Abstract
Background
Agriculture crops such as tomatoes and wheat are frequently targeted by insect pests which have a significant negative impact on the agricultural economies. The deployment of entomopathogenic fungi (EPF) for the control of the insect pests is an important alternative to synthetic insecticides. The EPF, Beauveria bassiana (Balsamo) Vuillemin, has been reported widely as a suitable biological control agent of many agricultural pests.
Results
In this study, B. bassiana SS-1 was isolated from local plant crops and its pathogenicity was assessed against the greater wax moth larvae Galleria mellonella (L.). The development of the pathogenic B. bassiana SS-1 on the insect was visualized using scanning electron microscopy (SEM). Results showed the ability of B. bassiana SS-1 to secrete extracellularly the important enzymes essential for insect cuticle penetration. B. bassiana SS1 recorded the maximum mean lipase (5.3 U/ml), protease (32.13 U/ml), and chitinase activities (2.95 U/ml). The endophytic pathogenic fungus B. bassiana SS-1 demonstrated pathogenicity against the fourth instar larvae of G. mellonella showing LC50 at 2.47 × 102 conidia/ml and LC95 at 3.98 × 105 conidia/ml. The SEM results showed physical contact with B. bassiana SS-1 hyphae on the surface of the G. mellonella larvae. Thus, the isolated EPF B. bassiana SS-1, even endophytic, could be a promising biocontrol agent to manage agricultural insect pests.
Conclusion
This study provided a comprehensive characterization of the pathogenicity of B. bassiana SS-1 with its microbiological characteristics. Future studies are needed to focus on the detection of highly virulent isolates against different insect pests and to assess their field contribution as a favorable biological control agent.
Similar content being viewed by others
Background
Natural resources are important source for agro-ecological crop protection using indigenous biological control agents (Rioba and Stevenson 2020). The agro-ecological crop protection offers sustainable management with slight ecological impacts and cost effective compared to chemical pesticides (Waqas et al. 2021). Consequently, there is a need for inexpensive alternatives to the synthetic chemical insecticides to overcome the challenges of pest's sustainable management (Gustianingtyas et al. 2020). Although heavy infestations of insect pests worldwide may have justified chemical control, their persistent applications are unsustainable because they induce the insecticide resistance and cause biodiversity decline (Waqas et al. 2021). Entomopathogenic fungi (EPF) are considered for the insect pest control, mainly include Beauveria bassiana (Balsamo) Vuillemin (Mishra et al. 2015). They have been associated with flora in the field (Guo et al. 2020). However, the co-occurrence of pathogens and development of fungal infection in the host is largely influenced by the environmental conditions (Guo et al. 2020). These potent fungal strains could be suitable candidates for fabricating biopesticides in an integrated strategy to control several pests. However, further research is required to evaluate and validate the laboratory findings globally. Biological control agents are not fully accepted by farmers because of their slow rate of insect elimination and sensitivity to environmental conditions (Rioba and Stevenson 2020). However, the agro-ecological management of insect pests is not only economic beneficially, but it is also critical for the biodiversity conservation (Paddock et al. 2021).
Endophytic fungi can colonize inside plants without damaging effects on plant health (González-Mas et al. 2019). In addition, they secrete secondary metabolites, which induce the immune defense mechanism of the plant against insect herbivores, thus indirectly promoting plant growth (Jia et al. 2016). B. bassiana is an EPF that can colonize endophytically different plant species successfully and exhibit remarkable protective effects against different pests (Oliveira et al. 2014). B. bassiana can penetrate the insect cuticle and subsequently germinates (Mohamed et al. 2018). It produces extracellular proteases, lipases, and chitinases that work together to overcome the chitinous and proteinaceous components of the insect cuticle and enables hyphal access to the hemolymph of the insect (Gebremariam et al. 2022). These unique aggressive features elucidate the importance of B. bassiana for integrated pest management on different crops. The objectives of this study were to isolate a prospective endophytic entomopathogen strain able to endophytically recolonize plants and then, subsequently evaluate its capacity to infect the larvae of Galleria mellonella (L.) (Honeycomb moth).
Methods
Ethical permission
Before beginning the study project, approval from the University's ethical review committee was obtained under the No. 06/2023/0059.
Sampling
The two agronomic plants species Triticum aestivum L. and Solanum lycopersicum L. were freshly collected from the tentative farm of Assiut University, Egypt. Plants with no visible symptoms of the disease were freshly collected twice monthly, brought to the laboratory in plastic bags, and processed immediately. The fungal endophytes were isolated from the leaves, stems, and roots of the collected plants according to Arnold et al. (2001) with some modifications. Plant tissues were carefully washed with tap water, sterilized with 70% ethanol for 2–3 min and rinsed in sodium hypochlorite solution (5%) for 3 min. The samples were washed twice with sterilized distilled (SD) water and kept in a laminar flow chamber to dry (Filip et al. 2003). The sterilization efficiency of the plant surface was confirmed according to (Schulz et al. 1993). For each plant sample, four segments (2 cm2) were placed on, two types of media, potato dextrose agar (PDA, Sigma-Aldrich) and Difco™ Sabouraud Dextrose Agar (SDA). The cultures were incubated at 25 °C and regularly examined for two weeks to observe any hyphae arising from the surface-sterilized plant segments. The developed fungal growth was subcultured on a new antibiotic-free PDA, using a tiny isolation needle before identification.
Identification and preparation of B. bassiana
Generally, all the isolated endophytic fungi were identified, using microscopic examination in the Assiut University Moubasher Mycological Centre (AUMMC). Beauveria bassiana was isolated and identified based on its characteristic morphological and cultural features, e.g., color, colony, powdery nature, and the distinctive zigzag-shaped conidiogenous cells (Moubasher 1993). The isolated strain of B. bassiana was activated on PDA media; the conidia of 10 Petri dishes cultured with B. bassiana were harvested after two weeks, using a sterile scalpel blade. These conidia were suspended in 25 ml SD water containing 0.01% Tween 80, and the conidial suspension was vortexed for 5 min for even conidia distribution. The number of conidia was estimated using an improved Neubauer hemocytometer (Marienfeld, Germany).
Induction and assessment of endophytic potentiality
Wheat and tomato plants were subjected to endophytic colonization by B. bassiana. Surface-sterilized seeds were cultured in sterile soil pots of 1 kg capacity in the greenhouse (five plants per pot). At the first true leaf stage of the plant, two weeks after planting, and 5 mL of conidial suspension of B. bassiana (3 × 108 conidia ml−1) was applied to the plant foliage using a manual atomizer. The control pots were sprayed with SD water containing 0.01% Tween 80. All pots were kept covered with polyethylene bags for 48 h to maintain adequate humidity. One day before inoculation, each pot was irrigated using SD water. The colonization of B. bassiana in leaves, stems, and roots were detected 5, 10, 15, 20, and 25 days after inoculation. The plant samples were examined randomly; the different plant tissue was cut into 1 cm2 and subjected to endophytes assay. Surfaces of the plant segment, five segments for each sample, were sterilized according to Arnold et al. (2001) as described above. The data were measured according to Petrini and Fisher (1987) equation:
Pathogenicity bioassay against G. mellonella larvae
G. mellonella were reared in laboratory at 33 °C and 70% RH. Old honeybee combs were used to feed G. mellonella larvae till adult emergence. Twenty adults were introduced into a glass jar (10 l) containing blocks of old honeybee combs (5 × 5 × 1.5 cm) and covered with gauze. After the period of the mating and oviposition, the wax blocks containing the eggs were placed into a smaller glass jar (2 l). The newly hatching larvae were provided with additional blocks of bee wax when needed. Once larvae reached the third instar, 30 larvae were divided into three groups of ten larvae each. On the other hand, different concentrations (9 × 10, 9 × 102, 9 × 103, 9 × 104, and 9 × 105 conidia ml−1) of the fungal spores' suspension were prepared; the larvae of each group were treated with their corresponding B. bassiana conidia concentration. Then, each group was settled into Petri dishes (10 cm in diameter) lined with sterile wetted filter paper. SD water containing 0.01% Tween 80 was served as a control and run in parallel with the conidia serial dilutions. The Petri dishes were incubated in the growth chamber (Scientific Co., Ltd., Korea) at 25 °C. The inoculated larvae were daily examined, the number of dead larvae was recorded, and the LC50 and LC95 were calculated.
Scanning electron microscopy
Scanning electron microscopy (SEM) technique was adopted to visualize the complete reappearance of B. bassiana on the insect. The infected G. mellonella larva was fixed in 2.5–5% glutaraldehyde in 0.1 M sodium cacodylate buffer at pH 7.3 for 2 h. The sample was dehydrated in a gradient methanol series and dried with liquid CO2 before the critical point. The dried sample was mounted on aluminum stubs with double-coated adhesive tape. coater and The sample was coated with a conductive material like gold (ca. 25 nm, 30 s) in a sputter examined with a SEM (JEOL JSM-5400 LV) equipped with a tungsten cathode. Samples were kept at room temperature in a desiccator until the achievement of the detection.
Enzymatic activity of Beauveria bassiana
The qualitative lipase activity of the isolated entomopathogenic endophyte was detected according to the method described by Ulman and Blasins (1974). The melted lipase medium was poured into vertical test tubes (each tube with 10 ml) under aseptic conditions. The tubes were inoculated with the isolated B. bassiana and then, incubated at 25 °C for 14 days, while the results were recorded weekly as the mean of three replicates. The quantitative enzyme detection was achieved, using the basal salt medium (BSM) according to the method modified by Sahin et al. (2000). The proteases activity detection was conducted using a casein media solution according to Oreilly and Day (1983). Quantitatively, the proteases produced by B. bassiana were detected by filtering the liquid cultures. 250 μl of the culture supernatant was added into Eppendorf tube containing 500 μl 1% (w/v) casein sodium salt and 500 μl (50 mM phosphate buffer, pH 7.0). The mixture was incubated in the water bath for 2 h. The reaction was terminated by adding 375 μl 20% (w/v) trichloroacetic acid. The tubes were kept in an ice bath for 30 min and then, centrifuged at 5000 × g for 15 min under cooling (Ramakrishna and Pandit 1988). The absorbance was measured at 280 nm; the amount present of free amino acids was calculated according to Boldizsár et al. (2013). The mineral medium containing (g/l) chitin, 5; KH2PO4,1; KCl, 0.5; MgSO4.7H2O, 0.5; FeSO4.7H2O, 0.01 and 15 g agar was used for chitinases detection (Anjani and Panda 1992). 3, 5-dinitrosalicylic acid (DNS) was used to measure the amount of reducing sugars liberated as a result of chitin degradation. The acid-swollen chitin (0.55 ml) was added into a test tube containing 0.30 ml of acetate buffer (50 mM, pH 4.75) and 0.15 ml of the crude enzyme extract and incubated at 47 °C for 1 h. The reaction was stopped by adding 1 ml of potassium sodium-tartrate reagent to the reaction solution. The mixture was centrifuged at 5000 rpm for 5 min. After centrifugation, 0.75 ml of the supernatant and 0.25 ml of DNS reagent were mixed and incubated in a boiling water bath for 5 min. The absorbance of the developed color was measured after cooling in room temperature. One unit of enzyme activity was defined as the amount of enzyme required for the formation of 1 μg of the product per min of the reaction. The intensity of the color change was measured using U.V. spectroscopy at 540 nm (Miller 1959).
Statistical analysis
The values of LC50, LC90, and slopes were determined by the computerized Probit analysis program (Robertson et al. 2007).
Results
Endophytic fungi
Endophytic fungi were isolated from healthy plant tissues collected from the experimental farm of Assiut University, Egypt. Data in Table 1 showed that 70 fungal isolates belonging to 23 fungal species were isolated from Triticum aestivum. The leaves of T. aestivum harbored a larger number of endophytic fungi (31 fungal isolates) than that of the stem (22 isolates) and the root (17 isolates). Fifty-six fungal isolates belonging to 13 fungal species were isolated from Solanum lycopersicum (Table 1). The leaves of S. lycopersicum harbored a larger number of endophytic fungi (32 isolates) than that of the stem (19 isolates) and the root (5 isolates). The results revealed that a total of 27 endophytic fungal species belonging to 12 genera were identified from the roots, stems, and leaves of the two agronomic plants. Only one incidence of EPF species was detected in the leaves of S. lycopersicum.
Beauveria bassiana SS-1
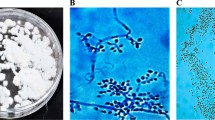
The microscopic examination of this fungal isolate showed woolly colonies that grew slowly. It has a white color at the beginning of the growth, turns to yellow later. Conidiogenous cells originated either single, in spirals from vegetative hyphae, or in clusters from swollen stalk cells. Conidiogenous cells differentiated into a subglobose to cylindrical venter or ellipsoidal and a filament form, zigzag-shaped rachis. Conidia hyaline are either ellipsoidal shaped, or globose shaped with slightly pointed or a rounded bases. These observations conformed to Domsch et al. (1980) when they described B. bassiana SS-1 (Balsamo) Vuillemin (Fig. 1).
Plant colonization by B. bassiana SS-1
Beauveria bassiana SS-1 succeeded to colonize tomato and wheat endophytically by the foliar spray method. Stems and leaves of the two tested plants showed the highest response toward B. bassiana SS-1 inoculation. However, the roots showed no response to this application, and the effect of B. bassiana as endophyte was detected to protect plant against insect pest (Wei et al. 2020). The untreated plant sections (control) showed no fungal growth of B. bassiana SS-1. Data in Fig. 2 show the colonization frequency of two weeks and the extent of B. bassiana SS-1 in the tissues of the plant. These results showed a frequent decrease over time in both plants; however, the colonization frequency in tomato tissues (leaves and stems) was higher than that in wheat.
Pathogenicity and enzymatic activity
The isolated strain of B. bassiana SS-1 showed high activity of extracellular secreted enzymes (Fig. 3). The activities of the secreted proteases and chitinases were detected as clear zones around B. bassiana SS-1 growth in the consistent assay media. The effects of lipase activity were detected as turbidity due to salt of fatty acids formation beneath the fungal growth according to Ulman and Blasins (1974). The endophytic B. bassiana SS-1 showed extracellular enzymatic activity after 7 days of inoculation, it produced 5.3 U/ml of lipase, 32.13 U/ml of proteases, and 2.95 U/ml of chitinases. The pathogenicity of B. bassiana SS-1 was detected against the fourth larval instar of G. mellonella. In the bioassay investigation, the B. bassiana SS-1 strain demonstrated high virulence against the fourth instar of the G. mellonella (L.) larvae (Fig. 4). The LC50 and LC90 values were 2.47 × 102 and 3.98 × 105 conidia/ml for B. bassiana SS-1, respectively. The slope was determined by a computerized probit analysis program as 14 ± 0.5416.
Qualitative assays of extracellular secreted enzymes of Beauveria bassiana. a formation of insoluble calcium salts by the released free fatty acids due to the action of the lipases. b Clear zone of hydrolysis produced due to degradation of the enzyme substrate (casein), incorporated in the agar plate by the action of proteases. c Clear zone on chitin agar plate due to chitin degradation
Discussion
Agricultural crops cultivated in Assiut, Egypt, were surveyed for natural entomopathogenic fungi incidence as endophytes. After the screening process, only one fungal isolate (B. bassiana SS-1) was detected as a candidate endophytic entomopathogen. The endophyte possesses a broad definition because of the multi-function it can perform such as latent saprophyte, latent pathogen, and mutualistic symbiont. However, specific endophytic fungi can produce toxic compounds which protect the host plant from pests attack (Saikkonen et al. 2010).
The biocontrol potential of B. bassiana as an endophyte appears promising although there remains ambiguity regarding the mode of action and location of the fungus in the plant (McKinnon et al. 2017). Currently, EPF are developed, marketed, and used as biopesticides to manage many insect species (Mantzoukas et al. 2022). Many EPF are applied as foliar and soil applications to manage agricultural pests more effectively (Camara et al. 2022). However, their efficiency is limited by adverse environmental conditions such as temperatures, humidity, and UV (Shaalan et al 2021). B. bassiana can be introduced as an endophyte in some plant species using different inoculation methods such as root dipping, seed coating, foliar spraying, and soil watering (Allegrucci et al. 2017).
Russo et al. (2019) firstly reported the damaging decrease in soybean leaves by H. gelotopoeon due to endophytic B. bassiana. The endophytic fungi B. bassiana has the potential to protect soybean plants against pest insects either by decreasing the preference and consumption of leaves by these insects or by the ingestion of plants inoculated with B. bassiana. Consequently, the number of lepidopteran individuals is decreased, and thus population increase is prevented. In this study, out of 27 endophytic fungal species belonging to 12 genera, only one B. bassiana species was successfully isolated from the leaves of tomato plant S. lycopersicum. The 10 studied isolates of B. bassiana by Sinno et al. (2021) were able to colonize tomato cv. Dwarf endophytically two weeks post-inoculum. Seven out of 10 strains were re-isolated from the roots of the inoculated plants (Sinno et al. 2021). The same authors reported that the endophytic EPF B. bassiana was a prospective biocontrol agent against either insects or fungal pathogens of tomatoes. The endophytic B. bassiana might protect tomatoes from fungal pathogens attack, such as Botrytis cinerea Pers., Fusarium oxysporum Schltdl, and Rhizoctonia solani Kühn plus pests, such as Bemisia tabaci Gennadius, Empoasca vitis Goethe, Spodoptera littoralis Boisduval, Helicoverpa zea Boddie, and Tuta absoluta Meyrick (Fergani and Refaei 2021). The endophytic colonization of tomato plant with B. bassiana is a promising method to manage the American tomato pinworm. B. bassiana could be detected as an endophyte for up to 30 days after inoculation (Silva et al. 2020). B. bassiana endophyte increased the resistance in tomato plants against Bemisia tabaci (Wei et al. 2020).
The endophytic ability of this particular fungal species was detected using the foliar spray as an inoculation method on tomato and wheat (Natalia et al. 2017). In this study, the isolated entomopathogen B. bassiana endophyte was able to establish itself in tomato plants through foliar inoculation. Shaalan et al. (2021) showed that seed submersion in conidial suspension caused the systemic colonization of the cucumber plant by B. bassiana and Metarhizium anisopliae, four weeks post-inoculation. Scanning electron microscopy displayed that conidia of both fungi genera directly penetrated seed epidermal cells within a day post-submersion. The fungus B. bassiana was frequently detected as an endophyte, which can colonize different plants in many ways and with different parts of the plant (Mantzoukas et al. 2021). The colonization frequency of the Beauveria in the different tissues of the same tomato plant differed significantly among tested strains and resulted higher in the stem compared to the leaves (Sinno et al. 2021). The present investigation showed that the isolated endophytic EPF from tomato crop had aggressiveness against great wax moth larvae (G. mellonella) (Fig. 4). Gençer and Bayramoğlu (2022) demonstrated that the B. bassiana endophyte isolates had the ability to G. mellonella control; these isolates are a safe alternative to chemical pesticides and can be recommended for use to protect stored wax products. The same authors showed that the LC50 for B. bassiana G-A and B. bassiana G-B against G. mellonella were 0.2 × 106 and 0.6 × 106 conidia/ml, respectively. The vital enzymes for the virulence of an EPF strain, e.g., chitinase activity were investigated through qualitative and quantitative assays. The proteases activity was detected using the degree of hydrolysis method at different durations as the following (12.18 ± 0.42 at 10 min, 14.48 ± 0.34 at 30 min, 23.33 ± 0.12 at 1 h, 25.48 ± 0.58 at 4 h and 32.13 ± 0.17 U/ml at 24 h), lipases specific activity (26.87 U/mg), and chitinases (2.95 ± 0.05 mg/ml). B. bassiana SS-1 demonstrated strong activity of enzyme production which may illustrate the high mortality of G. mellonella larvae at relatively low concentrations of spores' suspension. SEM technique showed that the B. bassiana SS-1 fungal units remerged from the cadaver of G. mellonella larvae (Fig. 5). Thus, the data encouraged the application of this virulent strain in the biocontrol process of the agricultural pest.
González-Mas et al. (2019) found that the plant emitted a different assortment of volatile compounds when it is colonized by B. bassiana than the control plant (un-colonized). Some of the emitted compounds were reported previously to be released as a response to herbivory and implicated in the natural enemy attraction. Some of the compounds have been also reported to have antimicrobial properties. Therefore, the endophytic association by B. bassiana might help to direct control insect pests and also increase the resistance of plant crops against agronomically insect herbivores and phytopathogens (González-Mas et al. 2019). Future research on improving fermentation and formulation technology together with the selection of virulent and stress-tolerant strains is needed to stimulate the widespread acceptance and utility of this fungus as a safe cost-effective mycoinsecticide.
Conclusion
This study aimed to isolate EPF from economic crops as an endophyte. B. bassiana SS-1 was isolated, identified, and characterized as an entomopathogenic endophyte from tomato plants. Its endophytic activity was detected in wheat and tomato plants. The data assert the potentiality of the endophytic B. bassiana and its promising application to control agricultural insect pests. Although more studies under field conditions are necessary to support the current finding, this seems to be an interesting tool and should be considered for pest biocontrol.
Availability of data and materials
All data and materials are available.
References
Allegrucci N, Velazquez MS, Russo ML, Perez E, Scorsetti AC (2017) Endophytic colonization of tomato by the entomopathogenic fungus Beauveria bassiana: The use of different inoculation techniques and their effects on the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). J Plant Prot Res 57:206–211
Anjani KJ, Panda T (1992) Studies on critical analysis of factors influencing improved production of protoplasts from Trichoderma reesei mycelium. Enzyme Microb Technol 14:241–248
Arnold AE, Maynard Z, Gilbert GS (2001) Fungal endophytes in dicotyledonous neotropical trees: patterns of abundance and diversity. Mycol Res 105:1502–1507
Boldizsár A, Simon S, Szirtes K, Soltész A, Szalai G, Keyster M, Ludidi N, Kocsy G (2013) Nitric oxide affects salt-induced changes in free amino acid levels in maize. J Plant Physiol 170:1020–1027
Camara I, Cao K, Sangbaramou R (2022) Screening of Beauveria bassiana (Bals.) (Hypocreales: Cordycipitaceae) strains against Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae) and conditions for large-scale production. Egypt J Biol Pest Control 32:85–92
Domsch KH, Gams W, Anderson TH (1980) Compendium of Soil Fungi. Vol. 1 and 2. Academic Press, New York. pp. 1–672
Fergani YA, Refaei EAE (2021) Pathogenicity induced by indigenous Beauveria bassiana isolate in different life stages of the cotton leaf worm, Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) under laboratory conditions. Egypt J Biol Pest Control 31:64–70
Filip V, Plockova M, Šmidrkal J, Špickova Z, Lzoch K, Schmidt S (2003) Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chem 83:585–593
Gebremariam A, Chekol Y, Assefa F (2022) Extracellular enzyme activity of entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae and their pathogenicity potential as a bio-control agent against whitefly pests, Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). BMC Res Notes 15(1):117
Gençer D, Bayramoğlu Z (2022) Characterization and pathogenicity of Beauveria bassiana strains isolated from Galleria mellonella L. (Lepidoptera Pyralidae) in Turkey. Egyptian J Biol Pest Control 32:99–105
González-Mas N, Cuenca-Medina M, Gutiérrez-Sánchez F, Quesada-Moraga E (2019) Bottom-up effects of endophytic Beauveria bassiana on multitrophic interactions between the cotton aphid, Aphis gossypii, and its natural enemies in melon. J Pest Sci 92:1271–1281
Guo Y, Takashima Y, Sato Y, Narisawa K, Ohta H, Nishizawa T (2020) Mycoavidus sp. strain B2-EB: comparative genomics reveals minimal genomic features required by a cultivable Burkholderiaceae-related Endofungal bacterium. Appl Environ Microbiol 86(18):1–16
Gustianingtyas M, Herlinda S, Suwandi S, Hamidson H, Hasbi SA, Verawaty M, Elfita A (2020) Toxicity of entomopathogenic fungal culture filtrates of lowland and highland soil of South Sumatra (Indonesia) against Spodoptera litura larvae. Biodiversitas 21(5):1839–1849
Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP (2016) A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol 7:906–919
Mantzoukas S, Lagogiannis I, Mpousia D, Ntoukas A, Karmakolia K, Eliopoulos PA, Poulas K (2021) Beauveria bassiana endophytic strain as plant growth promoter: the case of the grape vine Vitis vinifera. J Fungi 7:142–155
Mantzoukas S, Kitsiou F, Natsiopoulos D, Eliopoulos PA (2022) Entomopathogenic fungi: interactions and applications. Encyclopedia 2:646–656
McKinnon AC, Saari S, Moran-Diez ME, Meyling NV, Raad M, Glare TR (2017) Beauveria bassiana as an endophyte: a critical review on associated methodology and biocontrol potential. Biocontrol 62:1–17
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mishra S, Kumar P, Malik A (2015) Effect of temperature and humidity on pathogenicity of native Beauveria bassiana isolate against Musca domestica L. J Parasit Dis 39(4):697–704. https://doi.org/10.1007/s12639-013-0408-0. (Epub 2013 Dec 3. PMID: 26688637; PMCID: PMC4675588)
Mohamed HF, Sileem TM, El-Naggar SEM (2018) Effect of gamma irradiation and/or certain entomopathogenic fungi on the larval Mortality of Galleria mellonella L. Egypt J Biol Pest Control 28:95–102
Moubasher AH (1993) Soil fungi in Qatar and other Arab countries. The centre of Scientific and Applied Research, University of Qatar, Doha, Qatar.p.566
Natalia A, Maria SV, María LR, Emilia P, Ana CS (2017) Endophytic colonisation of tomato by the entomopathogenic fungus Beauveria bassiana: the use of different inoculation techniques and their effects on the tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae). J Plant Prot Res 57:331–337
Oliveira CM, Auad AM, Mendes SM, Frizzas MR (2014) Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Prot 56:50–54
Oreilly T, Day DF (1983) Effects of culture conditions on protease production by Aeromonas hydrophila. Appl Environ Microbiol 45:1132–1135
Paddock KJ, Pereira AE, Finke DL, Ericsson AC, Hibbard BE, Shelby KS (2021) Host resistance to Bacillus thuringiensis is linked to altered bacterial community within a specialist insect herbivore. Mol Ecol 30:5438–5453
Petrini O, Fisher PJ (1987) Fungal endophytes in Salicornia perennis. Trans Br Mycol Soc 87:647–651
Ramakrishna T, Pandit MW (1988) Self-association of α-chymotrypsin: effect of amino acids. J Biosci 13:215–222
Rioba NB, Stevenson PC (2020) Opportunities and scope for botanical extracts and products for the management of fall armyworm (Spodoptera frugiperda) for smallholders in Africa. Plants 9(2):207–223. https://doi.org/10.3390/plants9020207
Robertson JL, Savin NE, Russell RM, Preisler HK (2007) Bioassays with arthropods. (2nd ed.). CRC Press. ISBN: 9780849323317. p. 224
Russo ML, Scorsetti AC, Vianna MF, Allegrucci N, Ferreri NA, Cabello MN, Pelizza SA (2019) Effects of endophytic Beauveria bassiana (Ascomycota: Hypocreales) on biological, reproductive parameters and food preference of the soybean pest Helicoverpa gelotopoeon. J King Saud Univ Sci 31:1077–1082
Sahin N, Tamer AU, Bayar C (2000) Isolation, characterization, and identification of thiram-degrading microorganisms from soil enrichment cultures. Turkish J Biol 24:353–363
Saikkonen K, Saari S, Helander M (2010) Defensive mutualism between plants and endophytic fungi. Fungal Divers 41:101–113
Schulz B, Wanke U, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 97:1447–1450
Shaalan RS, Gerges E, Habib W, Ibrahim L (2021) Endophytic colonization by Beauveria bassiana and Metarhizium anisopliae induces growth promotion effect and increases the resistance of cucumber plants against Aphis gossypii. J Plant Prot Res 61:358–370
Silva ACL, Silva GA, Abib PHN, Carolino AT, Samuels RI (2020) Endophytic colonization of tomato plants by the entomopathogenic fungus Beauveria bassiana for controlling the South American tomato pinworm Tuta absoluta. Agric Biosci 1:3–11
Sinno M, Ranesi M, Di Lelio I, Iacomino G, Becchimanzi A, Barra E, Molisso D, Pennacchio F, Digilio MC, Vitale S (2021) Selection of endophytic Beauveria bassiana as a dual biocontrol agent of tomato pathogens and pests. Pathogens 10:1242–1261
Ulman V, Blasins G (1974) A simple medium for the detection of different lipolytic activity of microorganisms. Zintrabl Bakteriol Hyg II Abt A 229:264–267
Waqas MS, Shi Z, Yi TC, Xiao R, Shoaib AAZ, Elabasy ASS, Jin DC (2021) Biology, ecology, and management of cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Pest Manag Sci 77:5321–5333
Wei QY, Li YY, Xu C (2020) Endophytic colonization by Beauveria bassiana increases the resistance of tomatoes against Bemisia tabaci. Arthropod Plant Interact 14:289–300
Acknowledgements
The authors greatly acknowledge the staff of the Botany and Microbiology Department, Faculty of Science, Assiut University, Assiut, Egypt; and Plant Protection Research Institute, Agricultural Research Center, Egypt.
Funding
Funding is by the authors.
Author information
Authors and Affiliations
Contributions
SSEM contributed to supervision, visualization, writing—original draft, and project administration. MAAAR contributed to supervision, visualization, writing—original draft, and project administration. SHMH contributed to practical work, and writing—original Draft. KAH contributed to supervision, conceptualization, visualization, writing—review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Maraghy, S.S.M., Abdel-Rahman, M.A.A., Hassan, S.H.M. et al. Pathogenicity and other characteristics of the endophytic Beauveria bassiana strain (Bals.) (Hypocreales: Cordycipitaceae). Egypt J Biol Pest Control 33, 79 (2023). https://doi.org/10.1186/s41938-023-00690-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-023-00690-3