Abstract
Introduction
Conservative therapy is a viable option for patients with chronic subdural hematoma (cSDH) who express no, or only mild symptoms. It is not clear which factors are associated with success of conservative therapy. This study aims to determine conservative therapy's success rate and to identify features possibly associated with success.
Methods
A monocenter retrospective cohort study, including cSDH patients treated conservatively (wait-and-watch) from 2012 to 2022, was performed. The primary outcome was success of conservative therapy, defined as ‘no crossover to surgery’ during the follow-up period. Secondary outcomes were (1) factors associated with success, analyzed with univariate and multivariable logistic regression analyses, (2) 30-day mortality (3) time to crossover and (4) reasons for crossover.
Results
We included 159 patients. Conservative therapy was successful in 96 (60%) patients. Hematoma volume (OR 0.79, 95% CI 0.69–0.92) and hypodense hematoma type (OR 3.57, 95% 1.38–9.23) were associated with success. Thirty-day mortality rate was 5% and the median duration between diagnosis and surgery was 19 days (IQR 8–39). Clinical deterioration was the most frequent reason for crossover (in 61/63 patients, 97%) and was accompanied by radiological hematoma progression in 42 patients (67%).
Conclusion
In this selected group of patients, conservative therapy was successful in 60%. Smaller hematoma volume and hypodense hematoma type were associated with success. As time until crossover was approximately three weeks, deploying conservative therapy as primary treatment seems safe and could be rewarding as surgical complications can be avoided. Improvement in patient selection in future cohorts remains warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic subdural hematoma (cSDH) predominantly affects elderly and has an estimated incidence of 8.1–58 per 100,000 per year for patients older than 65 years [1, 2]. Because of the aging population the prevalence of cSDH is expected to increase rapidly in the near future [3]. Clinical presentation can differ concerning the type and severity of symptoms. Some patients experience none, or very mild symptoms.
Surgical therapy is the mainstay of treatment for patients with severe symptoms, such as diminished level of consciousness, hemiparesis or intractable headache [4]. However, especially in this often frail population, surgery comes with complications and an increased risk of mortality and disability, leaving patients dependent on care [5, 6]. Patients with no or relatively mild symptoms, can be managed conservatively by employing a ‘wait-and-watch’ or ‘wait-and-scan’ policy with regular outpatient clinic visits and additional CT-scans if necessary [7]. Strict criteria for conservative or surgical treatment, or general guidelines for cSDH treatment, are not available, leading to considerable treatment variation between hospitals and physicians [8,9,10].
Existing cSDH literature is mainly focused on surgically treated patients. Studies regarding the effect of conservative therapy are scarce and mostly case reports or series concerning selective, non-consecutive populations [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. A recent systematic review however, reported that success of conservative therapy could be achieved in 60% of all cases [32]. Unfortunately, consistent factors influencing success could not be established. In absence of these parameters, clinicians face challenges in adequately assessing whether the potential benefits and risks of surgery outweigh those of initiating or continuing conservative therapy. This study aims to evaluate the success of initial conservative therapy on a patient level, as well as on hematoma level, as patients with unilateral or bilateral hematoma are not equal, and to identify factors associated with success, in a large consecutive cohort of patients.
Methods
We performed a single-center, retrospective cohort study at the Amsterdam University Medical Centers, location AMC, a tertiary academic hospital. A list of all consecutive cSDH patients treated at our institution between 2012 and 2022 was comprised. Patients were screened for study eligibility (by MF and RL), using pre-specified in- and exclusion criteria. Patients, aged over 18 years, were included if they had a diagnosis of cSDH on radiological imaging, confirmed by a neuroradiologist, and if the primary treatment was conservative therapy, defined as ‘wait-and-watch therapy’. If there was uncertainty about whether a patient was eligible for inclusion, a third adjudicator (MS, neurosurgeon) was consulted for the final decision. Patients were excluded if conservative treatment contained medication (steroids or tranexamic acid) or any intervention (middle meningeal artery embolization), if they were included in an ongoing randomized controlled trial (TORCH-study [33]), if the cSDH developed after a craniotomy, if a cerebrospinal fluid shunt was in situ, or in case of withdrawal of therapy. In general, anticoagulant (AC) and antiplatelet (AP) therapy was discontinued at diagnosis. Only if there was a strong indication, such as a mechanical heart valve or recent myocardial or cerebral infarction, AC and AP therapy were continued. The local ethics committee determined that this study did not fall under the Medical Research Involving Human Subjects Act (WMO), and we obtained a waiver for official ethical approval (waiver number: W22_098#22.136).
Outcomes
The primary outcome was success of conservative therapy, defined as ‘no crossover to surgery’ during the follow-up period. Conservative therapy was also deemed unsuccessful for patients with a bilateral cSDH if unilateral surgical evacuation was performed during follow-up. In order to study hematoma-specific characteristics, success of conservative therapy was also analyzed per hematoma. For this analysis, patients with a bilateral hematoma were considered as two separate cases and success of conservative therapy could be achieved per hematoma. Secondary outcomes were factors associated with success of conservative therapy, 30-day mortality rate and for those who crossed over to surgery, the time-to-crossover and reasons for crossover to surgery.
Data collection
From the patients’ medical file we retrieved the patients demographics, medical history, use of anticoagulant or antiplatelet therapy, statins or ACE-inhibitors and clinical features at diagnosis, as well as reasons for crossover to surgery, the time between diagnosis and eventual surgery and 30-day mortality. An aSDH in history was defined as ‘an aSDH in the year prior to diagnosis of cSDH located on the same convexity as the cSDH’. Patients were classified as having a motor deficit at diagnosis if they exhibited any form of motor function loss (according to the Medical Research Council scale (MRC)). Radiological characteristics retrieved from reports included hematoma laterality, maximum diameter and presence and amount of midline shift. If one or multiple of these radiological variables were not described in the radiological report, these parameters were measured manually, as was hematoma volume using Brainlab software (Brainlab AG, Munchen, Germany) and hematoma type. Hematomas were categorized as either mixed or homogeneous [31, 34]. The homogeneous type was further classified based on Hounsfield Units (HU) into hypodense (HU < 25), isodense (HU 25–35) and hyperdense (HU > 35) hematomas (see Figs. 1 and 2). Data were stored using an online database (Castor EDC).
After diagnosis, patients were followed either by a neurologist or a neurosurgeon. Follow-up was primarily performed in our hospital, but in a small fraction of patients, follow-up was performed by the referring neurologist. Duration of follow-up was calculated from the date of diagnosis until date of last available outpatient clinic visit, or date of the last available head CT-scan. If follow-up data from another hospital was available, this was used.
Statistical analysis
Patient characteristics were compared using parametric and non-parametric tests. The normality of continuous variables was assessed with the Shapiro–Wilk test and considered normally distributed with a value > 0.9. A mean standard deviation (SD) were calculated for normally distributed variables. A median and interquartile range (IQR) was calculated for not normally distributed variables. Baseline characteristics between both groups (success vs. crossover) were compared using appropriate tests (Mann–Whitney U test, Chi-squared test, Fisher’s exact test and independent samples t test). Missing baseline values (see Table 1) were imputed 20 times using chained equations, assuming a missing at random (MAR) pattern. All available baseline variables, three auxiliary variables (center and year of diagnosis and presence of an arachnoid cyst) and the outcomes success of conservative therapy and 30-day mortality were used to impute missing values. Predictors for the success of conservative treatment per patient were assessed with univariate and multivariable logistic regression models. Variables with p < 0.2 in univariate analyses were included in the multivariable regression model. The pooled estimates across 20 imputed datasets of the multivariable analyses were calculated using Meng and Rubin’s rules. A similar, separate analysis, was performed to determine success of conservative therapy per hematoma. For this analysis, hematoma laterality (uni- vs. bilateral hematoma), hematoma type, hematoma diameter and volume were used to impute missing hematoma baseline values. All statistical analyses were performed with IBM SPSS statistics, version 28.0 and R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/) [35,36,37].
Results
Baseline characteristics
A total of 159 patients were included. The mean age of all patients was 70.5 years (SD 12.8). Of these 117 (74%) were male (Table 1). Nineteen (11.9%) patients had motor deficits at diagnosis. All patients, except one, had only a mild form (MRC > 3) of motor deficit. The patient with severe muscle weakness most likely suffered Todd’s paralysis since it appeared after a seizure. The weakness improved gradually after treatment with antiepileptic medication. Two patients had a MGS score of more than two at diagnosis. This high grade was based on multi-morbidity as one patient presented with additional pneumothorax, costal fractures and cerebral contusion, and one patient had concomitant skull fractures with cerebral contusions, costal fractures and a scapula fracture. Ninety-five (60%) patients had a unilateral cSDH and 64 patients had a bilateral hematoma. Thus, a total of 223 hematomas were included. Anticoagulant or antiplatelet therapy was discontinued in 64 out of 70 patients. The characteristics per hematoma are described in Table 2. Midline shift was present in 92 (58%) cases, with a median of 3 mm (IQR 0–5). Notably, nine of these cases had a shift exceeding 10 mm. Mean hematoma diameter and mean hematoma volume were 13.7 mm (SD 6.9) and 7.1 cl (SD 3.7), respectively.
Success rate and factors associated with success
Success of conservative therapy was achieved in 96 (60%) patients. Patients in the success group had a significantly smaller hematoma volume than in the crossover group (6.2 centiliters vs. 8.5 centiliters, p < 0.001), see Table 1. In multivariable regression analysis, only smaller hematoma volume was associated with success of conservative therapy (OR 0.79 95% CI 0.69–0.92, Table 3). Success of conservative therapy was achieved in 142 hematomas (64%). In multivariable regression analysis, hypodense hematoma type (OR 3.57, 95% CI 1.38–9.23) and volume (OR 0.76, 95% CI 0.67–0.87) were associated with success of conservative therapy (Tables 2 and 3).
Other outcomes
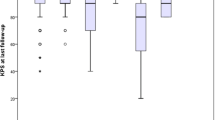
The median follow-up in the total study population was 74 days (IQR 28–133). The median follow-up of the success group was shorter than the crossover group (52 vs. 79 days). Seven (5%) patients died within 30 days after diagnosis (7.6% in the success group vs. 1.6% in the crossover group, p = 0.135). Five patients died from a cause not directly related to the cSDH (two due to sepsis, one aspiration pneumonia, one respiratory insufficiency and one gastrointestinal bleeding due to a peptic ulcer with aspiration). Four of these were initially hospitalized because of their cSDH. In two patients, the cause of death remained unknown. In the crossover group, clinical deterioration (61/63 (97%) patients) was the most common reason for surgery (Table 4). In those patients, clinical deterioration was accompanied by radiological progression of the hematoma in 42 patients (67%). Notably, pre-operative imaging was not repeated for all patients (n = 5, 8%) with clinical deterioration. For these patients, the average period between diagnosis and surgery was five days. In two patients, radiological growth of the hematoma was the only reason for crossover to surgery. In one of these patients an additional argument for surgery was that the neurological symptoms at diagnosis did not resolve over time. The median period between diagnosis and surgery was 19 days (IQR 8–39).
Discussion
In this retrospective, single-center cohort study, conservative treatment was successful in 60% of selected cSDH patients with no, or only mild, symptoms. Small hematoma volume and hypodense hematoma type were associated with success of conservative therapy.
Crossover rates reported in the literature are based on case reports, case series, and small retrospective cohort studies, but large prospective series are missing. Consequently, crossover rates vary considerably, from 0–7% [23, 25] to 84–93% [16, 22]. The main reason for this variation is selection bias, leading to study population heterogeneity. Some studies exclude patients with large hematomas or those with significant mass effect, resulting in a low crossover rate [25]. Other studies include only patients with a midline shift greater than 10 mm, resulting in a high crossover rate [22]. Severity of neurological deficits (quantified by MGS or GCS) is another critical factor influencing the crossover rate. In studies reporting crossover rates for patients with a maximum GCS or minimal MGS, rates are as low as 0–1%) compared to studies that include more severely affected patients [23, 30]. These variable in- and exclusion criteria complicate direct comparisons and prohibit drawing definitive conclusions. Our study did not apply these specific criteria as we aimed to quantify the crossover rate in a population representative of a significant portion of cSDH patients. Therefore, we argue that the crossover rate found in this study is a more accurate estimation than reported in existing studies.
Hematoma volume has been reported as a potential factor in predicting success of conservative therapy. The results, however, are conflicting. In a 2016 study by Kim et al., the group successfully treated with conservative therapy had a smaller hematoma volume, though the difference was not statistically significant (p = 0.146) [19]. In 2022, Wang et al. published one of the few larger studies focusing on predictive parameters for the outcome of conservative therapy [24, 27]. Among 98 patients, larger hematoma diameter and volume were significantly associated with crossover to surgery (thickness: OR 1.097, volume: OR 1.021). However, these results stem from a highly pre-selected population given that the data originated from a RCT. The study excluded patients on anticoagulant or antiplatelet medication, representing up to 50% of all cSDH patients [38,39,40]). Those on statins, accounting for approximately 30% of all cSDH patients, were also omitted. [40, 41]). Nonetheless, our study corroborates that hematoma volume influences the success of conservative therapy, even in a broader population.
Hypodense hematoma type was another factor associated with conservative therapy success. The underlying theory is that in hypodense hematomas, active bleeding components are absent [42, 43]. Therefore, hematoma growth and eventual surgery are not likely. Two studies have reported a positive association between hypodense hematoma density and success of conservative therapy [16, 31]. Unfortunately, the first study published did not measure HU, leading to methodological subjectivity and limited reproducibility [16]. In the second study, the method of hematoma classification was identical to the one used in this study [31]. A recent systematic review indicated that the major benefit of this classification method is its simplicity, especially in comparison to other, more detailed, available methods [44]. This simplicity reduces interobserver variability and enhances data consistency across studies. Given its straightforward implementation and precedent in prior research, we recommend that future studies adopt a similar classification approach.
Our study also shows that progression to surgery was a subacute process and did not occur directly or in the days shortly after diagnosis of the cSDH. The time until crossover found in this study concurs with a study by Jiang et al. and Rauhala et al. [24, 45]. The first study was a RCT in which the effect of atorvastatin on the crossover to surgery rate was investigated. In the placebo group, the mean time until crossover to surgery was 24 days (n = 98) [24]. In the second study, 223 patients were initially treated with conservative therapy, of whom 53 (24%) eventually required surgery. The average time between diagnosis and surgery was 24 days [45]. Considering the aforementioned study results and the provided time frame of this study, we conclude that wait-and-watch therapy can be safely deployed as initial treatment for this group of cSDH patients.
While neurologists and neurosurgeons at our institution collaborate closely for cSDH treatment, the decision for surgery lies with the attending neurosurgeon. Studies have shown that this decision is predominantly based on subjective features such as treatment center culture, surgeon preference and intuition [46, 47]. Clinical deterioration, frequently paired with radiological progression, was the prevalent reason for crossover in our study. Interestingly, motor deficit at diagnosis, which most surgeons often interpret as an indication for surgery, was evenly distributed amongst both groups. This implies that presence of (mild) motor deficits at diagnosis is not an indication for surgery alone. However, the total group of patients in which motor deficit was present was relatively small, so caution is required with interpretation of these results. Secondly, nine patients had a midline shift exceeding 10 mm at diagnosis. Five of these required eventual surgery, but in four success of conservative therapy was achieved. Deciding optimal treatment strategy is complex, especially in mildly symptomatic patients with such a midline shift. In order to prevent potential rapid neurological decline due to further brain herniation, most neurosurgeons would perform surgery. However, even in these cases success of conservative therapy can be achieved. Again, with such limited number of patients vast conclusions cannot be drawn and future research specifying indications for surgery is warranted.
Limitations
The most important limitation of this study is lack of clinical outcome and other outcomes related to functioning, independence or quality of life. Since this is a retrospective study, reliable assessment of such outcomes (e.g. through modified Rankin scale, mNIHSS, SF-36, or Barthel index) was not adequately documented. Secondly, because this study was conducted in a tertiary academic hospital, the results cannot be extrapolated directly onto general cSDH populations. Asymptomatic patients might have been overlooked, as they typically get managed by neurologists in referring centers instead of being sent to our institution. Therefore, the success rate found in this study probably underestimates the true success rate. Finally, the success group had a shorter follow-up compared to the crossover group. This reflects current standard care as patients were either referred back to the neurologist, or discharged of follow-up with instructions to regain contact if symptoms re-occurred or worsened. Standardized follow-up could solve these issues in future prospective and multi-centered studies.
Conclusion
In this single-center, retrospective study, conservative therapy of patients with a cSDH and no, or only mild, symptoms could be achieved without crossover-to-surgery in a slight majority of patients, even in the presence of (mild) motor deficits. Parameters associated with success were hematoma volume and hypodense hematoma type. As time to crossover in these selected patients is in the order of weeks, cautious follow-up appears to be safe and feasible. In order to determine the true efficacy of conservative therapy, especially considering clinical outcomes, future prospective studies are necessary. This requires a standardized, nationwide (and preferably international), and integrated approach.
Data availability
Not applicable.
Abbreviations
- AC:
-
Anticoagulant therapy
- AP:
-
Antiplatelet therapy
- aSDH:
-
Acute subdural hematoma
- BHC:
-
Burr hole craniostomy
- CI:
-
Confidence interval
- cSDH:
-
Chronic subdural hematoma
- COPD:
-
Chronic obstructive pulmonary disease
- CVA:
-
Cerebrovascular accident
- CT:
-
Computed tomography
- CL:
-
Centiliter
- eCRF:
-
Electronic case report form
- DVT:
-
Deep venous thrombosis
- EDC:
-
Electronic data capture
- HU:
-
Hounsfield Units
- IQR:
-
Interquartile range
- GCS:
-
Glasgow coma scale
- MAR:
-
Missing at random
- MGS:
-
Markwalder Grading scale
- MRC:
-
Medical Research Council scale
- MM:
-
Millimeter
- ML:
-
Milliliter
- OR:
-
Odds ratio
- PE:
-
Pulmonary embolism
- RCT:
-
Randomized controlled trial
- SD:
-
Standard deviation
- PE:
-
Pulmonary embolism
- SD:
-
Standard deviation
References
Mack J, Squier W, Eastman JT (2009) Anatomy and development of the meninges: implications for subdural collections and CSF circulation. Pediatr Radiol 39(3):200–210
Miah IP et al (2018) Dexamethasone therapy versus surgery for chronic subdural haematoma (DECSA trial): study protocol for a randomised controlled trial. Trials 19(1):575
Yang W, Huang J (2017) Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am 28(2):205–210
Henry J, et al. (2022) Management of chronic subdural hematoma: a systematic review and component network meta-analysis of 455 studies with 103 645 cases. Neurosurgery
Turrentine FE, Wang H, Simpson VB, Jones RS (2006) Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg 203(6):865–877
Portelli Tremont JN, Sloane PD (2022) Applying evidence-based principles to guide emergency surgery in older adults. J Am Med Dir Assoc 23(4):537–546
Soleman J, Nocera F, Mariani L (2017) The conservative and pharmacological management of chronic subdural haematoma. Swiss Med Wkly 147:w14398
Berghauser Pont LM et al (2013) Ambivalence among neurologists and neurosurgeons on the treatment of chronic subdural hematoma: a national survey. Acta Neurol Belg 113(1):55–59
Holl DC et al (2022) National survey on the current practice and attitudes toward the management of chronic subdural hematoma. Brain Behav 12(3):e2463
Laldjising ERA, Cornelissen FMG, Gadjradj PS (2020) Practice variation in the conservative and surgical treatment of chronic subdural hematoma. Clin Neurol Neurosurg 195:105899
Naganuma H et al (1986) Spontaneous resolution of chronic subdural hematomas. Neurosurgery 19(5):794–798
Horikoshi T et al (1998) Computed tomography characteristics suggestive of spontaneous resolution of chronic subdural hematoma. Neurol Med Chir (Tokyo) 38(9):527–32
Jones S, Kafetz K (1999) A prospective study of chronic subdural haematomas in elderly patients. Age Ageing 28(6):519–521
Parlato C, Guarracino A, Moraci A (2000) Spontaneous resolution of chronic subdural hematoma. Surg Neurol 53(4):312–5 (discussion 315-7)
Asghar M et al (2002) Chronic subdural haematoma in the elderly–a North Wales experience. J R Soc Med 95(6):290–292
Hirashima Y et al (2005) Effect of platelet-activating factor receptor antagonist, etizolam, on resolution of chronic subdural hematoma–a prospective study to investigate use as conservative therapy. Neurol Med Chir (Tokyo) 45(12):621–6 (discussion 626)
Miranda LB, Braxton E, Hobbs J, Quigley MR (2011) Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg 114(1):72–76
Lee GS, Park YS, Min KS, Lee MS (2015) Spontaneous resolution of a large chronic subdural hematoma which required surgical decompression. J Korean Neurosurg Soc 58(3):301–303
Kim HC, Ko JH, Yoo DS, Lee SK (2016) Spontaneous resolution of chronic subdural hematoma : close observation as a treatment strategy. J Korean Neurosurg Soc 59(6):628–636
Prud’homme M, Mathieu F, Marcotte N, Cottin S (2016) A pilot placebo controlled randomized trial of dexamethasone for chronic subdural hematoma. Can J Neurol Sci 43(2):284–290
Asan Z (2018) Growth potential of subdural hematomas under clinical observation: which subdural hematomas tend to grow and why they do. World Neurosurg 113:e598–e603
Ban SP et al (2018) Middle meningeal artery embolization for chronic subdural hematoma. Radiology 286(3):992–999
Hou K et al (2018) Efficacy of reinforced restriction of physical activity on chronic subdural hematoma: prospective pilot study. World Neurosurg 110:e1011–e1016
Jiang R et al (2018) Safety and efficacy of atorvastatin for chronic subdural hematoma in chinese patients: a randomized clinicaltrial. JAMA Neurol 75(11):1338–1346
Petralia CCT et al (2020) Effect of steroid therapy on risk of subsequent surgery for neurologically stable chronic subdural hemorrhage-retrospective cohort study and literature review. World Neurosurg 138:e35–e41
Wang D, et al. (2022) Peripheral Monocyte Percentage as a Potential Indicator of Prognosis in Patients with Chronic Subdural Hematoma Receiving Conservative Therapy. World Neurosurg
Wang D, et al. (2022) Risk factor analysis of the conservative treatment in chronic subdural hematomas: a substudy of the ATOCH trial. Adv Ther
Tian Y et al (2022) Establishment and validation of a prediction model for self-absorption probability of chronic subdural hematoma. Front Neurol 13:913495
Delgado-López PD et al (2009) Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur) 20(4):346–359
Parry D, et al. (2023) Asymptomatic chronic subdural haematoma - does it need neurosurgical intervention? Br J Neurosurg:1–6
Zhang X, et al. (2023) Factors influencing wait-and-watch management in mild primary chronic subdural hematoma: a retrospective case-control study. Acta Neurol Belg
Foppen M, et al. (2023) Success of conservative therapy for chronic subdural hematoma patients: a systematic review. Front Neurol 2023
Immenga S et al (2022) Tranexamic acid to prevent operation in chronic subdural haematoma (TORCH): study protocol for a randomised placebo-controlled clinical trial. Trials 23(1):56
Chon KH, Lee JM, Koh EJ, Choi HY (2012) Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 154(9):1541–1548
M.W.H. (2021) psfmi: prediction model pooling, selection and performance evaluation across multiply imputed datasets. 2021.
Team R (2020) RStudio: Integrated Development for R. RStudio. PBC, Boston
van Buuren S, Groothuis-Oudshoorn K (2011) Mice: multivariate imputation by chained equations in R. J Stat Softw 45(3):1–67
Gernsback J, Kolcun JP, Jagid J (2016) To drain or two drains: recurrences in chronic subdural hematomas. World Neurosurg 95:447–450
Häni L et al. (2019) Subdural versus subgaleal drainage for chronic subdural hematomas: a post hoc analysis of the TOSCAN trial. J Neurosurg: 1–9
Klein J, Mauck L, Schackert G, Pinzer T (2021) Do statins reduce the rate of revision surgery after chronic subdural hematoma drain? Acta Neurochir (Wien) 163(7):1843–1848
Guidry BS et al (2021) Statins as a medical adjunct in the surgical management of chronic subdural hematomas. World Neurosurg 149:e281–e291
Jeong SI et al (2014) Clinical analysis of risk factors for recurrence in patients with chronic subdural hematoma undergoing burr hole trephination. Korean J Neurotrauma 10(1):15–21
Ko BS et al (2008) Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc 43(1):11–15
Miah IP et al (2021) Radiological prognostic factors of chronic subdural hematoma recurrence: a systematic review and meta-analysis. Neuroradiology 63(1):27–40
Rauhala M et al (2020) Chronic subdural hematoma-incidence, complications, and financial impact. Acta Neurochir (Wien) 162(9):2033–2043
van Essen TA et al (2019) Variation in neurosurgical management of traumatic brain injury: a survey in 68 centers participating in the CENTER-TBI study. Acta Neurochir (Wien) 161(3):435–449
van Essen TA et al (2022) Surgery versus conservative treatment for traumatic acute subdural haematoma: a prospective, multicentre, observational, comparative effectiveness study. Lancet Neurol 21(7):620–631
Acknowledgements
We would like to acknowledge Koos Zwinderman (statistician) and Nick van Es (specialist internal medicine) for their help with the statistical analysis of this paper.
Funding
No funding was acquired for this study. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this study a waiver for approval by the local ethics committee was obtained.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Foppen, M., Lodewijkx, R., Bandral, H.V. et al. Factors associated with success of conservative therapy in chronic subdural hematoma: a single-center retrospective analysis. J Neurol 271, 3586–3594 (2024). https://doi.org/10.1007/s00415-024-12307-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12307-2