Abstract
Background
Foreign body reaction in brain tissue is a very rare immune response that has not been well studied. Hemostatic material has been reported as a possible trigger of this response in other organs and could be detected by [18F]fluorodeoxyglucose positron emission tomography/computerized tomography ([18F]FDG PET/CT), but there is no reported experience about the role of [18F]fluorocholine in this finding. [18F]Fluorocholine has the potential to differentiate viable central nervous system tumors from other entities, so it is frequently used in the follow-up of neurosurgery patients.
Case presentation
A right frontoparietal neoplastic lesion was found in a young-aged patient with analgesic refractory headache. Surgical resection and postsurgical radiotherapy were performed, and the pathologist analysis turned out a cellular ependymoma with signs of anaplasia. In the follow-up, an magnetic resonance imaging (MRI) showed a suspicious lesion, so a [18F]fluorocholine PET/CT was performed. Increased uptake was described in the right parietal region on the margin of the residual cystic lesion. The patient got a complete resection which was confirmed later by MRI. In the pathology analysis, a focally congestive cerebral parenchyma with a central histiocytic reaction to a foreign body area was described.
Conclusions
Following the experience of the current case report, [18F]fluorocholine PET/CT could also show a false positive related to foreign body reaction. This entity should be considered to avoid unnecessary major surgery on our patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Central nervous system (CNS) tumors are a challenging pathology, which requires a very short time of action due to their high mortality. The global burden of CNS tumors has increased during the last 25 years. Also, the lack of specific symptoms and signs makes imaging diagnosis a mainstay in CNS tumor management. The relation between the incidence and the mortality of CNS cancer guides the importance of early diagnosis to improve the survival of these patients [1]. Of all CNS tumors, gliomas are one of the most aggressive histological lines and the second most frequent brain tumor [2].
The first step for the brain tumor diagnosis comprises the use of magnetic resonance imaging (MRI) and computerized tomography (CT). MRI is the technique of choice due to its ability to obtain functional data on cellularity (MRI diffusion), vascularization (MRI perfusion), and metabolism (MRI spectroscopy) which are essential nowadays [3]. However, due to MRI-recognized limitations on viable tumor and treatment-induced lesions differentiation, there is a need to develop new ways of diagnosis and characterizing CNS tumors [4]. Effective management of high-grade gliomas is critical, primarily during the postoperative phases, wherein MRI findings may yield ambiguous results [5].
A step beyond radiological imaging, nuclear medicine has an important role. Positron emission tomography (PET) is a molecular imaging technique that complements MRI in the study of gliomas, in situations such as treatment response evaluation [6]. [18F]Fluorodeoxyglucose (FDG) is the most used radiotracer in PET/CT imaging, but jeopardizes the signal/background ratio due to high physiological uptake in normal brain parenchyma. Therefore, depending on the histopathologic nature of the lesion and the clinical question, other radiopharmaceuticals could be useful [7]. [18F]Fluorocholine is a radiotracer that gets trapped to form a major membrane phospholipid by choline kinase, being a membrane proliferation marker [8]. About the [18F]fluorocholine biodistribution, it does not cross the blood–brain barrier, so it is helpful to study central nervous system lesions with rupture of this barrier, such as primary brain tumor or metastasis [9, 10]. Also, [18F]fluorocholine has the potential to differentiate viable tumors from other entities, such as radionecrosis [11,12,13], and could fine-tune the diagnosis in complement with the MRI. This synergy between MRI and [18F]fluorocholine PET/CT could be particularly useful in glioma posttherapy follow-up [14].
The documentation of foreign body reactions has been extensively documented in diverse tissues and in response to various postsurgical materials. This reaction, characterized by macrophages and giant cells, a crucial part of the healing process that follows the introduction of medical devices or biomaterials [15, 16]. However, such a phenomenon remains understudied in the CNS.
Case presentation
We present a young-aged patient with a headache resistant to analgesic treatment, nausea, and vomiting who had an episode of sensitivity loss and speaking difficulties. After an urgent cranial CT, a right frontoparietal lesion was diagnosed. The patient was taken to the neurosurgery department and a cranial MRI was performed which confirmed the presence of a neoplastic lesion. The surgery was guided by neuronavigation with 5-aminolevulinic acid and cortical and subcortical stimulation. Intraoperatively, a hard consistency heterogeneous tumor was found with a capsule in some parts of its extension. The 5-aminolevulinic acid showed diffuse infiltration of white matter. The surgical wound was closed with cellulose derivate hemostatic material (Surgicel®), and the dura mater was sutured with braided silk and adhesive fibrinogen/thrombin matrix. The histopathological analysis resulted in a cellular ependymoma with signs of anaplasia (World Health Organization, WHO grade III). After the surgery, the patient underwent radiotherapy with some autolimited episodes of hemiparesis and dysarthria. Besides, he presented a somesthetic crisis (high sensibility including cutaneous and kinesthetic senses) in his left arm, for which he refused pharmacological antiepileptic treatment. Post-surgical MRI showed signs of probable complete tumor resection. Written consent was obtained for every procedure.
Successive cranial MRI studies over the following 3 years showed superficial parenchymal enhancement in the surgical bed, initially attributed to post-surgery irritative changes, but which progressively acquired a more nodular and conspicuous morphology over a cystic lesion (Fig. 1). Further studies were recommended to complete the evaluation under the suspicion of recurrence. A [99mTechnetium]Technetium-metoxiisobutilisonitrile single-photon emission computerized tomography/computerized tomography ([99mTc]Tc-MIBI SPECT/CT) was reported as no significant uptake.
Brain MRI studies. T1-3D (T1-weighted with a 3-dimensional acquisition) post-gadolinium in the axial plane from (A) postsurgical control (2 years after first surgery), B postsurgical control (2 years and a half after first surgery), and (C) postsurgical control (3 years after first surgery). A lesion can be seen in the surgical bed (white arrow) that progressively adopts a more nodular morphology with better marked off peripheral contrast uptake
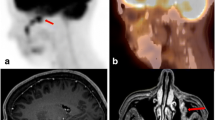
The patient was presented to the multidisciplinary committee, and it was decided to perform a [18F]fluorocholine PET/CT to assess the metabolic behavior of the radiological image. The brain [18F]fluorocholine PET/CT showed increased uptake of punctate morphology at the level of the right parietal cortex, on the external margin of the residual cystic lesion (Fig. 2). After this finding, a functional MRI (f-MRI) was performed to assess the possibility of a rescue surgery. The described lesion did not present any anatomical relation with the brain motor cortex, so the patient was proposed for a resection. The patient accepted and he was programmed for the surgery.
This second surgery was performed with a neuronavigator to localize the lesion and under neurophysiological control. The motor region was identified, located anterior to the injury (as indicated by imaging f-MRI) and a macroscopically complete resection was performed. In the postoperative period, the patient did not present any neurological symptoms and was discharged without any incidence.
One month after the surgery, the patient had a postsurgical MRI that confirmed the complete resection of the contrast-enhanced nodule but with an enhancement around the loculum and the meninx, which was blamed on the postsurgical changes. Microscopically, it was described as a pseudoencapsulated lesion, surrounded by brain parenchyma constituted by reactive astrocytes, without atypia nor mitosis. In the central area, foamy-cytoplasm cell proliferation was shown, being cluster of differentiation-68 (CD68) positive. Also, few lymphocytes against foreign body from the previous surgery were found (Fig. 3). The histopathological analysis concluded that the piece had focally congestive cerebral parenchyma and a central area with histiocytic reaction to a foreign body with an absence of malignant neoplastic cells.
Anatomopathological image of the second surgery piece. Arrows point to different cellular structures: textiloma (red arrow), foamy histiocytes staining with CD68 (black arrow), and reactive brain parenchyma (green arrow). A Hematoxylin–eosin × 10; B hematoxylin–eosin × 20; C hematoxylin–eosin × 40; D immunohistochemistry
Foreign body reactions after surgery have been described in different tissues in the human body and against different kinds of materials [15]. This entity is one of the factors that push to improve the research in the biocompatibility of surgical products [17]. However, foreign body reactions have not been well studied in the CNS and they are very rarely reported, mostly as individual case reports or case series studies. Among these reports, we find the use of material for endovascular therapy [18, 19] or hemostasis intra-operative chemical agents [20]. The authors of the present case report hypothesize that this reaction could be triggered by two potentially harmful surgical materials. The first considered possibility is that neurosurgical patties released microscopical fibers that brought out the foreign body reaction, however, the literature does not describe this possibility [21]. This fiber was not found in the histological analysis and some authors defend the strength and durability of the cellulose that compacts the cotton fibers [22]. Our second hypothesis was that cellulose derivate hemostatic material was used by the neurosurgeons in the first surgical intervention. This hemostatic material has been frequently applied in our center's neurosurgery interventions since 2016. Foreign body reactions against these derivates have been reported in the literature by several clinical cases [23]. In [18F]FDG PET/CT studies a similar reaction has been described in other organs such as lungs [24]. Also, our patient histological pattern could fit adequately in this context [25].
Intracranial foreign body reaction is a rare immune response and usually appears within weeks to months after surgery with clinical. Imaging features of the entity could be similar to tumor progression [26]. It has been reported that it could share radiologic characteristics with neoplasms in CT and MRI studies as the presence of a granuloma with peripheral contrast-enhanced in CT [27]. MRI also could show well-circumscribed masses with central hypointensity and peripheral contrast enhancement that in several cases could be confused with a tumoral lesion [28]. This false-positive diagnosis could make the patient undergo surgery without real need [29].
In our knowledge and after bibliographic research, it seems that there is not enough literature that describes the behavior of [18F]fluorocholine in the study of foreign body reaction, nor PET/CT role in the management of these patients. Despite the lack of specific literature, the clinical case reported by Jang et al. [20] remarked that the MRI spectroscopic study showed elevated choline in comparison with the rest of the measurable amino acids. Some authors pointed out that the normalized standard uptake value mean (SUVmean) from [18F]fluorocholine and normalized integral values of choline in spectroscopy could show a positive correlation [30]. There is a need for more studies that gather a significant sample to clarify the role of [18F]fluorocholine in the differential diagnosis of foreign body reaction. Faced with the lack of evidence, it seems that histopathologic analysis is the gold standard to differentiate between neoplastic tissue and foreign body reaction [31].
Conclusions
The introduction of new procedures and materials in surgical practice could induce diagnostic mistakes in imaging techniques, and this fact must be considered to avoid unnecessary invasive approaches. There is a need to report false-positive explorations to reduce these avoidable interventions. Foreign body reaction in central nervous tissue could be shown as an uptake focus in [18F]fluorocholine PET/CT, mimicking a neoplastic lesion.
Availability of data and materials
The data are available in the clinical history of the Andalusian health service.
Abbreviations
- 99mTc:
-
Metastable technetium
- [18F]FDG PET/CT:
-
[18F]Fluorodeoxyglucose positron emission tomography/ computerized tomography
- CD:
-
Cluster of differentiation
- CNS:
-
Central nervous system
- CT:
-
Computerized tomography
- FDG:
-
Fluorodeoxyglucose
- f-MRI:
-
Functional magnetic resonance imaging
- MIBI:
-
Metoxiisobutilisonitrile
- MRI:
-
Magnetic resonance imaging
- PET:
-
Photon emission tomography
- SPECT:
-
Single-photon emission computerized tomography
- SUV:
-
Standard uptake value
- WHO:
-
World Health Organization
References
GBD2016 Brain and Other CNS Cancer Collaborators. Global, regional, and national burden of the brain and other CNS cancer, 1990–2006: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):376–93.
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncol. 2015;17(Suppl 4):iv1–62.
Fink JR, Muzi M, Peck M, Krohn KA. Multimodality brain tumor imaging: MRI Imaging, PET, and PET/MRI imaging. J Nucl Med. 2015;56(10):1554–61.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28(11):1963–72.
Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20(4):485–502.
Mansoor NM, Thust S, Militano V, Fraioli F. PET imaging in glioma: techniques and current evidence. Nucl Med Commun. 2018;39(12):1064–80.
Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabeled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–57.
Taywade SK, Damle NA, Behera A, Devasenathipathy K, Bal C, Tripathi M, et al. Comparison of 18F-fluorocholine positron emission tomography/computed tomography and four-dimensional computed tomography in the preoperative localization of parathyroid adenomas- initial results. Indian J Endocrinol Metab. 2017;21(3):399–403.
Spaeth N, Wyss MT, Pahnke J, Biollaz G, Lutz A, Goepfert K, et al. Uptake of 18F-fluorocholine, 18F-fluoro-ethyl-l-tyrosine and 18F-fluoro-2-deoxyglucose in F98 gliomas in the rat. Eur J Nucl Med Mol Imaging. 2006;33(6):673–82.
Calabria FF, Barbarisi M, Gangemi V, Grillea G, Cascini GL. Molecular imaging of brain tumors with radiolabeled choline PET. Neurosurg Rev. 2018;41(1):67–76.
Spaeth N, Wyss MT, Weber B, Scheidegger S, Lutz A, Verwey J, et al. Uptake of 18F-Fluorocholine, 18F-Fluoroethyl-l-Tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: implications for separation of radiation necrosis from tumor recurrence. J Nucl Med. 2004;45(11):1931–8.
Grkovski M, Kohutek ZA, Schöder H, Brennan CW, Tabar VS, Gutin PH, et al. 18F-Fluorocholine PET uptake correlates with pathologic evidence of recurrent tumor after stereotactic radiosurgery for brain metastases. Eur J Nucl Med Mol Imaging. 2020;47(6):1446–57.
De Zwart PL, van Dijken BRJ, Holtman GA, Stormezand GN, Dieckx RAJO, van Laar PJ, et al. A diagnostic accuracy of pet tracers for the differentiation of tumor progression from treatment-related changes in high-grades glioma: a systematic review and metaanalysis. J Nucl Med. 2020;61(4):498–504.
Filippi L, Frantellizzi V, Vincentis G, Schillaci O, Evangelista L. Clinical applications of TSPO PET for glioma imaging: current evidence and future perspective-a systematic review. Diagnostics (Basel). 2023;13(10):1813.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100.
Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A. 2017;105(3):927–40.
Major MR, Wong VW, Nelson ER, Longaker MT, Gurtner GC. The foreign body response: at the interface of surgery and bioengineering. Plast Reconstr Surg. 2015;135(5):1489–98.
Dinesh SK, Lee SY, Thomas J. A case of mistaken identity: intracranial foreign body reaction after AVM embolisation mimicking a glioma. J Clin Neurosci. 2008;15(4):463–5.
Lopez-Calle J, Colasanti R, Chian C, Choque-Velasquez J. Foreign body granuloma reaction after endovascular therapy of an unruptured right frontal arteriovenous malformation. J Cerebrovasc Endovasc Neurosurg. 2020;22(4):267–72.
Jang SW, Kim SJ, Kim SM, Lee JH, Choi CG, Lee DH, et al. MRI spectroscopy and perfusion MRI imaging finding of intracranial foreign body granuloma: a case report. Korean J Radiol. 2010;11(3):359–63.
Stratton-Powell AA, Anderson IA, Timothy J, Kapur N, Culmer P. Neurosurgical patties: adhesion and damage mitigation. J Neurosurg. 2015;123(1):153–60.
Menovsky T, Ridder DD, de Vries J. Neurosurgical patties and their intraoperative interaction with neural tissue. Surg Neurol. 2009;71(3):326–9.
Staglianò S, D’Arco F, Tan AP, Jeelani O, Morana G, Mankad K. Haemostatic material (Surgicel®) mimicking residual tumour: magnetic resonance imaging findings in operated pediatric neuro-oncology cases. Quant Imaging Med Surg. 2018;8(9):971–8.
Ruiz-Zafra J, Rodriguez-Fernández A, Sánchez-Palencia A, Cueto A. Surgical adhesive may cause false positives in integrated positron emission tomography and computed tomography after lung cancer resection. Eur J Cardiothorac Surg. 2013;43(6):1251–3.
Jaramillo-Jimenez E, Gupta M, Snipes G, Cheek BS, Michael CB, Navarro-Montoya AM, et al. Textiloma mimicking a recurrent high-grade astrocytoma: a case report. J Neurol Surg Rep. 2020;81(1):e7–9.
Winter SF, Forst DA, Oakley DH, Batchelor TT, Diatrich J. Intracranial foreign body granuloma mimicking brain tumor recurrence: a case series. Oncologist. 2021;26(5):e893–7.
Cruz JP, Marotta T, O’Kelly C, Holtmannspöter M, Saliou G, Willinsky R, et al. Enhancing brain lesions after endovascular treatment of aneurysms. AJNR Am J Neuroradiol. 2014;35(10):1954–8.
Yoon MA, Kim E, Kwon BJ, Kim JE, Kang HS, Park JH, et al. Muslinoma and muslin-induced foreign body inflammatory reactions after surgical clipping and wrapping for intracranial aneurysms: imaging findings and clinical features. J Neurosurg. 2010;112(3):640–7.
Kothbauer KF, Jallo GI, Siffert R, Jimenez E, Epstein FJ. Foreign body reaction to hemostatic material mimicking recurrent brain tumor Report of three cases. J Neurosurg. 2001;95(3):503–6.
Wetter A, Grüneisen J, Fliessbach K, Lütje S, Schaarschmidt B, Umutlu L. Choline-based imaging of prostate cancer with combined [18F] Fluorocholine PET and 1h MRI spectroscopy by means of integrated PET/MRI. Clin Imaging. 2017;42:198–202.
Ayata P, Schaefer A. Innate sensing of mechanical properties of brain tissue by microglia. Curr Opin Immunol. 2020;62:123–30.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, MGR, TRS and EMTI; methodology, JPMB, TRS, NCT and CECS; writing—original draft preparation, TRS, JPMB, NCT and CECS; writing—review and editing, EMTI and MGR; visualization, TRS, EMTI and MGR; supervision, JPMB and MGR; project administration, MGR and TRS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained from the patient involved in the study. Approved by the Ethic Committee of the Hospital Virgen de las Nieves (07/02/2022). The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rudolphi-Solero, T., Triviño-Ibáñez, E.M., Martínez-Barbero, J.P. et al. [18F]Fluorocholine PET/CT false positive: foreign body reaction mimicking anaplastic glioma progression. A case report. Egypt J Neurol Psychiatry Neurosurg 59, 131 (2023). https://doi.org/10.1186/s41983-023-00731-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00731-6