Abstract
Background
Chronic subdural hematoma (cSDH) is a collection of old blood in the subdural space and has a relatively high estimated incidence, especially among the elderly and men, possibly due to falls, anticoagulant use, or age as independent factors. The subdural evacuating port system (SEPS) offers a minimally invasive solution for cSDH treatment.
Objective
The objective of our meta-analysis is to review the literature and assess the safety and efficacy of SEPS as a first-line treatment for cSDH.
Method
We conducted an exhaustive literature search to explore outcomes resulting from the implementation of SEPS as the initial treatment for cSDH. The main focus was on treatment success, comprising both symptom improvement and the absence of additional operating room interventions. Supplementary outcomes encompassed factors such as discharge arrangements, length of hospital stay (LOS), recurrence of hematoma, and any associated complications.
Result
A total of 15 studies, involving 1146 patients who underwent SEPS placement, satisfied the inclusion criteria. The combined rate of achieving a successful outcome stood at 0.79 (95% CI 0.75–0.83). The occurrence of delayed hematoma recurrence was found to be 0.155 (95% CI 0.101–0.208). Meanwhile, the aggregated inpatient mortality rate was 0.017 (95% CI 0.007–0.031). In terms of complications, the rates were 0.02 (95% CI 0.00–0.03) for any acute hemorrhage, 0.01 (95% CI 0.00–0.01) for acute hemorrhage necessitating surgery, and 0.02 (95% CI 0.01–0.03) for seizures. Notably, SEPS placement is associated with a success rate of 79% and exceptionally low incidences of acute hemorrhage and seizure.
Conclusion
SEPS is a viable first-line treatment for cSDH, supported by its minimally invasive nature, avoidance of general anesthesia, high success rate, and favorable safety profile.
Similar content being viewed by others
Introduction
A chronic subdural hematoma (cSDH) is a collection of blood in the subdural space that is 3 weeks old [1]. The incidence of cSDH has been estimated at 1.72–20.6 sufferers per 100,000 people, which is certainly a high incidence [2]. In addition, cSDH risk is relatively higher in the elderly and men than in the young or women [3, 4], which may be due to falls [5], use of anticoagulant therapy [6], or age as independent risk factors [7, 8]. cSDH has been found to progress in distinct steps from the time of its emergence to the time of its presentation and even thereafter. Therefore, its development is called a dynamic process [9]. Clinically, the progression of the hematoma is divided into three stages: the initial traumatic event, the latency period, and the clinical presentation period [10]. Patients may present without symptoms, or if symptoms are present, they can range from mild, such as headaches, to severe, such as ataxia, Parkinson's symptoms, etc. [11]. Various treatment modalities for cSDH have been described and have evolved over time, each having its advantages and disadvantages, such as. Surgical evacuation with burr holes, which is associated with higher recurrence rates [12], and middle meningeal artery embolization, which can be used either as a primary approach or as a surgical procedure for recurrent hematomas [13, 14]. However, with recent developments and to provide patients with the best possible care, new devices, and techniques are being discovered and experimented with, including the Subdural Evacuating Port System [SEPS], a new device intended to simplify the treatment of cSDH [15]. SEPS is a new technique with the advantage that it is the least invasive approach in the treatment of cSDH and can be performed under local anesthesia [16]. Today, SEPS is receiving greater attention in various hospitals and clinical settings due to its various advantages over traditional approaches such as burr-hole craniotomies, ranging from a shorter hospital stay to better postoperative prophylaxis to a reduced risk of complications such as seizures. SEPS also reduced the risk of cSDH recurrence by a significant proportion [17], originally estimated at up to 12–20% [18, 19]. However, because the technique is new, many institutes will hesitate to adopt it and think twice about what's best for their patients when some traditional approaches like Middle Meningeal artery (MMA) embolization and craniotomy are already available. Our study's objective is to conduct a systematic review of outcomes among patients who have utilized SEPS.
Procedure
The subdural evacuating port system (SEPS) is a minimally invasive procedure used for the treatment of chronic subdural hematoma. Before commencing the procedure, radiological imaging is performed to determine the precise location of the subdural fluid accumulation with maximum thickness.
Once the imaging is complete, the selected site is prepared with antiseptic solutions and draped to maintain a sterile field. Local anesthesia is administered at the chosen site using lidocaine with epinephrine to minimize bleeding and provide pain control. An incision of approximately five millimeters is made through the layers of the skin, subcutaneous tissue, galea, and periosteum. A self-retaining scalp retractor is then used to maintain access and visualization. Next, a twist drill hole is carefully created in the skull, allowing access to the underlying dura. The dura is incised using a unipolar cautery, providing a controlled opening for the SEPS placement. The SEPS device consists of a stainless-steel evacuating port, which is inserted into the twist drill hole in the skull. The other ends of the device include silicone tubing and a suction reservoir bulb, which are connected and securely fastened using silk ties. Once the SEPS is properly positioned and secured, the wound is closed, and a dressing is applied to protect the site. Following the procedure, the patient is kept in a supine position for a period of six to eight hours to ensure proper fluid drainage and minimize the risk of complications. After this period, the patient can gradually begin to mobilize under medical supervision.
The SEPS allows continuous drainage of the subdural fluid, which facilitates the gradual re-expansion of the compressed brain tissue. It is important to note that the SEPS procedure is performed using sterile techniques and adheres to the principles of patient safety and optimal surgical outcome [16, 20, 21].
Method
Search strategy
We conducted a comprehensive exploration of various databases, including but not limited to PubMed, Google Scholar, and Cochrane Library. This exhaustive search, encompassing all available data, was carried out on July 18, 2023. The complete MeSH phrase, which can be found in Additional file 1, was employed for reference. Specifically, we utilized the MeSH phrases "Subdural evacuating portal system OR SEP AND Chronic subdural hematoma OR CSDH" during the search process. The search was exclusively conducted in English. The references within these materials were diligently sought and reviewed by the authors. This meticulous approach was undertaken to ensure the inclusion of all relevant articles and to prevent the inadvertent omission of any pivotal studies. To ensure a thorough approach, we also considered supplementary sources to identify pertinent records. The search strategy was independently formulated by two authors, Dr. MAS, and SMSA adhering to specified criteria. Any inconsistencies or uncertainties that arose were harmonized through consensus discussions involving MSM, a third investigator.
Inclusion and exclusion criteria
The selection process for appropriate studies adhered to specific Population, Intervention, Comparison, and Outcome (PICO) criteria. Inclusion involved studies that explored the utilization of SEPs as the intervention for the treatment of chronic subdural hematoma (cSDH) within a population aged 18 years and above. Preference was given to research plans employing case series or case–control methodologies while focusing on investigations that assessed the implementation and outcomes of SEPs in cSDH cases. On the other hand, exclusion criteria comprised studies involving patients below 18 years of age, as well as materials categorized as "letter to the editors," editorials, review articles, or correspondence. This systematic approach aimed to ensure the selection of relevant and valuable studies while excluding materials that didn't align with the study's scope and objectives.
Protocol
We adhered to the recommended procedures outlined in the Cochrane Handbook of Systematic Review and Interventions for conducting systematic reviews and meta-analyses. Additionally, we applied the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) principles throughout our study. To ensure transparency and rigor, our study protocol was submitted to Prospero (CRD42023449993) and underwent comprehensive discussion and evaluation.
Data extraction
MAS and SMSA independently extracted the data from the selected studies. To help settle any disputes or controversies, a third author, AH, was consulted. The data were reviewed for duplicate research after being extracted. The extracted data included the name of the author, publication year, sample size, presenting symptoms, the characteristics of the hematoma (volume, thickness, and midline shift), the type and duration of the intervention (SEPs), and the results (Seizure Rate, Non-routine discharge Disposition, the proportion with recurrence, Overall Hemorrhage Rate, Reoperation for Hematoma, Length of Hospital Stay, Success Rate and Hemorrhage Rate requiring Surgery).
Data analysis
Data integration and analysis were carried out using OpenMeta-Analyst, independently conducted by author MSM. Descriptive statistics encompassed study-specific sample sizes for weighing means and standard deviations, while proportions were computed by relating patient numbers. Binary outcomes employed the Der Simonian-Laird approach, and continuous outcomes utilized a weighted mean technique within a random effects model, yielding combined estimates and 95% confidence intervals. Heterogeneity was assessed through the Higgins I2 statistic, with values above 50% indicating significance. Sensitivity analysis using leave-one-out identified heterogeneity-contributing studies, with a resulting plot in Additional file 1.
Quality assessment and risk of bias
AH conducted an independent assessment of study quality using the Newcastle Ottawa scale (Fig. 2). This evaluation considered aspects such as outcome data participant blinding, random sequence generation, outcome assessment, and other potential validity concerns. Each study was assigned a risk of bias grade, categorized as low, high, or unsure, for each variable. Discrepancies that emerged during this evaluation were resolved through consensus with a third researcher, MAS. Publication bias was appraised via funnel plot, Egger's test, and the Duval trim and fill method, visually presented in Additional file 1.
Results
Search result
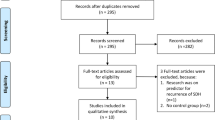
A total of 15 studies met inclusion criteria and were included in the meta-analysis. This included ten case series and five observational studies, all of which were retrospective (Fig. 1). All the included studies are of high methodological quality, assessed by Newcastle Ottawa scale and shown in Fig. 2.
Description of studies
The research encompassed a selection of 15 studies, which collectively involved 1146 patients with a mean age of 71 ± 5 years (ranging from 56.4 to 83.9). Neurologic deficit (focal paresis or aphasia) emerged as the most prevalent presenting symptom among the twelve studies, accounting for 44.2% of cases, followed by altered mental status (34.1%), headache (34.1%), ataxia/gait disturbance (30.9%), and seizure (2%). The average Glasgow Coma Scale (GCS) score at presentation was 13.7 ± 0.5, with a range of 13.2 to 14.5. On presentation, close to 43.6% of patients were using antiplatelet or anticoagulant medication. Septations were found in a minority of patients (25.7%), while over half of the patients had isodense/subacute, mixed density, or acute on chronic appearing subdural hematomas (58.3%). The average follow-up duration was 3.35 ± 1.9 months, ranging from 1 to 11 months. The patients underwent SEPS placement with the administration of local anesthesia and moderate sedation. In some studies, the positioning of patients during the procedure varied. Two patients were placed flat, while in one study, patients were positioned in the Trendelenburg position. Baseline characteristics of included studies is shown in Table 1.
Proportion of successful outcomes
The data regarding proportion of successful outcomes was extracted from 14 studies. The pooled success rate was 79%. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p = 0.002, I2 = 60%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled rate 0.79, 95% CI 0.74–0.83, p = 0.002) (Fig. 3). Leave one out analysis was done and plot was included in the Additional file 1.
Length of stay
The data for length of stay was extracted from 6 studies. The length of stay was found to be 6.75 days. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p < 0.001, I2 = 97%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled LOS 6.75, 95% CI 5.51–7.99, p < 0.001) (Fig. 4).
Discharge disposition
The data for non-routine discharge disposition was extracted from 4 studies. The non routine discharge disposition was found to be 35.3%. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p < 0.001, I2 = 91%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled rate 0.35, 95% CI 0.21–0.50, p < 0.001) (Fig. 5).
Hematoma recurrence
The data for delayed hematoma was extracted from 10 studies. The pooled rate of hematoma recurrence was found to be 16%. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p < 0.001, I2 = 72%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled rate 0.16, 95% CI 0.10–0 21, p < 0.001) (Fig. 6).
Hematoma reoperation
The data for Reoperation of hematoma was extracted from 3 studies. The pooled rate of hematoma reoperation was found to be 9%. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p < 0.019, I2 = 75%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled rate 0.09, 95% CI 0.01–0.18, p < 0.019) (Fig. 7).
Inpatient mortality
The data for inpatient mortality was extracted from 7 studies. The pooled rate of inpatient mortality was found to be 1.7%. Upon conducting a sensitivity analysis, the results revealed insignificant heterogeneity between the studies (p = 0.618, I2 = 0%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically insignificant (pooled rate 0.02, 95% CI 0.01–0.03, p = 0.618) (Fig. 8).
Hemorrhage rate
The data for overall hemorrhage rate was extracted from 8 studies. The pooled rate of hemorrhage rate was found to be 2%. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p < 0.227, I2 = 25%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled rate 0.02, 95% CI 0.01–0.03, p = 0.227) (Fig. 9).
Hemorrhage rate requiring surgery
The data for hemorrhage rate requiring surgery was extracted from 7 studies. The pooled hemorrhage rate requiring surgery was found to be 5%. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p < 0.001, I2 = 88%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled rate 0.05, 95% CI 0.01–0.08, p < 0.001) (Fig. 10).
Seizure rate
The data for seizure rate was extracted from 8 studies. The pooled seizure rate was found to be 2%. Upon conducting a sensitivity analysis, the results revealed significant heterogeneity between the studies (p < 0.106, I2 = 41%). Despite this heterogeneity, the analysis indicated that the difference between studies was statistically significant (pooled rate 0.02, 95% CI 0.01–0.04, p < 0.106). Study, conducted by Golub in 2020, exclusively examined the utilization of anti-epileptic medications. In this study, all patients received prophylactic treatment with levetiracetam for a duration of 14 days (Fig. 11).
Discussion
Chronic subdural hematoma (cSDH) refers to a confined accumulation of fluid, blood, and degraded blood components located between the arachnoid and dura mater layers covering the brain's surface [35, 36]. The preferred surgical approaches for treating this condition have been a subject of ongoing debate. Historically, craniotomy and bur hole surgery were the primary treatment modalities [37,38,39]. However, recently, procedures such as SEPS and other minimally invasive techniques like middle meningeal artery embolization have gained traction among surgeons [40, 41]. The objective of our meta-analysis is to assess the potential of SEPS as a primary treatment option for CSDH. Despite a prior meta-analysis incorporating SEPS outcomes, numerous new series have emerged since that study was published. In this comprehensive review, which includes data from 15 studies involving 1146 patients who underwent SEPS treatment, we observed significantly favourable results in terms of procedural success, accompanied by low rates of morbidity and mortality.
The success rate of SEPS in our analysis was found to be 79%, consistent with previous studies [25]. However, variations in success rates have been observed across different studies, with Tanveer et al. reporting a higher success rate of 93% attributed possibly to a younger population [27]. Conversely, other studies showed lower success rates around 60% [28]. Mooney and colleagues, as well as Ortiz and Bazler's studies, revealed success rates below 70% [23, 24, 28]. Mooney's study included a population with a high prevalence of hypertension (90%) and traumatic brain injury (TBI) patients [23]. Ortiz's study also exhibited a relatively low success rate and necessitated subsequent procedures; notably, none of the patients in their study underwent craniotomy [24]. Comparatively, MMA embolization demonstrated a higher success rate of 93% [42]. Notably, while surgical evacuation has a relatively low failure rate of 3–5%, it is accompanied by a higher risk of side effects [43, 44].
The management of seizure rates in cSDH patients has evolved through approaches like SEPS and Middle Meningeal Artery (MMA) embolization [45]. Our analysis revealed a seizure rate of 2% following SEPS, a figure comparable to rates observed with burr-hole craniostomy [BHC] or craniotomy [46, 47]. Golub et al. identified a significantly lower seizure incidence with SEPS compared to craniotomy [17, 21]. However, variations in seizure rates are evident across studies, indicating the need for further investigation [16, 22, 31].
Regarding hematoma recurrence, our meta-analysis indicated a pooled recurrence rate of 16% following SEP procedures. Notably, individual studies showed varying recurrence rates, emphasizing the effectiveness of SEP in managing cSDH [17, 31, 34]. Haemorrhage rates necessitating surgical intervention were found to be 5%, with reoperation rates for hematoma at 9%. The consideration of non-routine discharge disposition in cSDH management revealed rates ranging from 18.9% to 52%, underscoring the complexity of patient outcomes [21, 23, 26]. Furthermore, the length of stay (LOS) following SEPS treatment was found to be 6.75 days, which aligns with some surgical studies but is notably lower than others [48,49,50].
Limitations
This study is constrained by several limitations. Firstly, we conducted a single-arm analysis and omitted data that directly contrasts SEPS with alternative treatments or control groups. Drawing comparisons with published cohorts that underwent procedures like BHC or craniotomy might be flawed due to disparities in baseline patient characteristics. Those patients might have been chosen for those treatments based on larger hematoma volumes or worse neurological conditions, inherently raising their chances of an unfavorable outcome. Secondly, not all the studies we included provided information on all the variables of interest. Certain outcomes, such as Length of Stay (LOS) and post-treatment disposition, were only documented in a subset of the analyzed studies, introducing variability. Likewise, there were inconsistencies among studies regarding the clinical or radiographic criteria used to define a successful outcome. While each study referred to symptomatic or radiographic improvement in chronic subdural hematoma (cSDH), slight variations in these criteria contributed to the heterogeneity of the composite outcome measure. Lastly, the strength of meta-analyses hinges on the quality of the studies they encompass. In this instance, all 12 studies were retrospective, potentially introducing bias in the selection of patients for SEPS and consequently influencing the outcomes.
The limitations inherent to our investigation of chronic subdural hematoma management using the subdural evacuating port system encompass a paucity of available research, dependence on case series and case–control studies, data availability challenges, and the difficulty of sourcing sufficient randomized controlled trials (RCTs). Furthermore, our search was limited to English language studies, thus excluding any relevant research published in other languages from our results.
Conclusions
In conclusion, our study supports SEPS as a viable first-line approach for cSDH treatment due to its minimally invasive procedure and lack of requirement for general anaesthesia, enhancing its safety profile. The substantial success rate and favourable safety record underscore its potential advantages. Nevertheless, comprehensive randomized clinical studies are crucial to authenticate and contrast these findings against conventional surgical choices and emerging innovative techniques like MMA embolization.
Abbreviations
- cSDH:
-
Chronic subdural hematoma
- SEPS:
-
Subdural evacuating port system
- LOS:
-
Length of hospital stay
- TBI:
-
Traumatic brain injury
- MMA:
-
Middle Meningeal Artery
- BHC:
-
Burr-hole craniostomy or craniotomy
- RCTs:
-
Randomized controlled trials
- GCS:
-
Glasgow Coma Scale
- AMS:
-
Altered mental state
References
Májovský M, Netuka D. Chronic subdural hematoma - review article. Rozhl Chir. 2018;97(6):253–7.
Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020;141:339–45.
Uno M, Toi H, Hirai S. Chronic subdural hematoma in elderly patients: Is this disease benign? Neurol Med Chir. 2017;57(8):402–9.
Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci. 2018;50:7–15.
Ha V-AT, Nguyen TN, Nguyen TX, Nguyen HT, Nguyen TT, Nguyen AT, et al. Prevalence and factors associated with falls among older outpatients. Int J Environ Res Public Health. 2021;18(8):4041.
Bartolazzi F, Ribeiro ALP, de Sousa WJFN, Vianna MS, da Silva JLP, Martins MAP. Relationship of health literacy and adherence to oral anticoagulation therapy in patients with atrial fibrillation: a cross‐sectional study. J Thromb Thrombolysis. 2021:1–7.
Nyberg L, Boraxbekk CJ, Sörman DE, Hansson P, Herlitz A, Kauppi K, et al. Biological and environmental predictors of heterogeneity in neurocognitive ageing: Evidence from Betula and other longitudinal studies. Ageing Res Rev. 2020;64:101184.
Rauhala M, Helén P, Huhtala H, Heikkilä P, Iverson GL, Niskakangas T, et al. Chronic subdural hematoma—incidence, complications, and financial impact. Acta Neurochir. 2020;162(9):2033–43.
Cecchini G. Chronic subdural hematoma pathophysiology: a unifying theory for a dynamic process. J Neurosurg Sci. 2017;61(5):536–43.
Yang W, Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am. 2017;28(2):205–10.
Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg. 2016;11(4):330–42.
Liu W, Bakker NA, Groen RJM. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures: a systematic review. J Neurosurg. 2014;121(3):665–73.
Link TW, Boddu S, Marcus J, Rapoport BI, Lavi E, Knopman J. Middle meningeal artery embolization as treatment for chronic subdural hematoma: a case series. Oper Neurosurg. 2018;14(5):556–62.
Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017;101:520–7.
Lollis SS, Wolak ML, Mamourian AC. Imaging characteristics of the subdural evacuating port system, a new bedside therapy for subacute/chronic subdural hematoma. AJNR Am J Neuroradiol. 2006;27(1):74–5.
Liu T, Gao Z, Zhou J, Lai X, Chen X, Rao Q, et al. Subdural evacuating port system with subdural thrombolysis for the treatment of chronic subdural hematoma in patients older than 80 years. Front Neurol. 2023;14:1068829.
Golub D, Ashayeri K, Dogra S, Lewis A, Pacione D. Benefits of the subdural evacuating port system (SEPS) procedure over traditional craniotomy for subdural hematoma evacuation. The Neurohospitalist. 2020;10(4):257–65.
Oh HJ, Lee KS, Shim JJ, Yoon SM, Yun IG, Bae HG. Postoperative course and recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2010;48(6):518–23.
González-Vargas PM, Thenier-Villa JL, Calero Félix L, Galárraga Campoverde RA, Martín-Gallego Á, de la Lama Zaragoza A, et al. Factors that negatively influence the Glasgow Outcome Scale in patients with chronic subdural hematomas. An analytical and retrospective study in a tertiary center. Interdiscip Neurosurg. 2020;20:100.
Golub D, Ashayeri K, Dogra S, Lewis A, Pacione D. Benefits of the SUBDURAL EVACUATING PORT SYstem (SEPS) procedure over traditional craniotomy for subdural hematoma evacuation. Neurohospitalist. 2020;10(4):257–65.
Hoffman H, Ziechmann R, Beutler T, Verhave B, Chin LS. First-line management of chronic subdural hematoma with the subdural evacuating port system: Institutional experience and predictors of outcomes. J Clin Neurosci. 2018;50:221–5.
Mohan A, Malnik S, Grady C, Lucke-Wold B, Kubilis P, Hoh BL. Inversed probability case-control analysis of operative burr hole evacuation versus subdural evacuating port system for chronic subdural hematomas: Clinical and economic outcomes. Clin Neurol Neurosurg. 2022;220:107356.
Mooney J, Erickson N, Saccomano B, Maleknia P, Fisher WS 3rd. Predictors and outcomes of subdural hematomas managed via subdural evacuation port system. World Neurosurg X. 2023;17:100145.
Ortiz M, Belton P, Burton M, Litofsky NS. Subdural drain versus subdural evacuating port system for the treatment of nonacute subdural hematomas: a single-center retrospective cohort study. World Neurosurg. 2020;139:e355–62.
Hoffman H, Jalal MS, Bunch KM, Chin LS. Management of chronic subdural hematoma with the subdural evacuating port system: systematic review and meta-analysis. J Clin Neurosci. 2021;86:154–63.
Flint AC, Chan SL, Rao VA, Efron AD, Kalani MA, Sheridan WF. Treatment of chronic subdural hematomas with subdural evacuating port system placement in the intensive care unit: evolution of practice and comparison with bur hole evacuation in the operating room. J Neurosurg. 2017;127(6):1443–8.
Tanweer O, Frisoli FA, Bravate C, Harrison G, Pacione D, Kondziolka D, et al. Tranexamic acid for treatment of residual subdural hematoma after bedside twist-drill evacuation. World Neurosurg. 2016;91:29–33.
Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015;123(5):1209–15.
Safain M, Roguski M, Antoniou A, Schirmer CM, Malek AM, Riesenburger R. A single center’s experience with the bedside subdural evacuating port system: a useful alternative to traditional methods for chronic subdural hematoma evacuation. J Neurosurg. 2013;118(3):694–700.
Neal MT, Hsu W, Urban JE, Angelo NM, Sweasey TA, Branch CL Jr. The subdural evacuation port system: outcomes from a single institution experience and predictors of success. Clin Neurol Neurosurg. 2013;115(6):658–64.
Singla A, Jacobsen WP, Yusupov IR, Carter DA. Subdural evacuating port system (SEPS)–minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg. 2013;115(4):425–31.
Rughani AI, Lin C, Dumont TM, Penar PL, Horgan MA, Tranmer BI. A case-comparison study of the subdural evacuating port system in treating chronic subdural hematomas. J Neurosurg. 2010;113(3):609–14.
Kenning TJ, Dalfino JC, German JW, Drazin D, Adamo MA. Analysis of the subdural evacuating port system for the treatment of subacute and chronic subdural hematomas. J Neurosurg. 2010;113(5):1004–10.
Asfora WT, Schwebach L. A modified technique to treat chronic and subacute subdural hematoma. Surg Neurol. 2003;59(4):329–32.
Patil H, Gupta R. A comparative study of bolus dose of hypertonic saline, mannitol, and mannitol plus glycerol combination in patients with severe traumatic brain injury. World Neurosurg. 2019;125:e221–8.
Uno M. Chronic subdural hematoma-evolution of etiology and surgical treatment. Neurol Med Chir. 2023;63(1):1–8.
Duerinck J, Van Der Veken J, Schuind S, Van Calenbergh F, van Loon J, Du Four S, et al. Randomized trial comparing burr hole craniostomy, minicraniotomy, and twist drill craniostomy for treatment of chronic subdural Hematoma. Neurosurgery. 2022;91(2):304–11.
Thavara BD, Kidangan GS, Rajagopalawarrier B. Comparative study of single Burr-Hole craniostomy versus twist-drill craniostomy in patients with chronic subdural hematoma. Asian J Neurosurg. 2019;14(2):513–21.
Chari A, Kolias AG, Santarius T, Bond S, Hutchinson PJ. Twist-drill craniostomy with hollow screws for evacuation of chronic subdural hematoma. J Neurosurg. 2014;121(1):176–83.
Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg. 2019;122:613–9.
Okuma Y, Hirotsune N, Sato Y, Tanabe T, Muraoka K, Nishino S. Midterm follow-up of patients with middle meningeal artery embolization in intractable chronic subdural hematoma. World Neurosurg. 2019;126:e671–8.
Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP, et al. Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J Neurointerv Surg. 2021;13(10):951–7.
Sattari SA, Yang W, Shahbandi A, Feghali J, Lee RP, Xu R, et al. Middle meningeal artery embolization versus conventional management for patients with chronic subdural hematoma: a systematic review and meta-analysis. Neurosurgery. 2023;92(6):1142–54.
Ozevren H, Cetin A, Hattapoglu S, Baloglu M. Burr hole and craniotomy in the treatment of subdural hematoma: a comparative study. Niger J Clin Pract. 2022;25(7):1056–60.
Brinjikji W. Particle theory in middle meningeal artery embolization for chronic subdural hematoma. Interv Neuroradiol. 2022;28(2):131.
Yuan Y, Peizhi Z, Xiang W, Yanhui L, Ruofei L, Shu J, et al. Intraoperative seizures and seizures outcome in patients undergoing awake craniotomy. J Neurosurg Sci. 2019;63(3):301–7.
Sastry RA, Pertsch N, Tang O, Shao B, Toms SA, Weil RJ. Frailty and outcomes after craniotomy or craniectomy for atraumatic chronic subdural hematoma. World Neurosurg. 2021;145:e242–51.
Roohollahi F, Kankam SB, Shafizadeh M, Khoshnevisan A. A prospective randomized controlled trial of the effect of the number of burr hole on chronic subdural hematoma recurrence: an institutional experience. Clin Neurol Neurosurg. 2023;226:107624.
Smith MD, Kishikova L, Norris JM. Surgical management of chronic subdural haematoma: One hole or two? Int J Surg. 2012;10(9):450–2.
Kaliaperumal C, Khalil A, Fenton E, Okafo U, Kaar G, O’Sullivan M, et al. A prospective randomised study to compare the utility and outcomes of subdural and subperiosteal drains for the treatment of chronic subdural haematoma. Acta Neurochir. 2012;154(11):2083–8 (discussion 8–9).
Acknowledgements
Not applicable.
Funding
The authors received no extramural funding for the study.
Author information
Authors and Affiliations
Contributions
MAS did the conceptualization. SPNA and SMSA conducted the literature search and screening. Drafting of the manuscript was done by MAS, AH, AR, SMSA, and NA. Analysis was done by M.S.M. MAS and MSM performed the editing and supervision. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests and there is no conflict of interest.
Compliance with instructions to authors
We hereby affirm that this manuscript has been meticulously prepared in strict accordance with all prescribed instructions provided to the authors.
Authorship confirmation and approval
We confirm that the authorship requirements have been diligently met, and the final version of the manuscript has been unanimously approved by all contributing authors.
Publication status
We certify that this manuscript is entirely original and has not been published previously, nor is it currently under consideration by any other journal.
Reporting checklist
We followed the PRISMA Guidelines for this systemic review and Meta-analysis and the checklist has been included in the files.
Ethical consideration
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary material encompassing the PubMed search string, funnel plots, Egger test results, and sensitivity analysis plots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shafique, M.A., Mustafa, M.S., Haseeb, A. et al. Subdural evacuating port system for chronic subdural hematoma: a systematic review and meta-analysis of clinical outcomes. Egypt J Neurosurg 38, 76 (2023). https://doi.org/10.1186/s41984-023-00251-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-023-00251-8