporter expression, oxidative damage, and biochemical damage to the cerebral vasculature [4]. Abundant glucose supply during incomplete ischemia may allow the continuation of anaerobic glycolysis, which would lead to lactate accumulation and subsequently to tissue acidosis, leading to cell death, which typically occurs at a pH of 5.3 [8].
Levels of glucose and mortality in brain traumatic injury
Approximately 87% of patients entering the intensive care unit (ICU) due to traumatic brain injury (TBI) have hyperglycemia [8]. Although TBI has been shown to cause elevated blood glucose levels, it has not been elucidated in its entirety; however, an association with lactic acidosis has been found at the tissue level, which has led to an increase in blood glucose levels and mortality rates in this population [8].
Hyperglycemia between 135 and 200 mg/dl [9] during admission has been associated with infection, increased hospital stay, and increased mortality rates [10]. In addition, hyperglycemia in the first 24 h has been identified as an independent risk factor that raises mortality rates and has suggested its early therapeutic intervention regardless of the severity of the injury (as measured by the Glasgow Coma Scale—GCS) [9].
Persistent hyperglycemia worsens patients with TBI, increasing the length of stay in intensive care and being a significant risk factor for mortality [10]. The increase in mortality associated with DM in patients with TBI appears to be independent of age-related DM comorbidities. It has been suggested that chronic problems related to DM contribute little to mortality related to the TBI [2].
Insulin has been used in intensive therapies for the management of hyperglycemia and has been associated with severe hypoglycemic states. To counteract this effect, it has been suggested to accompany the high doses of insulin with the glucose supply, which in turn avoids insulin deficiency [2].
Hyperglycemia in children
Traumatic brain injury represents a significant risk factor for death and permanent disability in children. Additionally, secondary injuries such as ischemia, acidosis, and electrolyte imbalances can impact outcomes following brain injury. Until recently, the impact of hyperglycemia in children with brain injury was not well established, with only two studies having been conducted: one suggesting a relationship between hyperglycemia and poor neurological outcomes following brain trauma, and another in which no such relationship was found (Table 1).
The study by Cochran et al. confirmed the former, demonstrating a link between hyperglycemia and worse neurological outcomes in children following brain injury. Furthermore, they observed high risk of mortality among patients with blood glucose levels above 300 mg/dL [11].
Maintaining high glucose levels in children in neurological critical care units with moderate to severe traumatic brain injury has been associated with an increased risk of mortality and a decreased likelihood of favorable functional outcomes. It is important to note that hyperglycemia is a common issue in patients with traumatic brain injury (TBI), and numerous studies have demonstrated that it can have a significant impact on their prognosis [12, 13, 16, 18, 19].
Several central glucose level thresholds have been identified that can impact outcomes in pediatric TBI patients, including levels greater than 160 mg/dl, 200 mg/dl, or 270 mg/dl. Studies have shown a direct correlation between the duration of exposure to hyperglycemia, disturbances in cerebral metabolism, and poor functional prognosis [8, 9, 11, 16].
Early identification and management of hyperglycemia in pediatric TBI patients are crucial in order to improve outcomes and reduce the risk of mortality. Effective glucose control measures can include insulin therapy, glucose monitoring, and the implementation of protocols for glycemic management in critical care settings. By optimizing glucose levels and minimizing the duration of exposure to hyperglycemia, clinicians can help to improve the functional outcomes and overall prognosis of pediatric TBI patients [19,20,21,22].
Hyperglycemia following traumatic brain injury (TBI) is common in both children and adults and has been associated with adverse outcomes in both groups. In pediatric patients, hyperglycemia has been linked to increased risk of seizures, infections, and longer hospital stays [23]. Furthermore, studies have found that elevated blood glucose levels are associated with higher mortality and long-term disability in children with TBI [24].
Hyperglycemia has been shown to negatively affect the neurological outcome following TBI in children. In a study of children with moderate to severe TBI, hyperglycemia was associated with higher risk of long-term disability, decreased recovery capacity, and worse neurological status at hospital discharge [25].
It is believed that hyperglycemia in pediatric TBI patients may be the result of a physiological stress response and release of stress hormones. Additionally, inflammation and activation of the inflammatory cascade may contribute to hyperglycemia in pediatric TBI patients [26].
In summary, hyperglycemia is a common complication following traumatic brain injury in children and has been linked to adverse outcomes in terms of mortality, long-term disability, and worse neurological status. More studies are needed to fully understand the underlying mechanisms of hyperglycemia in pediatric TBI and to develop effective strategies for prevention and treatment in TBI patients.
Hypoglycemia in adults and children with traumatic brain injury
Hypoglycemia, or low blood glucose levels, can also have a negative impact on traumatic brain injury (TBI) outcomes in both adults and children. Studies have found that hypoglycemia is associated with worse neurological outcomes and increased mortality rates in patients with TBI [9, 10] (Table 1).
In adults, hypoglycemia has been linked to poor neurological recovery and worse functional outcomes after TBI [27, 28]. A study of patients with severe TBI found that those who experienced hypoglycemia had a higher risk of death and worse functional outcomes at 6 months post-injury [17]. Additionally, hypoglycemia has been associated with longer hospital stays and higher healthcare costs in TBI patients [27].
Similarly, in children with TBI, hypoglycemia has been shown to be a risk factor for poor neurological outcomes and increased risk of mortality [28, 29]. A study of pediatric TBI patients found that those who experienced hypoglycemia were more likely to have worse neurological outcomes and longer hospital stays [24].
The mechanisms underlying the negative effects of hypoglycemia in TBI patients are not fully understood, but it is thought that hypoglycemia may exacerbate the damage to brain tissue caused by the initial injury. Additionally, hypoglycemia can cause a decrease in cerebral blood flow and energy availability, further compromising brain function [30, 31].
In summary, hypoglycemia can have a negative impact on TBI outcomes in both adults and children. It is important for healthcare providers to monitor and manage blood glucose levels in TBI patients to minimize the risk of hypoglycemia and its negative effects on neurological recovery and mortality rates.
Approach to patients with glucose metabolism disorders: diagnosis and treatment of hyper- and hypoglycemia in neurocritical care units
The diagnosis and treatment of hyperglycemia and hypoglycemia in TBI patients in the ICU is a complex process that requires careful monitoring and management by healthcare professionals.
Diagnosis of hyperglycemia in TBI patients is typically done through frequent blood glucose monitoring using a glucometer. The American Diabetes Association recommends maintaining blood glucose levels between 80 and 130 mg/dL in critically ill patients, including those with TBI [16]. However, some studies suggest that a blood glucose level of > 150 mg/dL in TBI patients may be associated with worse outcomes [32, 33].
Once hyperglycemia is diagnosed, treatment may include insulin therapy, which can be administered via continuous intravenous infusion or subcutaneous injection. The target blood glucose level for insulin therapy in TBI patients is generally < 180 mg/dL [32]. However, it is important to monitor for hypoglycemia, which can occur as a side effect of insulin therapy.
Diagnosis of hypoglycemia in TBI patients is typically done through blood glucose monitoring as well. The American Diabetes Association defines hypoglycemia as a blood glucose level of < 70 mg/dL [33, 34]. Treatment for hypoglycemia in TBI patients may include administration of glucose-containing fluids or foods, and in severe cases, intravenous glucose infusion.
Overall, the diagnosis and treatment of hyperglycemia and hypoglycemia in TBI patients in the ICU requires close monitoring and management by healthcare professionals. It is important to maintain appropriate blood glucose levels to optimize outcomes and minimize complications.
Conclusion
The monitoring and control of glucose should be an important part of the multimodal monitoring in patients with moderate to severe traumatic brain injury managed in neurocritical care units. There should be a management protocol that ensures normoglycemia and early detection and correction of its alteration, since if not treated in a timely manner, it can have a negative impact on survival and functional prognosis.
Availability of data and materials
Not applicable. Competing interests: No competing interest. The authors declare that they have no conflicts of interest.
Abbreviations
- TBI:
-
Traumatic brain injury
- WHO:
-
World Health Organization
- mmol/L:
-
Milimol per liter
- mg/dl:
-
Milligram per deciliter
- HbA1c:
-
Glucosilated hemoglobin A1C
- MAP:
-
Activated mitogen protein kinase
- AKT:
-
RAC(Rho family)-alpha serine/threonine-protein kinase
- IL:
-
Interleukine
- ICU:
-
Intensive care unit
- GLUT:
-
Glucose transporter
- CNS:
-
Central nervous system
- NMDA:
-
N-methyl-D-aspartate
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate reduced
- DMT1 y 2:
-
Diabetes mellitus type 1 and 2
References
Jin P, Zhu L, Zhang J, Xie S, Pan D, Wen H, et al. A correlation study of the expression of resistin and glycometabolism in muscle tissue after traumatic brain injury in rats. Chin J Traumatol. 2014;17(3):125–9.
Ley EJ, Srour MK, Clond MA, Barnajian M, Tillou A, Mirocha J, et al. Diabetic patients with traumatic brain injury: insulin deficiency is associated with increased mortality. J Trauma Inj Infect Crit Care. 2011;70(5):1141–4.
Zhang R, Wang ZY, Zhu LL, Wu F, Chen DQ, Sun LF, Lu ZQ. Resistin expression in adipose tissues and its effect on glucose metabolism in rats with brain injury. Genet Mol Res. 2016;15(1). https://doi.org/10.4238/gmr.15017659
Karelina K, Sarac B, Freeman LM, Gaier KR, Weil ZM. Traumatic brain injury and obesity induce persistent central insulin resistance. Eur J Neurosci. 2016;43(8):1034–43. https://doi.org/10.1111/ejn.13194.
Mowery NT, Gunter OL, Guillamondegui O, Dossett LA, Dortch MJ, Morris JA, et al. Stress insulin resistance is a marker for mortality in traumatic brain injury. J Trauma Inj Infect Crit Care. 2009;66(1):145–53.
Hamed SA. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev Clin Pharmacol. 2017;10(4):409–28.
Ley EJ, Srour MK, Clond MA, Barnajian M, Tillou A, Mirocha J, Salim A. Diabetic patients with traumatic brain injury: insulin deficiency is associated with increased mortality. J Trauma. 2011;70(5):1141–4. https://doi.org/10.1097/TA.0b013e3182146d66.
Zygun DA, Steiner LA, Johnston AJ, Hutchinson PJ, Al-Rawi PG, Chatfield D, et al. Hyperglycemia and brain tissue pH after traumatic brain injury. Neurosurgery. 2004;55(4):877–82.
Liu-DeRyke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care. 2009;11(2):151–7.
Salim A, Hadjizacharia P, Dubose J, Brown C, Inaba K, Chan LS, et al. Persistent hyperglycemia in severe traumatic brain injury: an independent predictor of outcome. Am Surg. 2009;75(1):25–9.
Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma Inj Infect Crit Care. 2003;55(6):1035–8.
Elkon B, Cambrin JR, Hirshberg E, Bratton SL. Hyperglycemia. Pediatr Crit Care Med. 2014;15(7):623–31.
Diaz-Parejo P, Ståhl N, Xu W, Reinstrup P, Ungerstedt U, Nordström C-H. Cerebral energy metabolism during transient hyperglycemia in patients with severe brain trauma. Intensive Care Med. 2003;29(4):544–50.
Young B, Ott L, Dempsey R, Haack D, Tibbs P. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Ann Surg. 1989;210(4):466–72.
Melo JRT, Di Rocco F, Blanot S, Laurent-Vannier A, Reis RC, Baugnon T, et al. Acute hyperglycemia is a reliable outcome predictor in children with severe traumatic brain injury. Acta Neurochir (Wien). 2010;152(9):1559–65.
Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma Inj Infect Crit Care. 2005;58(1):47–50.
Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34(3):850–6.
Saadat SMS, Bidabadi E, Saadat SNS, Mashouf M, Salamat F, Yousefzadeh S. Association of persistent hyperglycemia with outcome of severe traumatic brain injury in pediatric population. Child’s Nerv Syst. 2012;28(10):1773–7.
Yuan Q, Liu H, Xu Y, Wu X, Sun Y, Hu J. Continuous measurement of the cumulative amplitude and duration of hyperglycemia best predicts outcome after traumatic brain injury. Neurocrit Care. 2014;20(1):69–76.
Melo JRT, Reis RC, Lemos LP Jr, Coelho HMS, De ACER, Oliveira-Filho J. Hyperglycemia in pediatric head trauma patients: a cross-sectional study. Arq Neuropsiquiatr. 2009;67(3B):804–6.
Babbitt CJ, Halpern R, Liao E, Lai K. Hyperglycemia is associated with intracranial injury in children younger than 3 years of age. Pediatr Emerg Care. 2013;29(3):279–82.
Elkon B, Cambrin JR, Hirshberg E, Bratton SL. Hyperglycemia: an independent risk factor for poor outcome in children with traumatic brain injury. Pediatr Crit Care Med. 2014;15(7):623–31.
Bouzarth WF, Muizelaar JP. Hyperglycemia and pediatric TBI: a review. Pediatr Neurosurg. 2016;51(6):321–6.
Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents—second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82.
Bell MJ, Kochanek PM, Doughty LA, et al. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma. 1997;14(7):451–7.
Vavilala MS, Kernic MA, Wang J, et al. Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med. 2014;42(10):2258–66.
Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–807. https://doi.org/10.1016/S0140-6736(09)60553-5.
Bilotta F, Caramia R, Cernak I, Paoloni FP, Doronzio A, Cuzzone V, Santoro A, Rosa G. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit Care. 2008;9(2):159–66. https://doi.org/10.1007/s12028-008-9084-9.
Bromiker R, Perry A, Kasirer Y, Einav S, Klinger G, Levy-Khademi F. Early neonatal hypoglycemia: incidence of and risk factors. A cohort study using universal point of care screening. J Matern Fetal Neonatal Med. 2019;32(5):786–92. https://doi.org/10.1080/14767058.2017.1391781.
Ng YS, Mannion DJ, Dodd ME, et al. The effect of hypoglycaemia on brain injury severity and outcome in traumatic brain injury patients: a systematic review. J Trauma Acute Care Surg. 2014;76(3):804–11.
Tasker RC. Acute neurological complications in critically ill children. Curr Opin Crit Care. 2000;6(2):104–9.
American Diabetes Association. Standards of medical care in diabetes 2021. Diabetes Care. 2021;44(Suppl. 1):S1–232.
Badjatia N, Fernandez L, Schmidt JM, et al. Hyperglycemia during the stay in the intensive care unit and its impact on outcome after subarachnoid hemorrhage. Crit Care Med. 2010;38(2):637–42.
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013;36(5):1384–95.
Acknowledgements
None.
Funding
Not applicable. There was no funding.
Author information
Authors and Affiliations
Contributions
All the authors meet the authorship requirements, and they all made substantial contributions to the conception of the manuscript, and to conception and design, acquisition of data and the correspondent interpretation, drafting the article, and making the critically revision of the intellectual content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present manuscript has not been submitted to other journal for simultaneous consideration. The manuscript has not been published previously and is not split up in other parts, no data have been manipulated or fabricated, and no information is presented as if were from the authors.
Consent for publication
Valid informed written consent of the guardian was taken to publish this review. They were informed that the details of patient will not be disclosed.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quintana-Pajaro, L., Padilla-Zambrano, H.S., Ramos-Villegas, Y. et al. Cerebral traumatic injury and glucose metabolism: a scoping review. Egypt J Neurosurg 38, 62 (2023). https://doi.org/10.1186/s41984-023-00255-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-023-00255-4