Abstract
Background
Entomopathogenic nematodes (EPNs) have been regarded as the most convenient strategy for insect pest management. The native strains of EPNs: Heterorhabditis bacteriophora EUPT-SD, H. bacteriophora EUPT-R, H. bacteriophora EUPT-KN, H. bacteriophora EUPT-K and H. bacteriophora EUPT-H isolated from mid-Himalayan region of Himachal Pradesh were tested in laboratory for their multiplication and virulence against 3rd and 4th larval instars of the tobacco cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae), the serious polyphagous pest affecting a wide range of agricultural crops worldwide.
Results
All the EPN strains were effective against 3rd and 4th larval instars of S. litura. Insect mortality reached 90–96% after 96 h at nematode concentrations of 150 infective juveniles (IJs)/ml. The insect mortality was also recorded at low concentrations of IJs, but the most exposure period was required. High virulence was shown by H. bacteriophora EUPT-SD 96 and 94%, followed by H. bacteriophora EUPT-R 92 and 90%, H. bacteriophora EUPT-KN 92 and 90%, H. bacteriophora EUPT-K 92 and 90% and H. bacteriophora EUPT-H 92 and 90%, respectively, against 3rd and 4th larval instars in terms of reproductive potential and killing. All the insects were alive in the absolute control.
Conclusion
Utilization of EPNs for the management of S. litura may be the best method to overcome the insect resistance problems and to manage the population of this insect pest. It may be an effective method and may be a partial substitute of synthetic insecticides, thus minimizing the excessive use of synthetic chemicals. The results demonstrated the potential of indigenous EPNs isolates against S. litura, but before further recommendation, multiplication field trials need to be conducted to confirm their efficacy at farm level.
Similar content being viewed by others
Background
The tobacco cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae), is a serious insect pest that feeds upon a wide variety of crops having very high fecundity with elevated reproduction rates and migratory behaviour. Young larvae feed on leaves, and 4th and 5th larval instars cause maximum damage by completely skeletonized the leaves, which declined the photosynthesis ability of infected plants (Yadav et al. 2017). In Himachal Pradesh, the control strategies applied to manage the population of these insect pests are generally chemicals based which are hazardous to living beings and costly. Applications of chemical-based synthetic insecticides are the solution only for shorter duration that ultimately resulted in negative impacts over the biodiversity (Thakur et al. 2022a). However, insect pest population was triggered by biological opponents such as parasitoids, pathogens and predators. The microorganism-based biocides are the best method to overcome all negative issues (Thakur et al. 2020) that ultimately promote the sustainability through natural farming practices (Thakur et al. 2022b).
EPNs were reported as an eminent biocontrol agent against a broad spectrum of insect pests accompanied with S. litura (Tomar et al. 2022a). EPNs in families: Steinernematidae and Heterorhabditidae, premise in several soil types (Hominick 2002). Host range of steinernematids and heterorhabditids is very broad, and it includes over 200 different insect species (Hasan et al. 2009). The infective juveniles (J3) have the potential to invade the insect body via natural routes and the natural body openings, i.e. mouth, spiracles, cuticle and anus (Askary and Ahmad 2020). The juvenile stage along with its bacterial endosymbiont kills the insects efficiently through invading haemocoel by regurgitating endosymbiont (Tomar et al. 2022b). These endosymbiont releases toxic compounds inside insect body, which finally kills the insects via toxaemia (Gaugler 2002). As the insect killed, the body becomes soft but not decayed due to the production of antibiotics and secondary metabolites by the bacteria. Infection with steinernematids resulted in ochre, black or yellow brown colour cadaver, while heterorhabditids infected cadaver showed brick-red, purple, red, sometimes green or orange colourations (Sundarababu and Sankaranarayanan 1998). The insect management by the use of EPNs depends upon the developmental stages of insects (Jackson and Brooks 1995). Environmental pollution caused by synthetic chemicals is a major public concern nowadays for which a greener and cleaner tactic is required by the use of entomopathogenic microbial biopesticides. The applications of EPNs are an influential, efficient and eco-friendly approach for insect pest management (Thakur et al. 2021). Earlier EPNs along with entomopathogenic fungus and bacteria have been reported to cause the highest mortality against Spodoptera larvae (Tomar et al. 2022c) in bioassay as well as in polyhouse and field conditions (Thakur et al. 2022c). EPNs are mass produce, formulate and applied easily, therefore being used throughout the world against the soil dwelling and foliar crop insect pests (Tomar and Thakur 2022). In the present investigation, EPNs (Heterorhabditis bacteriophora) collected from various regions of mid-Himalaya were comprehensively compared and evaluated for its biocidal and reproductive potential against S. litura larvae. Furthermore, this study established the most virulent strain of H. bacteriophora for the eradication of S. litura problem under integrated pest management (IPM) programs.
Methods
Culturing and multiplication of entomopathogenic nematodes
The EPNs were isolated from the soil samples collected from five districts namely Solan, Shimla, Kangra, Kullu and Hamirpur districts of Himachal Pradesh. The collected soil samples were kept into the zip lock polythene bags labelled with information such as soil type, locality, type of fruit orchard, etc. The samples were brought to the Zoology laboratory and kept at low temperature and were processed within 3–5 days. The soil samples were taken out from the bags, and the debris was removed. The soil was filled into the plastic containers and the last instar of the greater wax moth, and Galleria mellonella larvae were added into these containers. The dead cadavers were removed from the containers every day and subjected to white trap. Nematodes were collected via white trap methodology (White 1927). Further, the EPNs were nurtured using G. mellonella larvae at 27 ± 1 °C with RH 55 ± 10% in the laboratory (Orozco et al. 2014). EPNs stock was perpetuated and stored in distilled water (Woodring and Kaya 1988). The collected isolates were named as EUPT-SD from district Solan, EUPT-R from district Shimla, EUPT-KN from district Kangra, EUPT-K from district Kullu and EUPT-H from district Hamirpur, respectively.
Identification of entomopathogenic nematodes
Nematode identification was done on the basis of their morphological characteristics. For this, the nematodes were killed; fixed and morphological observations were recorded based upon their taxonomical keys (Poinar Jr 1975).
Collection of the host insect (Spodoptera litura)
Larvae of S. litura were collected directly from the agricultural fields, while adults were collected using light traps. The larvae were transferred to the plastic vials (30 ml), and the adults were kept into a chimney. The castor leaves were provided to the larvae, and sucrose solution was given to the adults for feeding. A piece of paper was also kept inside the chimneys where females lay eggs. The eggs were further transferred to the moist tissue paper where they hatched into 1st instar larvae. The emerged adults were further transferred on the tender castor leaves upon, which they feed and grow into further instars. This way the culture was propagated in the laboratory at 55 ± 10% RH and 27 ± 1 °C.
Biocidal predisposition of nematodes towards Spodoptera litura
The insect killing competence of different strains of H. bacteriophora collected from various localities was evaluated under a laboratory bioassay experiment against 3rd and 4th larval instars of S. litura. The laboratory-reared larvae S. litura were kept over the Whatman filter paper No. 1 inside a Petri plate along with diet (castor leaves). The nematode suspensions collected from five different districts named: Solan (EUPT-SD), Shimla (EUPT-R), Kangra (EUPT-KN), Kullu (EUPT-K) and Hamirpur (EUPT-H), were applied into the Petri plate at different concentrations such as 30, 60, 90, 120 and 150 IJs/ml along with absolute control. The insect death rate was noticed after every 24 up to 96 h. The treatments were replicated 5 times, and the experiment was accomplished for twice. The data recorded over the two experiments were pooled and subjected to the statistical analysis.
Nematodes multiplication on Spodoptera litura
The dead insect cadavers were collected from the bioassay experiment and were kept in the white trap. The trap consists of two different sized Petri plates in which the smaller one of 60 mm diameter was lined with one Whatman filter paper No. 1 that was placed inside a larger Petri plate of 100 mm diameter. The larger Petri plate was filled with distilled water in order to maintain the moisture content on the filter paper. The dead cadavers recovered from the bioassay study were washed with distilled water twice and then kept over the moist filter paper. The Petri plate was covered and kept inside the incubator. The emerging nematodes were moved into the water in the surrounding region. These were harvested from the water and kept into the storage bottles. The harvesting was done up to 10 days until all the EPNs were collected. The data on the nematode emergence and reciprocation on 3rd and 4th larval instars were recorded and subjected to statistical analysis.
Statistical analysis
Statistical analyses were performed on the data collected over the insect mortality and nematode reciprocation. The analysis of variance (ANOVA) was used to conclude the EPNs’ biocontrol efficacy and reproduction rates against S. litura. Corrected mortality was calculated using Abbott’s formula (Abbott 1925). Probit analysis was performed, and the median lethal concentration (LC50) was calculated. The calculated data were signified as means ± standard error.
Results
Entomopathogenic nematodes (EPNs) were isolated via soil baiting technique using G. mellonella larvae, followed by the white trap methodology; nematode isolation was done from various localities among five districts of Himachal Pradesh. The morphological measurements of the mounted specimens were taken, and the isolated EPNs were identified as H. bacteriophora based upon their morphological observations. The isolated nematodes were further multiplied on G. mellonella larvae and were kept into the roux bottles, and their insecticidal activities were evaluated (Fig. 1).
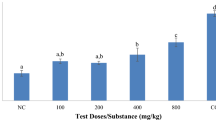
Biocontrol potential of different strains of H. bacteriophora collected from the different localities was assessed in the laboratory. The comparative analysis on the bioefficacy study showed that locally available H. bacteriophora strain EUPT-SD caused maximum larval mortality 96% (F = 20.52, df = 5, P < 0.05) and 94% (F = 23.76, df = 5, P < 0.05) amongst 3rd and 4th larval instars of S. litura in the inoculum of 150 IJs/ ml after 96 h. of nematode inoculation (Fig. 2). A significant difference amongst the larval mortality was caused by five different nematode inoculums. Considering the data based upon probit analysis, the calculated median lethal concentrations LC50 = 36.76 IJs (95% FL: 29.14–46.37) slope 1.98 ± 0.25 amongst 3rd instar larvae and LC50 = 37.83IJs (95% FL: 29.70–48.19) slope 1.87 ± 0.25 for the 4th instar larvae after 96 h (Table 1). The Shimla isolate H. bacteriophora strain EUPT-R caused 92% and 90% high larval mortality in the nematode inoculum concentration of 150 IJs after 96 h. The recorded mortality data showed significant variations upon exposure of different nematode inoculum concentration (F = 16.74, df = 5, P < 0.05) amongst the 3rd and (F = 16.74, df = 5, P < 0.05) 4th larval instars (Fig. 2). Based upon the probit of killing, LC50 value for 3rd instar S. litura larvae were 41.33 IJs with 95% FL: 32.58–52.41, slope 1.88 ± 0.25 and LC50 = 43.36IJs with 95% FL: 33.83–55.57, slope 1.77 ± 0.24 for 4th instar larvae after 96 h (Table 1).
Percent mortality caused by Heterorhabditis bacteriophora strains in 3rd and 4th larval instars of Spodoptera litura (A and B) Mortality caused by Heterorhabditis bacteriophora EUPT-SD; (C and D) Mortality caused by Heterorhabditis bacteriophora EUPT-R; (E and F) Mortality caused by Heterorhabditis bacteriophora EUPT-KN; (G and H) Mortality caused by Heterorhabditis bacteriophora EUPT-K; and (I and J) Mortality caused by Heterorhabditis bacteriophora EUPT-H
The EPN isolates from district Kangra, H. bacteriophora strain EUPT-KN, also exhibited 92 and 90% larval mortality amongst both the instars, respectively (Fig. 2). The insect mortality increased as the inoculum concentration raised and also the exposure period enhanced. Statistically significant results were obtained from the bioassay experiment (F = 19.93, df = 5, P < 0.05) in 3rd and (F = 26.15, df = 5, P < 0.05) in 4th larval instars. In the log probit analysis, the LC50 value for 3rd instar larvae was 46.81 IJs with 95% FL: 37.63–58.22, slope 2.04 ± 0.25 and for 4th instar larvae was 50.06 IJs with 95%FL: 33.83–55.57, slope 1.99 ± 0.24 after 96 h (Table 1). Similarly, the Kullu isolate, H. bacteriophora strain EUPT-K, also caused the highest 92 and 90% mortality amongst both larval instars, respectively. Significant variations were observed after treatment with different concentrations of nematode juveniles (F = 16.63, df = 5, P < 0.05) among 3rd instar larvae and (F = 24.92, df = 5, P < 0.05) for 4th instar larvae (Fig. 2). The mortality rate further increased with the increase in the inoculum concentration. The calculated LC50 = 44.86IJs with 95% FL: 36.306–55.43, slope 1.99 ± 0.27 among 3rd instar larvae and LC50 = 52.98IJs with 95% FL: 41.65–67.383, slope 1.79 ± 0.24 among 4th instar larvae after 96 h (Table 1).
The Hamirpur isolate H. bacteriophora strain EUPT-H again showed maximum larval mortality 92% (F = 21.30, df = 5, P < 0.05) and 90% (F = 29.15, df = 5, P < 0.05), respectively, for both larval instars of Spodoptera (Fig. 2). All the insects were alive in the absolute control. The calculated LC50 value was 42.78IJs with 95% FL: 33.92–53.94, slope 1.93 ± 0.25 for 3rd instar larvae and LC50 = 46.80IJs with 95% FL: 37.38–58.59, slope 1.97 ± 0.27 against 4th instar larvae after 96 h (Table 1). Significant variations were observed between the insect mortality data upon treatment with different concentrations of infective juveniles.
EPNs multiplication in insect larvae was also evaluated through comparing the number of emerging nematodes from each larval instar. The data were collected regularly until all the EPNs were harvested from the white trap. The data recorded from the present study denoted that the later instar (4th instar larvae) produced more number of nematodes as compared to the younger instar (3rd instar larvae) (Figs. 3 and 4). Among the different strains of EPNs, the highest number of nematodes was procured from the H. bacteriophora EUPT-H and H. bacteriophora EUPT-SD upon treatment with 150 IJs/ml concentration against 4th instar larvae of S. litura.
Discussion
Different strains of EPNs, H. bacteriophora strains, were isolated from different mid-Himalayan regions of Himachal Pradesh, and the virulence of these strains was evaluated against the 3rd and 4th larval instars of S. litura by conducting a bioassay experiment. The results revealed that the native population of H. bacteriophora strain EUPT-SD caused the maximum larval mortality 96 and 94% amongst both larval instars. Other isolates including H. bacteriophora strain EUPT-R, H. bacteriophora strain EUPT-KN, H. bacteriophora strain EUPT-K and H. bacteriophora strain EUPT-H showed almost similar effect causing 92 and 90% mortality, respectively. All the treatments showed significant mortality rates in the insects. Earlier, many researchers have reported the biocontrol potential of EPNs against the S. litura larvae (Yan et al. 2020). The present investigation is also supported by Park et al. (2001) who reported that small instars were more prone EPNs to infection due to their sensitive skin. Acharya et al. (2020) who evaluated the virulence potential of four EPN species such as H. indica, H. bacteriophora, Steinernema longicaudum and S. carpocapsae against all larval instars of tobacco cutworm and reported more mortality amongst younger larval instars than the older ones. These findings are similar to the present findings. Similar findings were observed by Burana et al. (2022). Obtained results are also in line with the earlier findings of Sun et al. (2021) who evaluated the virulence potential of Heterorhabditis and Steinernema against 5th instar the S. litura larvae and recorded over 90% mortality in S. litura after the exposure of 72 h. Javed et al. (2022) also recorded similar observations.
During the present investigation, it was also observed that increased nematode inoculum concentration and the enhanced exposure period resulted in maximum larval mortality. The results are in conformity with the earlier research work carried by Holajjer et al. (2014). The results are also supported by the findings of Acharya et al. (2020). They observed that the insect mortality increased with the raise in the nematode inoculum and time interval. The lowest median lethal concentration LC50 = 36.76IJs of 3rd instar larvae and LC50 = 37.83IJs of 4th instar larvae was recorded, when different concentrations of H. bacteriophora strain EUPT-SD were inoculated to the larval instars. Similar observations were recorded by Umamaheswari et al. (2006) who reported LC50 = 3.53 IJs/larva upon treatment with six different isolates of EPNs. Burana et al. (2022) reported LD50 = 15.84 dauer juvenile/larva in 1st instar and LD50 = 40.34 dauer juvenile/larva among 3rd instar larvae upon treatment with S. siamkayai. The results are also supported by Dichusa et al. (2021) who had reported the insect mortality range between 0 and 100% upon treated with seven species of EPNs with LC50 value 7.13 ± 1 IJs/larva. Thakur et al. (2022a) recorded LC50 = 59.95 IJs/larvae in 3rd instar larvae and LC50 = 50.91 IJs/larvae among 4th instar larvae of S. litura under the laboratory conditions.
The study on the nematode reciprocation denoted that larger instars of S. litura produced more population of EPNs and the young instars produced much lower nematode concentration. The present finding supported by the earlier findings of Park et al. (2001) who reported the highest number of nematodes from 5 and 6th larval instars of S. litura. Holajjer et al. (2014) reported that the number of nematode inocula added to infect the S. litura larvae does not affect the population of nematode juveniles and does not interfere in reproduction and multiplication of the EPNs. Safdar et al. (2018) observed maximum 25,786 nematodes produced from 5th instar of S. litura, followed by younger instar (4th instar larvae) containing 17,500 nematodes, followed by 3rd instar larvae 12,642 nematodes and 2nd ones 9652 nematodes. The reproduction potential of the EPNs fluctuates with respect to species, isolates, invasion rates, size of the host, host susceptibility and diverse environmental conditions as humidity and temperature (Askary and Ahmad 2021).
Conclusion
It has been concluded from the present study that the native strains of EPNs were highly virulent and can cause maximum larval mortality amongst the 3rd and 4th larval instars of S. litura. Moreover, the insect mortality rate increased as the exposure time exceeded. It was further observed that the youngest instar larvae (3rd instar) were more susceptible towards the EPNs infection than the oldest instar larvae (4th instar). Further, the nematode reciprocation was much high in the largest instar larvae than the smallest ones. So it can be demonstrated that utilization of EPNs for the management of S. litura is the best method to overcome the insect resistance problems and to eradicate this insect pest population. It is an effective method and may be a partial substitute in place of synthetic insecticides, thus minimize the excessive use of synthetic chemicals.
Abbreviations
- H. bacteriophora :
-
Heterorhabditis bacteriophora
- Df:
-
Degree of freedom
- IJs:
-
Infective juveniles
- EPNs:
-
Entomopathogenic nematodes
- BCAs:
-
Biocontrol agents
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Acharya R, Yu Y-S, Shim J-K, Lee K-Y (2020) Virulence of four entomopathogenic nematodes against the tobacco cutworm Spodoptera litura Fabricius. Biol Cont 150:104348
Askary TH, Ahmad MJ (2020) Efficacy of entomopathogenic nematodes against the cabbage butterfly (Pieris brassicae (L.)(Lepidoptera: Pieridae) infesting cabbage under field conditions. Egyptian J Biol Pest Cont 30:1–7
Askary TH, Ahmad MJ (2021) Biocidal efficacy of some native isolates of entomopathogenic nematodes against oriental armyworm, Mythimna separata Walker (Lepidoptera: Noctuidae). Indian J Nematol 51:67–73
Burana K, Ehlers RU, Nimkingrat P (2022) Entomopathogenic nematodes for control of the cotton cutworm Spodoptera litura in marigolds. J Appl Entomol 146:415–423
Dichusa CA, Ramos R, Aryal S, Sumaya NPD, Sumaya NH (2021) Survey and identification of entomopathogenic nematodes in the province of Cotabato, Philippines, for biocontrol potential against the tobacco cutworm, Spodoptera litura (Fabricius)(Lepidoptera: Noctuidae). Egyptian J Biol Pest Cont 31:1–10
Gaugler R (2002) Entomopathogenic nematology. CABI Publications, pp 1–388
Hasan W, Singh C, Askary T (2009) Entomopathogenic nematodes—as a biocontrol agent for insect pests of various crops. Indian Farming Digest 42:15–18
Holajjer P, Patil JB, Harish G, Nataraja M, Jasrotia P, Savaliya S (2014) Evaluation of entomopathogenic nematodes, Steinernema carpocapsae and Heterorhabditis indica for their virulence against Spodoptera litura. Annals Plant Protec Sci 22(1):163–165
Hominick WM (2002) Biogeography. Entomopathogenic Nematology 1:115–143
Jackson JJ, Brooks MA (1995) Parasitism of western corn rootworm larvae and pupae by Steinernema carpocapsae. J Nematol 27:15
Javed S, Khanum TA, Ali A (2022) Storage and efficacy of entomopathogenic nematode species as a biocontrol agent against the armyworm, Spodoptera litura (Fabricius)(Lepidoptera: Noctuidae). Egyptian J Biol Pest Cont 32:1–5
Orozco RA, Lee M-M, Stock SP (2014) Soil sampling and isolation of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae). J Visual Exp. https://doi.org/10.3791/52083
Park SH, Yu YS, Park JS, Choo HY, Bae SD, Nam MH (2001) Biological control of tobacco cutworm, Spodoptera litura Fabricius with entomopathogenic nematodes. Biotechnol Bioprocess Engin 6:139–143
Poinar GO Jr (1975) Description and biology of a new insect parasitic Rhabditoid, Heterorhabditis bacteriophora N. Gen., N. Sp.(Rhabditida; Heterorhabditidae N. Fam.). Nematologica 21(4):463–470
Safdar H, Javed N, Khan SA, Arshad M (2018) Reproduction potential of entomopathogenic nematodes on armyworm (Spodoptera litura). Pak J Zool 50:771–774
Sun B et al (2021) Evaluation of indigenous entomopathogenic nematodes in Southwest China as potential biocontrol agents against (Lepidoptera: Noctuidae). J Nematol 53:1–17
Sundarababu R, Sankaranarayanan C (1998) Biological control of insects using nematodes. In: Recent advances in plant nematology CBS Publishers and Distributors, New Delhi, pp 153–170
Thakur N, Kaur S, Tomar P, Thakur S, Yadav AN (2020) Microbial bi-opesticides: current status and advancement for sustainable agriculture and environment. In: Rastegari AA, Yadav AN, Yadav N (eds) Trends of microbial biotechnology for sustainable agriculture and biomedicine systems: diversity and functional perspectives. Elsevier, Amsterdam, pp 243–282
Thakur N, Tomar P, Kaur S, Jhamta S, Thakur R, Yadav AN (2021) Entomopathogenic soil microbes for sustainable crop protection. In: Yadav AN (ed) Soil microbiomes for sustainable agriculture. Springer, pp 529–571
Thakur N, Kaur S, Kaur T, Tomar P, Devi R, Thakur S, Tyagi N, Thakur R, Mehta DK, Yadav AN (2022a) Organic agriculture for agro-environmental sustainability. In: Soni R, Suyal DC, Yadav AN, Goel R (eds) Trends of applied microbiology for sustainable economy. Academic Press, pp 699–735
Thakur N, Tomar P, Kaur S, Kumari P (2022b) Virulence of native entomopathogenic nematodes against major lepidopteran insect species of tomato (Solanum lycopersicum L.). J Appl Biol Biotechnol 10:6–14
Thakur N, Tomar P, Sharma S, Kaur S, Sharma S, Yadav AN, Hesham AE-L (2022c) Synergistic effect of entomopathogens against Spodoptera litura (Fabricius) under laboratory and greenhouse conditions. Egypt J Biol Pest Cont 32:1–10
Tomar P, Thakur N (2022) Isolation and evaluation of Heterorhabditis bacteriophora strain-S26 as biocontrol agents against Pieris brassicae L. under laboratory conditions. Indian J Nematol 52:49–58
Tomar P, Thakur N, Sharma A (2022a) Infectivity of entomopathogenic nematode against the cabbage butterfly (Pieris brassicae L.) in polyhouse and in field condition. Egypt J Biol Pest Cont 32:1–7
Tomar P, Thakur N, Yadav AN (2022b) Endosymbiotic microbes from entomopathogenic nematode (EPNs) and their applications as biocontrol agents for agro-environmental sustainability. Egypt J Biol Pest Cont 32:1–19
Tomar P, Thakur N, Yadav AN (2022c) Indigenous entomopathogenic nematode as biocontrol agents for insect pest management in hilly regions. Plant Sci Today 8:51–59
Umamaheswari R, Sivakumar M, Subramanian S (2006) Biocontrol efficacy of entomopathogenic nematodes on Spodoptera litura (Lepidoptera: Noctuidae) in blackgram. Indian J Nematol 36:19–22
White G (1927) A method for obtaining infective nematode larvae from cultures. Sci 66:302–303
Woodring JL, Kaya HK (1988) Steinernematid and heterorhabditid nematodes: a handbook of biology and techniques. Southern cooperative series bulletin (USA), pp 1–30
Yadav S, Patil J, Sharma H (2017) Bio-efficacy of Steinernema carpocapsae against Spodoptera litura under laboratory condition. J Pure Appl Biosci 5:165–172
Yan X, Shahid Arain M, Lin Y, Gu X, Zhang L, Li J, Han R (2020) Efficacy of entomopathogenic nematodes against the tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). J Econ Entomol 113:64–72
Acknowledgements
The study was carried out in the Zoology laboratory at Eternal University, Baru Sahib, Himachal Pradesh. Authors acknowledge the financial assistance provided by Department of Science and Technology, Govt. of India (SP/YO/506/2018-G). The authors are also thankful to Vice Chancellor, Eternal University, Baru Sahib, for providing necessary laboratory facilities.
Funding
This work belongs to the project that has been funded by Department of Science and Technology, Govt. of India (SP/YO/506/2018-G).
Author information
Authors and Affiliations
Contributions
NT gave the concept. PT performed the experiment, wrote the manuscript and did the statistical analysis. Both the authors have read the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
The authors declare that the submitted manuscript is the authors' original research work and it has not been submitted for publication elsewhere.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomar, P., Thakur, N. Biocidal potential of indigenous isolates of Entomopathogenic Nematodes (EPNs) against tobacco cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Egypt J Biol Pest Control 32, 107 (2022). https://doi.org/10.1186/s41938-022-00607-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00607-6