Abstract
Purpose
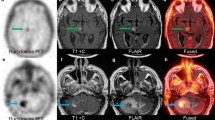
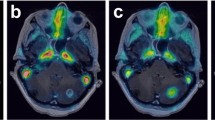
Radiographic changes of brain metastases after stereotactic radiosurgery (SRS) can signify tumor recurrence and/or radiation necrosis (RN); however, standard imaging modalities cannot easily distinguish between these two entities. We investigated whether 18F-Fluorocholine uptake in surgical samples of the resected lesions correlates with pathologic evidence of recurrent tumor and PET imaging.
Methods
About 14 patients previously treated with SRS that developed radiographic changes were included. All patients underwent a preoperative 40-min dynamic PET/CT concurrent with 392 ± 11 MBq bolus injection of 18F-Fluorocholine. 18F-Fluorocholine pharmacokinetics were evaluated by standardized uptake value (SUV), graphical analysis (Patlak plot; KiP) and an irreversible two-compartment model (K1, k2, k3, and Ki). 12 out of 14 patients were administered an additional 72 ± 14 MBq injection of 18F-Fluorocholine 95 ± 26 minutes prior to surgical resection. About 113 resected samples from 12 patients were blindly reviewed by a neuropathologist to assess the viable tumor and necrotic content, microvascular proliferation, reactive gliosis, and mono- and polymorphonuclear inflammatory infiltrates. Correlation between these metrics 18F-Fluorocholine SUV was investigated with a linear mixed model. Comparison of survival distributions of two groups of patients (population median split of PET SUVmax) was performed with the log-rank test.
Results
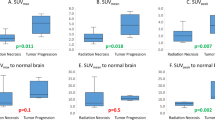
Exactly 10 out of 12 patients for which surgical samples were acquired exhibited pathologic recurrence. Strong correlation was observed between SUVmax as measured from a surgically removed sample with highest uptake and by PET (Pearson’s r = 0.66). Patients with 18F-Fluorocholine PET SUVmax > 6 experienced poor survival. Surgical samples with viable tumor had higher 18F-fluorocholine uptake (SUV) than those without tumor (4.5 ± 3.7 and 2.6 ± 3.0; p = 0.01). 18F-fluorocholine count data from surgical samples is driven not only by the percentage viable tumor but also by the degree of inflammation and reactive gliosis (p ≤ 0.02; multivariate regression).
Conclusions
18F-Fluorocholine accumulation is increased in viable tumor; however, inflammation and gliosis may also lead to elevated uptake. Higher 18F-Fluorocholine PET uptake portends worse prognosis. Kinetic analysis of dynamic 18F-Fluorocholine PET imaging supports the adequacy of the simpler static SUV metric.





Similar content being viewed by others
References
Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54.
Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–40.
Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neuro-Oncol. 2015;125:149–56.
Heper AO, Erden E, Savas A, et al. An analysis of stereotactic biopsy of brain tumors and nonneoplastic lesions: a prospective clinicopathologic study. Surg Neurol. 2005;64:S82–8.
Kongkham PN, Knifed E, Tamber MS, Bernstein M. Complications in 622 cases of frame-based stereotactic biopsy, a decreasing procedure. Can J Neurol Sci. 2008;35:79–84.
DeGrado TR, Coleman RE, Wang S, et al. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res. 2001;61:110–7.
DeGrado TR, Reiman RE, Price DT, Wang S, Coleman RE. Pharmacokinetics and radiation dosimetry of 18F-fluorocholine. J Nucl Med. 2002;43:92–6.
Kwee SA, Ko JP, Jiang CS, Watters MR, Coel MN. Solitary brain lesions enhancing at MR imaging: evaluation with fluorine 18 fluorocholine PET. Radiology. 2007;244:557–65.
Dunphy MPS, Harding JJ, Venneti S, et al. In vivo PET assay of tumor glutamine flux and metabolism: in-human trial of 18F-(2S,4R)-4-Fluoroglutamine. Radiology. 2018;287:667–75.
Grkovski M, Schwartz J, Gönen M, et al. Feasibility of 18F-Fluoromisonidazole kinetic modeling in head and neck cancer using shortened acquisition times. J Nucl Med. 2016;57:334–41.
Roivainen A, Forsback S, Grönroos T, et al. Blood metabolism of [methyl-11C]choline; implications for in vivo imaging with positron emission tomography. Eur J Nucl Med. 2000;27:25–32.
Kenny LM, Contractor KB, Hinz R, et al. Reproducibility of [11C]choline-positron emission tomography and effect of trastuzumab. Clin Cancer Res. 2010;16:4236–45.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–90.
Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16:e270–8.
Huguet F, Yorke ED, Davidson M, et al. Modeling pancreatic tumor motion using 4-dimensional computed tomography and surrogate markers. Int J Radiat Oncol Biol Phys. 2015;91:579–87.
Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol. 1998;19:407–13.
Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med. 2000;41:1861–7.
Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96:191–7.
Galldiks N, Stoffels G, Filss CP, et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med. 2012;53:1367–74.
Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49:694–9.
Sollini M, Sghedoni R, Erba PA, et al. Diagnostic performances of [18F]fluorocholine positron emission tomography in brain tumors. Q J Nucl Med Mol Imaging. 2018;62:209–19.
Rottenburger C, Hentschel M, Kelly T, et al. Comparison of C-11 methionine and C-11 choline for PET imaging of brain metastases: a prospective pilot study. Clin Nucl Med. 2011;36:639–42.
Hara T. 11C-choline and 2-deoxy-2-[18F]fluoro-D-glucose in tumor imaging with positron emission tomography. Mol Imaging Biol. 2002;4:267–73.
Lizarraga KJ, Allen-Auerbach M, Czernin J, et al. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med. 2014;55:30–6.
Cicone F, Minniti G, Romano A, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging. 2015;42:103–11.
Alkonyi B, Barger GR, Mittal S, et al. Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of α-11C-methyl-L-tryptophan PET. J Nucl Med. 2012;53:1058–64.
Sutinen E, Nurmi M, Roivainen A, et al. Kinetics of [(11)C]choline uptake in prostate cancer: a PET study. Eur J Nucl Med Mol Imaging. 2004;31:317–24.
Schaefferkoetter JD, Wang Z, Stephenson MC, et al. Quantitative 18F-fluorocholine positron emission tomography for prostate cancer: correlation between kinetic parameters and Gleason scoring. EJNMMI Res. 2017;7:25.
Grkovski M, Gharzeddine K, Sawan P, et al. 11C-Choline pharmacokinetics in recurrent prostate cancer. J Nucl Med. 2018;59:1672–8.
Lohmann P, Herzog H, Rota Kops E, et al. Dual-time-point O-(2-[(18)F]fluoroethyl)-L-tyrosine PET for grading of cerebral gliomas. Eur Radiol. 2015;25:3017–24.
Bansal A, Shuyan W, Hara T, Harris RA, Degrado TR. Biodisposition and metabolism of [(18)F]fluorocholine in 9 L glioma cells and 9 L glioma-bearing fisher rats. Eur J Nucl Med Mol Imaging. 2008;35:1192–203.
Slaets D, De Vos F. Comparison between kinetic modelling and graphical analysis for the quantification of [18F]fluoromethylcholine uptake in mice. EJNMMI Res. 2013;3:66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding information
This study was funded by the NIH grant 1R21 CA170289-01A1 (Principal Investigator, Kathryn Beal; corresponding, John L. Humm and Ronald G. Blasberg), R01 CA194321 (Principal Investigator: John L. Humm) and the MSK Radiochemistry & Molecular Imaging Probes Core, supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 (Principal Investigator: Craig B. Thompson).
Conflict of interest
RJY declares consulting and grant support from Agios and consulting for Puma, Icon, and NordicNeuroLab. Other authors declare no potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Memorial Sloan Kettering Cancer Center’s Institutional Review Board, Protocol #13-199; registered under ClinicalTrials.gov Identifier NCT02037945) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Brain
Electronic supplementary material
ESM 1
(PDF 67 kb)
Rights and permissions
About this article
Cite this article
Grkovski, M., Kohutek, Z.A., Schöder, H. et al. 18F-Fluorocholine PET uptake correlates with pathologic evidence of recurrent tumor after stereotactic radiosurgery for brain metastases. Eur J Nucl Med Mol Imaging 47, 1446–1457 (2020). https://doi.org/10.1007/s00259-019-04628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04628-6