Abstract
Background
The banana stem weevil, Odoiporus longicollis (Olivier), is a serious threat to banana cultivation world over. Since banana is a food crop, the use of naturally infecting biological control agents could be an effective alternative to manage the insect pest instead of harmful chemicals. Also, the efficacy of entomopathogenic fungi against O. longicollis was used in bioassay.
Results
Among the Beauveria bassiana isolates tested the median lethal concentration (LC50) 10.468 × 105 conidia ml−1 when treated with B. bassiana (NRCBEFPMP1), two other isolates of B. bassiana, namely NRCBEPF22 and NRCBEPF2, were also effective against O. longicollis and recorded LC50 of 12.617 × 105 and 12.891 × 105 conidia ml−1, respectively. The results of bioassay with different Metarhizium spp. showed variations in efficacy, where the most virulent isolate was M. quizhouense (NRCBEPF11) with LC50 8.050 × 105 conidia ml−1. Scanning electron microscopic analysis showed that B. bassiana and M. quizhouense caused infection by cuticle penetration and completed the infection process in 15 days. The composition of volatile organic compounds released by B. bassiana and M. anisopliae during pathogenesis showed that a significantly high number of known insect volatiles were present in infected insects. Consequently, these volatiles were emission in Insect attractant, Odorant receptor agonist, Plant hormone Plant, and Microbial Metabolites, through the biological activity, such as Methyl salicylate, Benzaldehyde, alpha-Terpineol, Limonene, Benzene, 1,2-dimethoxy, Phthalic acid, 1-Octadecene, Phenylacetaldehyde, 3-Octanone, Octanal, Methylheptenone and 2-Ethyl-1-hexyl alcohol.
Conclusion
Overall, the results show that EPF could significantly reduce damage by O. longicollis and produce a wide profile of secondary metabolites. Further, analysis was used for principal components to determine whether separated classes of fungi can be distinguished from one another based on their metabolite profiles.
Similar content being viewed by others
Background
Banana (Musa sp.) is the second most important fruit crop in India next to mango. Over nineteen species of insects have been reported to infest banana cultivars (Padmanaban et al. 2001). One important pest is the banana pseudostem borer, Odoiporus longicollis (Olivier) (Coleoptera: Curculionidae). Also known as banana stem weevil (BSW). It can cause substantial damage in terms of production and productivity of banana (David 2008). Recent reports suggest that O. longicollis is distributed over diverse geographical locations in India (Isahaque 1978) and serious crop loss due to BSW (Jayanthi and Varghese 1999).
In India, application of synthetic chemical insecticides is the most common option to control BSW (Thippaiah et al. 2011). Since banana is a food crop, safer control methods of BSW are needed. One important safe alternative is use of biological control for entomopathogenic fungi (EPF), as they can grow naturally in soils and are capable of infecting various insects (Sharmila and Mohan 2015). Researchers have tried to use Metarhizium anisopliae, and Beauveria bassiana and their related species to control O. longicollis (Velavan et al. 2021; Sripriya et al. 2000). In addition, foliar application of EPF formulation against BSW has also been attempted in the fields (Azam et al. 2010). Studies have also shown certain endophytic isolates of B. bassiana can be highly effective on O. longicollis, if allowed to colonize in early stages of crop (Alagesan et al. 2019). Padmanaban et al. (2019) could identify endophytic EPF within the Musa germplasm cultivars that induced natural protection against banana pests. While EPF are identified based on their morphological and molecular characteristics, species identification among Metarhizium spp. is difficult because of its structural simplicity and the lack of distinctive features (Velavan et al. 2021). Recently the diversity of Beauveria spp. occurring in India was studied through phylogenetic analysis (Kisaakye et al. 2021) and taxonomic status of Beauveria spp. occurring worldwide was attempted using both morphological and molecular methods (Rehner et al. 2011).
At National Research Centre on Banana in India, six EPF, namely B. bassiana, B. brongniartii, M. anisopliae, M. robertsii, M. quizhouense and M. pinghaense native isolates, have been tested against leaf and fruit scarring banana pest Basilepta subcostata. Thus, B. brongniartii, M. anisopliae and M. pinghaense strains were found effectively infecting B. subcostata (Viswakethu et al. 2021). Although a number of EPF have been evaluated to control insect pests of banana, very limited data is available on use of EPF against O. longicollis. The present study was undertaken to know the biological control ability of EPF strains, isolated from naturally infected pests of banana. Consequently, electron microscopy analyses of the infection process and identification of insect specific volatiles released during pathogenesis were also studied.
Methods
Entomopathogenic fungi
Twenty-seven native strains of EPF belonging to Beauveria bassiana, B. brongniartii, Metarhizium anisopliae M. robertsii, M. quizhouense and M. pinghaense were obtained from the culture collection of ICAR-National Research Centre on Banana (ICAR-NRCB), India. Naturally isolated at Odoiporus longicollis, Cosmopolites sordidus, Basilepta subcostata and Galleria mellonella identity was established by morphological and molecular studies (Table 1).
Rearing of Odoiporus longicollis
Specimens of banana stem weevils were collected from banana-growing regions of Thanjavur, Tiruchirappalli, and Theni in Tamil Nadu during 2017–2018. The weevils were maintained at Entomology Laboratory, Division of Crop Protection ICAR-NRCB, Tiruchirappalli. Banana pseudostem pieces (4–8 cm) were kept in plastic containers (10 kg volume) and weevils were released into the containers for feeding, mating and oviposition (Padmanaban et al. 2001). Pseudostems were changed once in 15 days. After hatching, for further development, the first instar grubs (larvae) were transferred on to fresh pieces of pseudostem. Larvae were reared in various stages viz. egg, 1st, 2nd, 3rd larval instars and adults. After adult emergence, they were placed in jars and supplied with cotton wicks saturated with 8–10% honey. Adults were fed with banana leaf sheath at 28 ± 1 °C, 75 ± 5% RH.
Bioassay
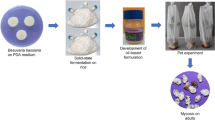
The EPF cultures were grown on potato dextrose yeast agar (PDAY) medium for 10–15 days at 25 ± 0.5 °C. Mycelial mats containing spores were harvested in 100 ml sterile distilled water in tubes with continuous stirring. The harvested contents were filtered through a single layer of muslin cloth to remove debris and mycelia. Conidial concentration was estimated using a hemocytometer under a light microscope (Olympus model BX100). Subsequently conidial suspensions (1 × 107, 1 × 106, 1 × 105, 1 × 104, and 1 × 103 conidia ml−1) were prepared with 0.1% TritonX-100, 0.2% Tween 80 and 0.1% glycerol. Adults of stem weevils were transferred aseptically to fresh plastic boxes (10 cm diameter and 30 cm height). The conidial suspensions of EPF isolates were swabbed on leaf sheaths (8–10 cm length) individually. For comparison, two commercial isolates, namely M. anisopliae (M1, M2) and B. bassiana (BCRL, TARI) were included in the experiment. Five replications were maintained for each treatment and each replication had 15 test adults. Leaf sheaths treated with water containing 0.1% Triton X-100, 0.2% Tween 80 and 0.1% glycerol served as control. Mortality was recorded every day up to 15 days after inoculation. Infected insect cadavers were transferred to the wet chamber and the pathogenic fungus was re-isolated and confirmed based on culture characteristics and spore morphology.
Volatile organic compound detection from insect–fungus interaction
Virulent EPF isolates, namely Beauveria bassiana, B. brongniartii, Metarhizium anisopliae, M. robertsii, M. quizhouense and M. pinghaense, were selected to identify secretion of volatile organic compounds (VOC) or organic compound emission of secondary metabolites during insect–fungus interaction. Infected dead insects were carefully returned to individual flasks and maintained without feeding under rearing conditions as previously described (Crespo et al. 2006). VOC were sampled from adults previously treated with either 1 × 108 conidia ml−1 or sterile distilled water containing 0.01% Tween-80 (controls). For each condition, one adult of known sex was gently placed in a 20-ml glass vial sealed with a Teflon cover having a rubber septum. The vial was vortexed for 10 s to elicit the release of volatiles; the released volatiles were immediately sampled from the headspace corresponding to the gaseous phase in contact with the insect sample (Arthur and Pawliszyn 1990). Three independent replicates (each replicate from a different individual) were performed for each condition (fungus-treated and untreated males and females). A vial containing no insect was used as controls. Volatile collections from EPF uninfected and infected adults were performed with a (polydimethylsiloxane PDMS) fiber with a 50-μm film thickness (Supelco, Inc., Bellefonte, PA, U.S.A.). The fiber had been previously conditioned according to the manufacturer’s instructions and was systematically reconditioned before each analysis.
Statistical analysis
The corrected mortality rate was calculated using the Abbotts formula. The lethal concentration LC50 was analyzed by using Probit analysis software in SPSS® version 25. The concentration responses of each replicate were observed for estimation of median lethal concentration (LC50) to kill 50% of exposed adults only. Principal components analysis (PCA), to illustrate the differences in the profiles of volatile compounds released during Insect–Fungal interaction, was also subjected to statistical analysis (Origin vs. OriginPro).
Results
Bioassay against Odoiporus longicollis
Twelve B. bassiana isolates that included two commercial (BCRL & TARI) and two B. brongniartii strains were tested against adults of O. longicollis. The adults of O. longicollis were susceptible to all the tested Beauveria spp. used in a concentration-dependent manner in five concentrations (1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107 conidia ml−1). High mortality was observed at low conidial concentrations that varied significantly (Table 2). It was observed that median lethal concentration (LC50) value for weevils was 10.468 × 105 conidia ml−1 in B. bassiana (NRCBEFPMP1) indicating that this isolate was the most virulent (Table 1). Two other isolates, namely (NRCBEPF22 and NRCBEPF2), were also virulent and showed LC50 of 12.617 × 105 and 12.891 × 105 conidia ml−1 (Table 2). The highest concentration requirement was exhibited by the two commercial strains BCRL and TARI and they exhibited the same LC50 value of 29.962 conidia ml−1. The results established that B. bassiana strains (NRCBEFPMP1, NRCBEPF22 and NRCBEPF2) were virulent and had potential to be deployed in the field.
Bioassay of 19 Metarhizium spp., to determine concentration dependent virulence against O. longicollis showed significant differences in mortality (Table 3). Probit analysis showed a variation in virulence among the different Metarhizium isolates. Based on LC50 values the most virulent isolate was M. quizhouense (NRCBEPF11) that exhibited the ability to kill 50% of the tested adults with 8.050 × 105 conidia ml−1. Other virulent isolates were M. pinghaense (NRCBEPF7), M. robertsii (NRCBEPF23) and M. robertsii (NRCBEPF24) with median lethal concentration (LC50) of 10.155, 10.497 and 12.443 × 105 conidia ml−1, respectively (Table 3). Hence, these isolates could be further tested in the field. The least virulent was M. anisopliae M1 (25.448 × 105 conidia ml−1). There was no evidence of mycosis in any control cadavers, whereas mycosis was confirmed on all dead O. longicollis treated with Beauveria or Metarhizium spp.
Scanning electron microscope (SEM) studies
SEM analysis was conducted by the model number TM3030 Plus (Hitachi) at Indian Institute of Horticultural Research, Bengaluru, India. SEM images of adults treated with strains of B. bassiana (NRCBEFPMP1) and M. quizhouense (NRCBEPF11) revealed that after adhesion of conidia onto the cuticle of O. longicollis, some of the conidia germinated and mycelia appeared after 10 days. The SEM studies revealed detailed information about the attachment and infection process of B. bassiana and M. quizhouense. In the sporulated cadavers, hyphae were detected in cuticle, inter-segments of legs, antenna and abdomen. Conidiogenesis was observed on the cuticle (Figs. 1 and 2). The images showed that the virulent EPF infected approximately 15 days to complete its infection process (Fig. 3).
SEM image showing the process of Beauveria bassiana infection in Odoiporus longicollis. A, B Conidia adhered to the surface of adults insect (2 mm & 500 μm), C, D the cuticle surface of the insect of hyphae (scale par 50 μm), E, F large amount formed in mycelia of B. bassiana (scale par 20 μm & 20 μm)
SEM image showing the process of Metarhizium quizhouense infection in Odoiporus longicollis. A, B Conidia adhered to the surface of adults insect (2 mm), C, D the cuticle surface of the insect of hyphae (scale par 50 µm & 30 µm), E, F large amount formed in mycelia of M. quizhouense (scale par 50 µm & 20 µm)
Bioassays performed in entomopathogenic fungi native isolated were affected by mummification of insects after 15 days of infection; a, b Beauveria brongniartii, and Beauveria bassiana cadaver fully covered by bright white mycelia power; c Metarhizium robertsii; d Metarhizium quizhouense; e Metarhizium pinghaense; f Metarhizium anisopliae the conidia formatted and the insect cadaver green
Identification of volatile organic compounds during pathogenesis
The composition of volatile organic compounds (VOC) released during pathogenesis by virulent EPF was investigated. Gas chromatography mass spectrometry (GC–MS) analyses showed that a significantly higher number of known insect volatile compounds were present among fungus-treated insects, when compared to untreated control. Insect toxic compounds were detected only in the volatiles of the fungus-exposed insects. These toxic compounds were identified as: Dihydrothiophenone, Benzaldehyde, Phenylacetaldehyde, Methylheptenone, 3-Octanone, 1-Hexanol, 2-ethyl-, Methyl salicylate, alpha-Terpineol, N-(3-Butenyl)-N-methylcyclohexanamine, Benzene, 1,2-dimethoxy-4-(methoxymethoxy), N,N'-Ethylenedi-beta-alanine, Palmitic acid, Caryophyllene oxide, 1-Octadecene, Hexadecanoic acid, Cyclohexane, 1,1'-dodecylidenebis [4-methyl] and these compounds were mainly categorized as insect repellents, attractants, plant metabolite, antimicrobial or antioxidants..
The PCA plot is presented in Fig. 4. PCA was applied to reduce the redundant information in data and to group the correlated responses into principal components (Hammoudaa et al. 2017). Each of the first principal components explains a variance of 88.53%. The relative peak areas of all identified volatile compounds were selected for the calculation of the main components. A multivariate analysis showed clear class separation of Metarhizium quizhouense species (Table 4). Slight differences were observed in the metabolite profiles of B. bassiana, B. brongniartii, M. robertsii, M. pinghaense and M. anisopliae.
Discussion
In the present study, 27 native strains of EPF belonging to B. bassiana, B. brongniartii, M. anisopliae, M. robertsii, M. quizhouense and M. pinghaense (Table 1) against adults of banana stem weevil O. longicollis were tested under laboratory conditions. Among the tested organisms, M. quizhouense (NRCBPF11) was observed to be the most virulent needing 8.050 × 105 conidia/ml to kill 50% of the beetles. However, isolates such as M. pinghaense, M. robertsii, B. brongniartii and B. bassiana were also found to be virulent (LC50 of 10.468 to 12.617 × 105conidia ml−1).
Bioassay studies showed that the dead adults were wholly covered by white or green conidia of the tested virulent fungi (Fig. 2). Fungal germination was also observed on dead insect, while the insect’s color became white with spongy mycelia of dark green or yellowish-green. The strategy of the present study was to see if adults get infected through treated leaf sheath and the results establish this fact. Systemic fungicides worked well in controlling the larval stages of O. longicollis as they feed on the inside of the leaf sheath. Adults were found feeding on fallen leaves and spraying these leaves helped infecting them. Studies showed that M. anisopliae and B. bassiana) were found to be infective at 1 × 108 conidia ml−1 against adults O. longicollis (Alagesan et al. 2019). In other studies, a high mortality was obtained with “beauvericide” B. bassiana-based formulation when used @ 1 × 107 spores ml−1 (Awasthi et al. 2017). Formulations of B. bassiana and M. anisopliae exhibited LC50 value of 1.25 × 106 conidia ml−1 against O. longicollis at 15 days (Sharmila and Mohan 2015). The present studies showed that Metarhizium spp. needed less conidia to cause 50% mortality. Similar results were obtained against other banana pests like Cosmopolites sordidus (stem borer) with conidial suspension (1.1 × 107 conidia ml−1) of B. bassiana (Lopes et al. 2011). Reports also suggested that with a high conidial concentration, a high mortality can be obtained (Akello et al. 2007). Further studies are needed to evaluate the virulent EPF so as to verify whether the mechanism observed in vitro occurs also in vivo.
Lozano-Soria et al. (2020) reported that in infected banana black weevil (BW), Cosmopolites sordidus and EPF can secrete (styrene, benzothiazole, camphor, borneol, 1,3-dimethoxy-benzene, 1-octen-3-ol and 3-cyclohepten-1-on, Alcohol (3-Octanone), and Phthalic acid) volatile compounds that can behave as attractants and repellents. This was also found in EPF infected banana fruit scarring beetle Basilepta subcostata Jac (Padamnaban et al. 2019). In another study Kavitha et al. (2020) could show that steroid in Stigmasterol-3-O-Glucoside was toxic to O. longicollis. Through, volatile was investigate in secondary metabolites, such as (Z)-9-octadecenamide, (Z,Z)-9,12-octadecadienoic acid (linoleic acid), (Z,Z,Z)-9,12,15-ocatadecadienoic acid (linolenic acid) and n-hexadecanoic acid (palmitic acid) for the identified and other invertebrates were evaluated as attractants or repellents (Rivero-Borja et al. 2018). VOCs mediate interactions between micro-organisms such as fungal insects that have been correlated with their pathogenic activity (Hummadi et al. 2021). Overall, EPF was emitted through the Volatile organic compounds (VOCs) can be used in chemotaxonomic profiling such as antifungal activity, phytotoxicity, symbiotic regulation, insect attractant, and repellent activities also other environmental parameters (Lobo et al. 2018).
In the present study, a total of 50 volatile compounds were identified from EPF infected banana stem weevil with major constituents having methyl group, methyl ester, aromatic aldehyde, carbon atoms, carboxyl groups, and alcohol. These compounds may alter the behavior of O. longicollis and hence some of the identified chemicals could interrupt and modify its behavior and in general its search ability for the host (banana) and could be served as a tool for management of O. longicollis. Recently researchers have reported the attraction of O. longicollis towards “male aggregation pheromone” such as “2-methyl-4-heptanol” but it was less effective but when it was used in combination with pseudostem extract it resulted in significant attraction (Palanichamy et al. 2019).
As the adults were targeted in this study, the infection process through SEM was targeted. During, infection process by both B. bassiana and M. anisopliae was typical with adhesion of conidia on surface, germination, penetration of the host cuticle and completion of life cycle by mycelial growth and sporulation on surface (Aw and Hue, 2017). Hydrophobins and cuticles were degrading enzymes such as chitinases, proteases and lipases are produced by EPF which help in penetration (Aw and Hue, 2017). The host was killed by the pathogen growth and release of fungal toxins in 15 days.
Conclusion
Biological control agents such as EPF could be used for management of banana pseudostem weevil. In the present study virulent isolates belonging to Metarhizium sp., and Beauveria sp., were identified as promising alternatives to chemical control. SEM studies showed that the infection process was completed in 15 days. The study had also identified volatiles produced during pathogenesis of virulent Beauveria sp. and Metarhizium sp. The development of possible new bio-control agents against the invasive banana pest is now possible with a better understanding of the potential use of indigenous EPF.
Availability of data and materials
All data of the study have been presented in the manuscript, and high-quality and grade materials were used in this study. Data associated with this study has been deposited at NCBI GenBank: Database under the accession numbers (NCBI Acc. MT645316, 318, 309; MT140307; MT151783, 786; MK899434; MK834817), M. anisopliae (MK834813, 805; MN888761, 763; MN892390, 391; MT140304, 308); B. brongniartii (MT151781, 784), M. robertsii (MN889408; MN892393, 394; MN893382, 380; MK836090), M. quizhouense (MN892392, MN893383) and M. pinghaense (MN892389).
Code availability
(Graph was generated using Origin Lab Professional Version 2021b software; URL link:www.OriginLab.com/2021b, accessed on 29 September 2021) software was used in the paper for statistical analysis.
Abbreviations
- VOC:
-
Volatile organic compounds
- SEM:
-
Scanning electron microscopic
- BSW:
-
Banana stem weevil
- LC50 :
-
Lethal concentration
- EPF:
-
Entomopathogenic fungi
- NRCB:
-
National Research Centre on Banana
- PDAY:
-
Potato dextrose yeast agar
- PCA:
-
Principal components analysis
- GC:
-
Gas chromatography
- MS:
-
Mass spectrometry
References
Akello J, Dubois T, Gold CS, Coyne D, Nakavuma J, Paparu P (2007) Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). J Invertebr Pathol 96:34–42
Alagesan A, Tharani G, Padmanaban B, Manivannan S, Jawahar S (2019) An assessment of biological control of the banana pseudostem weevil Odoiporus longicollis (Olivier) by entomopathogenic fungi Beauveria bassiana. Biocatal Agric Biotechnol 20:101262
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62(19):2145–2148
Aw KMS, Hue SM (2017) Mode of infection of Metarhizium spp. fungus and their potential as biological control agents. J Fungi. https://doi.org/10.3390/jof3020030
Awasthi NS, Sridharan S, Mohankumar S (2017) In vitro evaluation of native isolate of Metarhizium anisopliae (Metchinkoff) sorokin and its oil in water formulations against Odoiporus longicollis Olivier. J Biol Control 31(4):248–252
Azam M, Tara JS, Ayri S, Feroz M, Ramamurthy VV (2010) Bionomics of Odoiporus longicollis Olivier (Coleoptera: Rhynchophoridae) on banana plant (Musa paradisica). Mun Ent Zool 5(2):627–635
Crespo R, Pedrini N, Juárez MP, Bello GMD (2006) Volatile organic compounds released by the entomopathogenic fungus Beauveria bassiana. Microbiol Res 163:148–151
David BV (2008) Biotechnological approaches in IPM and their impact on environment. J Biopesti 1:01–05
Hammoudaa IB, Freitasb F, Ammara S, Gomes Da Silva MDR, Bouaziz M (2017) Comparison and characterization of volatile compounds as markers of oils stability during frying by HS–SPME-GC/MS and Chemometric analysis. J Chromatogr B 1068(1069):322–334
Hummadi EH, Dearden A, Generalovic T, Clunie B, Harrott A, Cetin Y et al (2021) Volatile organic compounds of Metarhizium brunneum influence the efficacy of entomopathogenic nematodes in insect control. Biol Control 155:104527
Isahaque NMM (1978) Note on the incidence of Odoiporus longicollis Olivier on banana in Assam. Pesticides 12(6):22–24
Jayanthi PDK, Verghese A (1999) Report of the occurrence of banana weevils in Bangalore. Insect Environ 4(4):153
Kavitha KJ, Anil John J, Evans DA (2020) Stigmasterol-3-O-glucoside, an allelopathic molecule responsible for pest resistance of Thenkaali (AAB), a Musa cultivar against Odoiporus longicollis Olivier. Cur Sci 118(6):946–953
Kisaakye J, Fourie H, Coyne D, Cortada L, Masinde S, Subramanian S, Haukeland S (2021) Evaluation of the Entomopathogenic Potential of Beauveria bassiana, Metarhizium anisopliae and Isaria fumosorosea for Management of Cosmopolites sordidus Germar (Coleoptera: Curculionidae). Agriculture 11:1290
Lobo LS, Girotti JR, Mijailovsky SJ, Fernandes ÉKK, Luz C, Pedrini N (2018) Synthesis and secretion of volatile short-chain fatty acids in Triatoma infestans infected with Beauveria bassiana. Med Vet Entomol. https://doi.org/10.1111/mve.12306
Lopes RB, Michereff-Filho M, Tigano MS, Oliveria JN, Lopez EL, Fancelli M, da Silva JP (2011) Virulence and horizontal transmission of selected Brazilian strains of Beauveria bassiana against Cosmopolites sordidus under laboratory conditions. B Insectol 64:201–208
Lozano-Soria A, Ugo Picciott U, Lopez-Moya F, Lopez-Cepero J, Porcelli F, Lopez-Llorca VV (2020) Volatile Organic Compounds from Entomopathogenic and Nematophagous Fungi, repel banana black weevil (Cosmopolites sordidus). Insects 11:509
Padmanaban B, Sundararaju P, Sathiamoorthy S (2001) Incidence of banana pseudostem borer, Odoiporus longicollis (Oliv.) (Curculionidae: Coleoptera) in banana peduncle. Indian J Entomon 63:204–205
Padmanaban B, Kamala Jaynth, PD, Bakthavatsalam N, Sarvan kumar P, Baskar N, Velavan V, Karthikeyan C, Uma S (2019) Role of host plant volatiles in adult attraction and auto dissemination of entomopathogenic fungi: A case study with Banana fruit scarring beetle Basilepta subcostata (Jac.). In: International conference on plant protection in horticulture: advances and challenges, 24–27 July 2019 in ICAR-IIHR, Bengaluru, India
Palanichamy S, Padmanaban B, Vaganan MM, Uma S (2019) Electrophysiological and behavioural responses of banana pseudostem weevil, Odoiporus longicollis Olivier (Coleoptera: Curculionidae) to aggregation pheromone, 2-methyl-4-heptanol and host plant kairomones. Cur Sci 116(10):1753–1757
Rehner SA, Minnis AM, Sung G, Jennifer J, Humber RA (2011) Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 103(5):1055–1073
Rivero-Borja M, Guzmán-Franco AW, Rodríguez-Leyva E, Santillán-Ortega C, Pérez-Panduro A (2018) Interaction of Beauveria bassiana and Metarhizium anisopliae with chlorpyrifos ethyl and spinosad in Spodoptera frugiperda larvae. Pest Manag Sci 74(9):2047–2052
Sharmila Bharathi C, Mohan B (2015) Bioefficacy of liquid Beauveria bassiana for the management of pseudostem borer Odoiporus longicollis (Olivier) in hill banana of Kolli hills. Asian J Plant Sci Res 5:55–60
Sripriya C, Padmanaban BS, Uma S (2000) Evaluation of banana (Musa sp.) germplasm against insect pests. Indian J Entomon 62:382–390
Thippaiah M, Ashok Kumar CT, Shivaraju C, Sudhir KS, Naveena NL (2011) Study of biology of banana pseudostem weevil, Odoiporus longicollis Oliv. Internat J Entomol 2:1–5
Velavan V, Rangeshwaran R, Sivakumar G, Sasidharan TO, Sundararaj R, Kandan A (2021) Occurrence of Metarhizium spp. Isolated from forest samples in South India and their potential in biological control of banana stem weevil Odoiporus longicollis Oliver. Egypt J Biol Pest Control 31:131–142
Viswakethu V, Balakrishanan P, Murugan L, Narayanaswamy B, Subbaraya U (2021) Entomopathogenic fungi as a promising biological control agent against banana fruit scarring beetle, Basilepta subcostata (Jac.) (Chrysomelidae: Coleoptera). Egypt J Biol Pest Control 31:53–59
Acknowledgements
We thank Director, ICAR-National Research Centre for Banana, Tiruchirappalli (Tamil Nadu). The authors are grateful to the Department of Biotechnology, New Delhi (BT/PR23236/NER95/596/2017), for providing financial support to carry out his work in 2018–2020. The authors thank Dr. Raju Karthic for the PCA analysis software (OriginLab) at ICAR National Research Center for Banana. Also, K. Balasubramanian, Technical Officer (T-5), Division of Crop Protection, Division of Plant Pathology, ICAR-IIHR Bengaluru has been helped by SEM Photo.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viswakethu, V., Ramasamy, V., Krishnamoorthy, S. et al. Effect of entomopathogenic fungi against banana pseudostem weevil Odoiporus longicollis (Olivier) and elucidation of infection process. Egypt J Biol Pest Control 32, 114 (2022). https://doi.org/10.1186/s41938-022-00611-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00611-w