Abstract
Background
The intracranial chondroma is a sporadic type of benign tumor with an incidence of less than 0.5% of all intracranial tumors. Furthermore, this type of tumor has been reported more frequently at the skull base, less at the falx cerebri, and exceptionally of the dura, when it happened to be intracranial.
Case presentation
We report a challenging diagnosis and the successful management of a rare case of intracranial dural chondroma in a 19-year-old student without past medical history, revealed by secondary epilepsy with behavioral and mood disorder without neurological deficit.
Conclusion
The dural origin of intracranial chondroma is a rare type of tumor with nonspecific clinical manifestations, and diagnosis confirmation requires a histopathological finding. Surgical gross total resection of the lesion is the golden standard of its management.
Similar content being viewed by others
Introduction
Chondromas are benign tumors composed of mature hyaline cartilage. They generally have limited growth potential and are not locally aggressive. Chondromas develop from nests of growth-plate cartilage that have become entrapped in the medullary canal of the metaphysis or the metaphyseal-diaphyseal junction. These hamartomatous proliferations persist as islands in the bone and then develop from enchondral ossification [1].
An intracranial chondroma is a benign cartilaginous tumor first reported by Hirschfeld in 1851. They are sporadic brain tumors, comprising approximately 0.3–0.5% of primary cerebral tumors. They commonly originate from the skull base but can infrequently arise from the falx, convexity dura, or ventricular ependyma. Diagnosis requires histopathologic confirmation, as patients present with nonspecific symptoms related to mass effect. Imaging characteristics often resemble meningiomas, oligodendrogliomas, and vascular malformations [2, 3]. There have been only 11 cases of intracranial chondromas of dural origin reported in the literature to the best of our knowledge.
We report a challenging diagnosis and the successful management of a rare case of intracranial dural chondroma in a 19-year-old student. Apart from the nonspecific aspect of the clinical findings, the radiological investigations did not help for a straightforward diagnosis orientation. The patient underwent surgery and promptly recovered after the gross total resection of the tumor.
Case presentation
Patient information
A 19-year-old student was admitted to our department for secondary epilepsy with behavioral and mood disorders without neurological deficits. His past medical, family, and psychosocial history were unremarkable. The patient was initially treated unsuccessfully at the psychiatry department, where medical treatment was provided for the persistence of the mood disorders.
Clinical findings
At the admission, the patient was conscious with a Glasgow Coma Scale of 15 with behavioral disorders such as disinhibition and impulsivity. There was no motor-sensory deficit. He denied any changes in speech and facial, visual, swallow, bladder, or bowel disturbance at the onset. He denied any trauma or other inciting event.
Diagnostic assessment
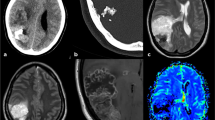
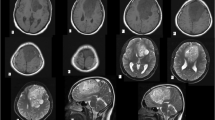
The cerebral MRI was done, and based on the extra-axial, left frontoparietal lesion found (Fig. 1), he was transferred to our department for management. The intracranial lesion was heterogeneous on T1 and T2-weighted imaging, slightly enhanced after Gadolinium injection, without clear evidence of a calcified component. A head CT-Scan was done to narrow our differential diagnosis window. This radiological investigation confirmed the calcifications previously suspected and shows a bony erosion of the parietal bone adjacent to the lesion (Fig. 2A). The blood laboratory test was normal. Since the lesion was of the cranial convexity, extra-axial, old with calcification, with mass effect on the cerebral parenchymal without its infiltration, we first thought of a meningioma or any other cerebral tumor of bone origins.
Therapeutic intervention
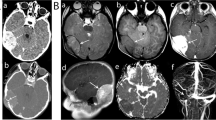
The patient underwent surgery with gross total resection of the lesion through the left frontoparietal approach. The lesion was inserted into the dura matter, round, hard, calcified, and irregular in shape with its extension to the adjacent parietal bone (Fig. 3). The sample was sent to the laboratory, and the histopathology report concluded with an intracranial chondroma (Fig. 4).
Follow-up and outcome
The patient did well in the postoperative period, with a CT-Scan confirming the total removal of the tumor with a slight stigma of hemorrhage (Fig. 2B). At three-month follow-up, the patient had completely recovered and resumed his daily duty. The head MRI at this time was normal without tumor recurrence (Fig. 5).
Discussion
Intracranial chondroma of dural origin is a rare and even exceptional type of chondromas. Our case is the 12th case ever reported in the literature since the first reported intracranial chondroma case by Hirschfeld in 1851. In our study, the patient presented with secondary epilepsy, behavioral, and mood disorders without neurological deficits.
The clinical symptoms are variable and due to mass effect depending on the size and location of the lesion. Some authors have described headache 52.5%, diplopia 11.9%, seizure 11.9%, papilledema 8.5%, weakness 8.5%, and dizziness 6.8%. The differentiation of these lesions from meningiomas through imaging is generally difficult. In most cases, the lesions are heterogeneous in T1 and T2 weighted. Chondromas are typically DWI hypointense with high apparent diffusion coefficient (ADC) values, while meningiomas are typically DWI hyperintense with low ADC values. These criteria can be helpful in the evaluation of differential diagnoses. Moreover, the tumor parenchyma appeared heterogeneously hypointense on T1WI and hyperintense or mixed hyperintense and hypointense on T2WI (Table 1). In contrast, the calcification appeared hypointense on T1WI and T2WI in five cases, demonstrating significant inhomogeneous enhancement on postcontrast MRI, which revealed the typical “punica granatum seeds” sign [3,4,5,6]. This is different from what we found in our case showing the heterogeneous hyperintensity on T1 and T2 weighted predominantly at the bony erosion side, which is later on found to be the calcified parietal bone infiltration of the dural lesion. These radiological findings are more characteristic of a meningioma. Even the cerebral CT-Scan could not make the difference between these two entities. Bone destruction occurs in over 50% of the cases, whereas irregular calcifications are seen in about 60% [7].
Since the tumors are slow-growing, the symptoms may not appear for many years. The parenchyma has adapted to the insidious and progressive mass effect. Thus, the intracranial chondromas are characterized by their large size at presentation. This item, even though not specific, can help differentiate them from meningiomas. In fact, our patient was a student at the university, with a good school record performance [11, 18], not affected by this slow-growing chondroma.
A gross total resection of the lesion with a rim of the dura mater and the defect patched with a dural graft was the main treatment in the 11 cases reported. With an uneventful postoperative period. The tumor was removed through a left frontoparietal craniotomy [12]. A white, very firm, frontoparietal dural-based lobulated mass was identified with the intradural component of the same aspect. The tumor was avascular but surrounded by multiple vessels. The tumor was removed enbloc from the underlying brain parenchyma that was under the mass effect without being infiltrated. This is followed by a cranioplasty during the same surgical procedure [13, 15, 19, 20]. The histopathological findings show a chondroid pattern of low cellularity and chondrocytes with a lack of atypia and mitosis, confirming the diagnosis of a benign lesion, a chondroma. The diagnosis of intracranial dural chondroma can be very tricky, and its differential diagnosis with other dural origins tumors like meningioma or even oligodendroglioma may not be certain before surgery. The clinical and radiological findings are not specific to intracranial dural chondroma. The CT-Scan can be very helpful in giving calcifications details, while histopathology confirms the diagnosis [3, 11,12,13,14,15, 17,18,19,20,21].
Conclusion
The dural origin of intracranial chondroma makes this cartilaginous lesion confusing with some other intracranial tumors. The radiological findings are more characteristic of a meningioma. Thus, this is a benign and rare tumor with a real diagnosis challenge. Even though the diagnosis can be suspected on the radiological investigation and confirmed by the histopathological examination after surgery, there are no specific clinical findings. Surgical gross total resection of the lesion is the golden standard of its management.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Palaniappan Lakshmanan. Chondroma: Background, Pathophysiology, Epidemiology. 2019 Nov 9 [cited 2021 May 4]; Available from: https://emedicine.medscape.com/article/1258109-overview#a2
Yeung JT, Krznarich TS, Moreno EA, Mukkamala A, Karim AS. Intracranial parafalcine chondroma in a pregnant patient. Surg Neurol Int. 2012;3:44. https://doi.org/10.4103/2152-7806.94930.
Sullivan JC, Goldsmith J, Rojas R, Varma H, Kasper EM. Intracranial dural parafalcine chondroma: case report and systematic review of the literature. World Neurosurg. 2019;122:1–7. https://doi.org/10.1016/j.wneu.2018.09.169.
Duan F, Qiu S, Jiang J, Chang J, Liu Z, Lv X, et al. Characteristic CT and MRI findings of intracranial chondroma. Acta Radiol. 2012;53(10):1146–54. https://doi.org/10.1258/ar.2012.120433.
Abeloos L, Maris C, Salmon I, Balériaux D, Sadeghi N, Lefranc F. Chondroma of the dural convexity: a case report and literature review. Neuropathology. 2012;32(3):306–10. https://doi.org/10.1111/j.1440-1789.2011.01264.x.
Furui T, Iwata K, Yamamoto H, Murakami A. A case of intracranial chondroma presenting with pontine hemorrhage. No shinkei geka Neurol Surg. 1990;18(6):543–6.
Feierabend D, Maksoud S, McLean AL, Koch A, Kalff R, Walter J. Giant convexity chondroma with meningeal attachment. Clin Neurol Neurosurg. 2018;169:37–40. https://doi.org/10.1016/j.clineuro.2018.03.027.
Nakazawa T, Inoue T, Suzuki F, Nakasu S, Handa J. Solitary intracranial chondroma of the convexity dura: case report. Surg Neurol [Internet]. 1993;40(6):495–8.
Oz Atalay F, Ozgun G, Tolunay S, Bekar A. Intracranial extra-axial chondroma: a case report. J Nippon Med Sch [Internet]. 2014;81(1):35–9.
Ramamurthi B, Iyer CGS, Vedachalam SP. Intracranial meningeal chondroma. J Neurosurg [Internet]. 1961;18(6):826–8.
Reinshagen C, Redjal N, Sajed DP, Nahed BV, Walcott BP. Intracranial dural based chondroma. J Clin Neurosci. 2016;25:161–3. https://doi.org/10.1016/j.jocn.2015.05.052.
Shrot S, Cohen AR, Rodriguez FJ, Berkowitz F, Soares BP, Huisman TA. Intracranial dural chondroma in a child—conventional and advanced neuroimaging characteristics and differential diagnosis. Neuroradiol J. 2018;31(4):386–9. https://doi.org/10.1177/1971400917712268.
Somerset H, Wilkinson CC, Kleinschmidt-Demasters BK. 18-year-old woman with a dural mass. Brain Pathol. 2013;23(1):113–6. https://doi.org/10.1111/bpa.12012.
Hong JT, Lee SW, Son BC, Sung JH, Choi HC, Kim MC. Delayed occurrence of intracranial supratentorial chondroma following compound depressed skull fracture. Acta Neurochir. 2005;147(3):343–5. https://doi.org/10.1007/s00701-004-0430-1.
Weng J-C, Li D, Li H, Ma J-P, Tian K-B, Wang L, et al. Surgical management and outcomes of intracranial chondromas: a single-center case series of 66 patients. World Neurosurg. 2017;108:264–77. https://doi.org/10.1016/j.wneu.2017.08.151.
Laghmari M, Metellus P, Fuentes S, Adetchessi T, Dufour H, Bouvier C, et al. Cranial vault chondroma: a case report and literature review. Neurochirurgie [Internet]. 2007;53(6):491–4. https://doi.org/10.1016/j.neuchi.2007.09.148.
Çolpan E, Attar A, Erekul S, Arasıl E. Convexity dural chondroma: a case report and review of the literature. J Clin Neurosci. 2003;10(1):106–8. https://doi.org/10.1016/S0967-5868(02)00281-3.
Patel A, Munthali L, Bodi I. Giant cystic intracranial chondroma of the falx with review of literature. Neuropathology. 2009;29(3):315–7. https://doi.org/10.1111/j.1440-1789.2008.00957.x.
Takizawa H, Sugiura K, Baba M, Chisiki T, Kamatsuka E, Tachisawa T, et al. Parasellar chondroma: a case report. Br J Neurosurg. 1987;1(1):147–51. https://doi.org/10.3109/02688698709034351.
Elhakeem AA, Essa AA, Soliman RK. Chondroma of the falx cerebri: a case report and review of literature. Neuropathology. 2019;39(6):461–6. https://doi.org/10.1111/neup.12598.
Xin Y, Hao S, Zhang J, Wu Z, Jia G, Tang J, et al. Microsurgical treatment of intracranial chondroma. J Clin Neurosci. 2011;18(8):1064–71. https://doi.org/10.1016/j.jocn.2010.12.028.
Acknowledgements
Not applicable
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
YCHD: Conceptualization, writing draft, reviewing and editing, visualization, supervision, validation, methodology. IEK: Writing, review & editing, FNI: Writing, review & editing. EAS: Writing & editing, MS: Writing & editing, ACEA: Writing & editing, MG: Supervision, Validation, & review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained from the patient prior to the submission of this article. Also, this article respects both the Consensus-based Clinical Case Reporting Guideline and the Recommendations for the Conducting, Reporting, Editing, and Publication of Scholarly Work in Medical Journals.
Consent for publication
Informed consent was obtained from the patient to publish his case.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dokponou, Y.C.H., El Kacemi, I., Imoumby, F.N. et al. Management and outcome of intracranial chondroma of the dural origin: case report and review of the literature. Egypt J Neurosurg 38, 19 (2023). https://doi.org/10.1186/s41984-023-00204-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-023-00204-1