Abstract
Background
Migraine and stroke are neurovascular diseases that have become a public health problem and a relatively high economic burden. The relationship between migraine and stroke has been described in the literature for a long time. Several studies reveal that migraine can be a risk factor for stroke.
Methods
We systematically searched PubMed using the PRISMA method and keywords according to MeSH terms to prepare this systematic review. We included published articles discussing migraine as a risk factor for ischemic stroke with a cohort study design and English article and listed the statistical value of the study.
Results
In our meta-analysis, we found that migraine significantly increases the incidence of ischemic stroke. There were 12 studies included in this systematic review, and a meta-analysis was performed. The results showed a significant association between migraine and ischemic stroke (HR 1.205, 95% CI 1.151–1.262 p = 0.000), migraine with aura and ischemic stroke (HR 1.442, 95% CI 1.241–1.675 p = 0.000), and migraine without aura and ischemic stroke (HR 1.126, 95% CI 1.048–1.211 p = 0.001). The exact mechanism of stroke caused by migraine is still unclear, although, in some theories, several mechanisms have been described that are thought to be the cause of stroke. Several important points of the cause of stroke in migraine are hemodynamic changes, endothelial dysfunction, cervical artery dissection, vascular reactivity, hypercoagulability, and abortive migraine drugs.
Conclusions
Both migraine with aura and without aura are risk factors for ischemic stroke. Several mechanisms that may cause ischemic stroke in migraine have been described, of which CSD-induced endothelial dysfunction is the primary pathophysiology of ischemic stroke in migraine.
Similar content being viewed by others
Introduction
Previous research has looked at the relationship between stroke and migraine. Stroke is the world's second most significant cause of death and the third leading cause of disability, with a prevalence of 2.9%, particularly among males. On the other hand, migraine affects roughly 13% of the population, with a female predominance, particularly among those of reproductive age. Although the origin of migraine-related stroke is unknown, it is thought that migraine might cause brain ischemia, leading to stroke. Migraine strokes are highly uncommon, accounting for only 0.8% of all strokes [1].
Migraine might be an unintentional risk factor for stroke, which increases with age. According to a prospective study, migraine doubled the chance of having an ischemic stroke [2]. Varying migraine subtypes have different reports. The relative risk (RR) of migraine with aura was 2.16 and increased with the number of attacks. In the case of migraine without aura, several systematic evaluations conclude that it is not a risk factor for stroke. However, this finding is debatable, because multiple cohort studies have found that migraine with and without aura are risk factors for stroke, with migraine with aura having a higher risk. Individually, migraine and stroke are diseases that frequently occur and become a primary social concern, so the interaction between these two diseases must be understood and researched [3].
Migraine is a neurological condition caused by cortical spreading depression (CSD). When CSD occurs, blood flow to the brain, particularly the posterior circulation, increases for around 1–2 min, causing tissue hyperoxia. It is immediately followed by neuronal suppression and hypoperfusion, which lasts 1–2 h and results in a 20–30% reduction in blood flow to the brain, resulting in an oligemic situation. Reduced blood flow alone is not enough to kill cells in typical circumstances. However, if the CSD situation is repeated, the neuron cells will grow more sensitive, and the ischemia threshold will drop, potentially resulting in cell death [4].
In migraine, endothelial dysfunction plays a part in the stroke process. During CSD, proinflammatory cytokines promote endothelial dysfunction, which results in reduced vascular responsiveness, such as vasodilation and vasoconstriction. Endothelial microparticles (EMP), endothelial progenitor cells (EPCs), high-sensitivity CRP (hs-CRP), and endothelin-1 are some of the biomarkers that indicate endothelial dysfunction in migraine (ET-1) [5].
Atherosclerosis is one of the risk factors for thrombus formation, which can lead to ischemic stroke. The link between migraine and atherosclerosis has been demonstrated in several investigations. The carotid intima–media thickness (CIMT) has been used in various analyses. CIMT has been shown to increase in multiple studies [6]. According to Besir and colleagues, CIMT values were more significant in migraine patients, with a mean CIMT value of 0.493 0.074 mm in migraine patients and 0.409 0.053 mm in control patients. According to one study, the frequency and length of migraines are also significant factors in the development of atherosclerosis [7].
Genetic Polymorphism in the MTHFR (methylenetetrahydrofolate reductase) gene is a gene that mediates the increased risk of ischemic stroke in migraine. MTHFR encodes enzymes for folate and homocysteine metabolism. Deletion of the angiotensin-converting enzyme (ACE-DD) gene polymorphism may also be a possibility. ACE-DD is associated with vWF, venous thrombophilia, hypercoagulability, increased vascular smooth muscle tone, and lacunar infarction. ACE-DD is also found in patients with migraines and is associated with increased attacks [8].
Ergotamine and triptans, used to treat migraines, have a vasoconstrictive effect. Dopamine receptors are affected by ergotamine. Cardiovascular diseases, such as coronary heart disease and cerebral vasospasm, can result from high doses. While multiple studies have linked triptans to stroke, no solid evidence has been found. Therefore, in prescribing this drug, it is necessary to know the right dose to prevent this unexpected effect. These drugs in appropriate doses and not misused, rarely cause a stroke [9].
Because there are several inconsistent reports of the association of migraine with stroke, we conducted an updated systematic review and meta-analysis to investigate the association of migraine as a risk factor for ischemic stroke for both migraine with aura and migraine without aura in several cohort studies.
Methods
Searching and screening
We systematically searched PubMed using the PRISMA method and keywords according to MeSH terms to prepare this systematic review. We use keywords 1. “MIGRAINE”; AND 2. “STROKE”; AND 3. “RISK FACTOR” on search engines. SPIDER format was used to establish a search strategy (Sample: Migraineurs, Phenomenon of Interest: Migraine with aura or migraine without aura, Design: an observational study, Evaluation: ischemic stroke, Research type: cohort studies).
Preliminary research and idea validation
We do a preliminary search to identify relevant articles, ensure the validity of the proposed idea, avoid duplication of previously addressed questions, and ensure that we have enough articles for conducting its analysis.
Inclusion and exclusion criteria
We included published articles discussing migraine as a risk factor for ischemic stroke with a cohort study design and English articles, and listed the statistical value of the study. We excluded published articles that did not describe migraine as a risk factor for ischemic stroke with a study design other than a cohort study, not an English article, and did not include the statistical value of the study.
Study selection and screening
The title and abstract were screened based on inclusion and exclusion criteria by two authors (first screening). After screening the title and abstract, two authors review the selected full text (second screening). If two authors disagree, the third author is consulted, and a decision is made by consensus. Articles were included in the meta-analysis when data-pooling and outcome measurement was identical.
Data extraction and quality assessment
Three reviewers (IMOA, EHT, and IPEW) collected and extracted data from selected articles. We extracted study design, study site, population size, age, migraine type, gender, length of follow-up, and adjustment data. We assessed the quality of each selected study using the Newcastle–Ottawa Quality Assessment Scale (NOS) instrument. The quality assessment carried out by three reviewers (IMOA, IPEW, and EHT) will then be confirmed fourth dan fifth reviewers (IASW and NPAPM).
Data items and collection
Three authors (IMOA, EHT, and IPEW) collected and extracted data from selected articles. The data were extracted for the following variables: study design, study site, population size, age, migraine type, gender, length of follow-up, adjustment data, and statistical data.
Statistical analysis
The statistical data were calculated using Comprehensive Meta-analysis V3 software.
Data checking
Due to the expected human error and bias, we do the data checking step, in which every included article is compared with its counterpart in an extraction sheet by evidence photos, to detect mistakes in data. If two authors disagree (IMOA and EHT), the third author (IPEW) is consulted, and a decision is made by consensus. If needed, fourth and fifth authors (IASW and NPAPM) were included.
Ethical approval
Ethical approval is not required.
Results
Literature search
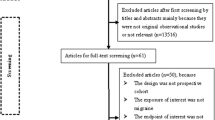
There are 1043 articles in our search engine. We sorted the articles only observational studies, so 469 articles were then screened based on title and abstract. After screening the titles and abstracts, we got 34 cohort study articles. Finally, 12 articles matched our inclusion and exclusion criteria, and data were extracted for this systematic review. The flow of the article search system can be seen in Fig. 1.
Quality assessment
The quality assessment of each study was assessed using Newcastle–Ottawa Quality Assessment Scale (NOS) instrument. The study with a total score range of 7–10 is high quality, 4–6 has a low risk of bias, and 1–3 has a high risk of bias. There were three studies with a score of 9/9, 4 studies with a score of 8/9, 3 studies with a score of 7/9, and 2 studies with a score of 6/9. Based on NOS, it is known that there are ten studies with high quality and two studies have a low risk of bias. The results of the quality assessment can be seen in Table 1.
Study characteristic
The cohort studies were conducted in Korea, the United States, the United Kingdom, Sweden, Finland, and Taiwan. The study subjects ranged from 6730 to 1,125,691 people, with a mean age ranging from 45.3 to 73.4 years. Lee and colleagues carried out the largest samples, and Tsai and colleagues carried out the smallest. Each study describes the relationship between migraine and ischemic stroke by statistical analysis using odds and hazard ratios, and variable adjustments have been made. Study characteristics and conclusions from each study can be seen in Table 2.
Main pooled results of meta-analysis
Several studies were combined for meta-analysis. There were nine studies to determine the association between migraine and ischemic stroke, seven studies for migraine with aura and stroke, and six for migraine without aura and ischemic stroke.
Migraine and ischemic stroke
Figure 2 shows the results of a random-effect models meta-analysis for migraine and stroke. Of the nine studies, five studies had insignificant results and four studies with significant results. When a meta-analysis was performed, the combined hazard ratio was significant (HR 1.205 95% CI 1.151–1.262 p = 0.000). Heterogeneity obtained p = 0.118, I2 = 37.5.
Migraine with aura and ischemic stroke
Figure 3 shows the results of a random-effect models meta-analysis for migraine and stroke. Of the seven studies, there were two studies with insignificant results and five studies with significant results. The combined hazard ratio was significant (HR 1.442, 95% CI 1.241–1.675 p = 0.000). Heterogeneity obtained p = 0.14, I2 = 45.5.
Migraine without aura and ischemic stroke
Figure 4 shows the results of a random-effect models meta-analysis for migraine and stroke. Of the 6 studies, there were five studies with insignificant results and one study with significant results. The combined hazard ratio was significant (HR 1.126, 95% CI 1.048–1.211 p = 0.001. Heterogeneity obtained p = 0.45, I2 = 0.
Discussion
In our meta-analysis, both migraine aura and without aura were significant risk factors for ischemic stroke. Migraine, in general, increased the risk of ischemic stroke by 1.2-fold (HR 1.205, 95% CI 1.151–1.262 p = 0.000), migraine with aura increased the risk of ischemic stroke by 1.4-fold (HR 1.442, 95% CI 1.241–1.675 p = 0.000), and migraine without aura increased the risk of ischemic stroke by 1.1-fold (HR 1.126, 95% CI 1.048–1.211 p = 0.001). The meta-analysis results of migraine in general and migraine with aura were relatively similar to previous studies. Different results were seen in the meta-analysis of the migraine without aura group. Many studies have revealed that migraine without aura does not increase the risk of ischemic stroke, but our meta-analysis suggests that migraine without aura is a risk factor for ischemic stroke. Migraine without aura can be a risk factor for ischemic stroke, even though it has a lower HR value than migraine with aura. Many cohort studies had a non-significant value or HR < 1 in the forest plot of the meta-analysis (Fig. 4). However, the combined hazard ratio shows significant results, because the study by Lee and colleagues has a much greater total population than other studies.
The exact mechanism of stroke caused by migraine is still unclear, although, in some theories, several mechanisms have been described that are thought to be the cause of stroke. In the studies included in this systematic review, cortical spreading depression is the underlying mechanism of migraine, which is the underlying cause of stroke in migraine [22]. As mentioned in the introduction section, several important points of the cause of stroke in migraine are hemodynamic changes, endothelial dysfunction, vascular reactivity, and abortive migraine drugs themselves.
In migraine, there is no specific advice for preventing ischemic stroke. It is preferable to alter migraine risk factors. Antiplatelet medications, such as aspirin, are not advised. As previously stated, the frequency of migraine attacks is linked to the risk of ischemic stroke. As a result, it is preferable to prevent migraine attacks by changing migraine risk factors to prevent ischemic stroke in migraine sufferers [2].
Conclusions
In our systematic review and meta-analysis, migraines with aura and without aura are associated with an increased risk of ischemic stroke. Preventing the migraine attack by modifying migraine risk factors is preferable to prevent ischemic stroke rather than antiplatelet agents.
Strengths and limitations
There are several strengths and limitations in our systematic review. First, our systematic review has many studies and a large sample population. Second, in this study, we carried out a quantitative analysis, namely, a meta-analysis, and obtained combined results between several studies, resulting in one statistical conclusion, so that the study value was higher. The limitation of our study is that some of the studies are older than the last 10 years. However, this is not a problem, because the number of studies published in the previous 10 years has seven, so five other studies over the last 10 years can be used as data reinforcement.
Availability of data and materials
Not applicable.
Abbreviations
- BA:
-
Basilar Artery
- BHI:
-
Breath Holding Index
- CI:
-
Confidence interval
- CIMT:
-
Carotid intima–media thickness
- CSD:
-
Cortical spreading depression
- EMP:
-
Endothelial microparticles
- EPCs:
-
Endothelial progenitor cells
- HR:
-
Hazard ratio
- hs-CRP:
-
High sensitivity C-reactive Protein
- HVI:
-
Hyperventilation Index
- I 2 :
-
Heterogeneity statistic
- MCA:
-
Middle cerebral artery
- MFV:
-
Mean flow velocity
- MVI:
-
Migraine Vascular Index
- NO:
-
Nitrite oxide
- OR:
-
Odds ratio
- PAF:
-
Platelet activating factor
- PCA:
-
Posterior cerebral artery
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RR:
-
Relative risk
- TCD:
-
Transcranial Doppler
- vWF:
-
Von-Willebrand Factor
References
Harriott AM, Barrett KM. Dissecting the association between migraine and stroke. Curr Neurol Neurosci Rep. 2015;15(3):5.
Lee MJ, Lee C, Chung CS. The migraine-stroke connection. J Stroke. 2016;18(2):146–56.
Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123(7):612–24.
Spalice A, Del Balzo F, Papetti L, Zicari AM, Properzi E, Occasi F, et al. Stroke and migraine is there a possible comorbidity? Ital J Pediatr. 2016;42(1):41.
Tietjen GE, Maly EF. Migraine and ischemic stroke in women. A narrative review. Headache. 2020;60(5):843–63.
Mason BN, Russo AF. Vascular contributions to migraine: time to revisit? Front Cell Neurosci. 2018;12:233.
Besir FH, Koçer A, Dikici S, Yazgan S, Ozdem Ş. The evaluation of atherosclerosis in migraine patients. Pain Pract. 2013;13(1):41–5.
Samaan Z, Gaysina D, Cohen-Woods S, Craddock N, Jones L, Korszun A, et al. Methylenetetrahydrofolate Reductase Gene Variant (MTHFR C677T) and migraine: a case control study and meta-analysis. BMC Neurol. 2011;11:66.
Potter PF. Triptans in migraine: the risks of stroke, cardiovascular disease, and death in practice. Neurol Neurosurg. 2006;2006:120–1.
Li L, Schulz UG, Kuker W, Rothwell PM. Age-specific association of migraine with cryptogenic TIA and stroke. Neurology. 2015;85(17):1444–51.
Monteith TS, Gardener H, Rundek T, Elkind MSV, Sacco RL. Migraine and risk of stroke in older adults. Neurology. 2015;85(8):715–21.
Androulakis XM, Kodumuri N, Giamberardino LD, Rosamond WD, Gottesman RF, Yim E, et al. Ischemic stroke subtypes and migraine with visual aura in the ARIC study. Neurology. 2016;87(24):2527–32.
Lantz M, Sieurin J, Sjölander A, Waldenlind E, Sjöstrand C, Wirdefeldt K. Migraine and risk of stroke: a national population-based twin study. Brain. 2017;140(10):2653–62.
Sumelahti ML, Sumanen MS, Mattila KJ, Sillanmäki L, Sumanen M. Stroke and cardiovascular risk factors among working-aged Finnish migraineurs. BMC Public Health. 2021;21(1):1088.
Kurth T, Michael G, Nancy RC, Giancarlo L, Hans-Christoph D, Julie EB. Migraine and risk of cardiovascular disease in women. JAMA Network. 2006;296(3):283–91.
Kurth T, Markus S, Giancarlo L, Julie EB. Migraine frequency and risk of cardiovascular disease in women. Am Acad Neurol. 2009;73(8):581–8.
Kurth T. Migraine and risk of cardiovascular disease in men. Arch Intern Med. 2007;167:795–801.
Kurth T, Slomke MA, Kase CS, Cook NR, Lee IM, Gaziano JM, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64(6):1020–6.
Stang PE, Carson AP, Rose KM, Mo J, Ephross SA, Shahar E, et al. Headache, cerebrovascular symptoms, and stroke: the atherosclerosis risk in communities study. Neurology. 2005;64(9):1573–7.
Lee SY, Lim JS, Oh DJ, Kong IG, Choi HG. Risk of ischaemic stroke in patients with migraine: a longitudinal follow-up study using a national sample cohort in South Korea. BMJ Open. 2019;9(4):e027701.
Tsai CL, Chou CH, Lee PJ, Yin JH, Chen SY, Lin CC, et al. The potential impact of primary headache disorders on stroke risk. J Headache Pain. 2016;17(1):108.
Sacco S, Kurth T. Migraine and the risk for stroke and cardiovascular disease. Curr Cardiol Rep. 2014;16(9):524.
Acknowledgements
We would like to thank Department of Neurology, Faculty of Medicine, Universitas Udayana/Sanglah General Hospital, Bali, Indonesia for support in making this review
Funding
No funding sources.
Author information
Authors and Affiliations
Contributions
IMO concept the idea and design of the article. EHT and IPEW analyzed the data of meta-analysis. IASW and NPAPM draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
I, on behalf of my co‐authors submit the following manuscript for publication consideration. I understand the objectives of the journal and have formatted the manuscript to fit the style and needs of the journal. I confirm that the manuscript has been prepared for and sent only to The Egyptian Journal of Neurology, Psychiatry and Neurosurgery for publication consideration and not submitted to any other journal or any other type of publication either by me or any of my co‐authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adnyana, I.M.O., Widyadharma, I.P.E., Tedyanto, E.H. et al. Migraine as a risk factor for ischemic stroke: a systematic review and meta-analysis of cohort studies. Egypt J Neurol Psychiatry Neurosurg 58, 125 (2022). https://doi.org/10.1186/s41983-022-00562-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-022-00562-x