Abstract
Background
The peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae), is a key pest of fruits in Egypt. Insect-pathogenic fungi are one of the biocontrol agents that increasingly substitute the traditional pesticides to overcome pesticide risks. Therefore, the present study aims to assess the fungal virulence of Beauveria bassiana (Balsamo) and Metarhizium anisopliae (Metchnikoff) against B. zonata pupae. Also, extended pathogenicity effect of these fungi on adult flies was studied.
Results
The results showed that M. anisopliae fungus had more pathogenicity to B. zonata pupae on the 2nd, 3rd, and 5th days post-treatment than B. bassiana. Pathogenicity fungal effects of treated larvae extended to the surviving adults. Fungal concentration and post-exposure interval reversely impacted the pupae by 63.88 and 63.59% mortality in the case of M. anisopliae and B. bassiana, respectively. The lethal concentration of treated fly by M. anisopliae (LC50 = 9.5 × 106 conidia/ml and LC90 = 9.9 × 107 conidia/ml) was lower than that of B. bassiana (LC50 = 5.1 × 107 conidia/ml and LC90 = 1.9 × 109 conidia/ml). Median lethal time (LT50) value was fungal species-dependent, and concentration. Metarhizium anisopliae was more virulent than B. bassiana; the lowest LT50 value was 9.48 days by M. anisopliae and 13.33 days by B. bassiana, depending on the fungal tested concentration of 2.3 × 106 conidia/ml.
Conclusions
The tested entomopathogenic fungi could be considered promising biocontrol agents against B. zonata and could be used for fly suppression through soil application in IPM programs.
Similar content being viewed by others
Background
Tephritid fruit flies are a group of economic insect pests that attack fruits and certain vegetables worldwide, causing direct and indirect economic injury (Lysandrou 2009). The genus Bactrocera has a wide range of hosts and invasive capabilities of certain species that are considered a severe threat to horticultural crops (Clarke et al. 2005). The peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae), is one of the significant economic pests present in West Asia, North Africa, and Southern Europe (Eppo 2010). It attacks over 40 host plants (Delrio and Cocco 2012) between fruits and vegetables. Furthermore, according to the European and Mediterranean Plant Protection Organization, it is classified as a quarantine A1 pest (Eppo 2010).
Extensive applications of conventional pesticides against fruit flies generate major environmental problems (Magaña et al. 2007), such as resistance development of field strain of the fly. Several efforts have been carried out to limit the usage of toxic pesticides to manage this pest, such as employing abiotic factors, soil water content, soil compaction, and biopesticides alternative to traditional insecticides (El-Gendy and AbdAllah 2020; El-Gendy et al. 2021).
Over the last decades, entomopathogenic fungi (EPF) have played a significant role in the natural biological control of many insects (Burges 1981). Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Clavicipitaceae) and Metarhizium anisopliae (Metchnikoff) Sorokin (Hypocreales: Clavicipitaceae) have an essential role in insect pests' control. B. bassiana is the only insect pathogen that can directly penetrate into its hosts through their cuticles and provides good prospects for managing B. zonata puparia in the soil (soil inocula) (Garrido-Jurado et al. 2011). B. bassiana is a safe biocontrol organism on non-target insects and mammals, including people (Zimmermann 2007). B. zonata adults and pupae were found susceptible to the EPF, such as M. anisopliae and B. bassiana (Rashad et al. 2015). Extended pathogenicity of B. bassiana and M. anisopliae to surviving adult flies treated in the larval stage is limited. Therefore, the present trial aimed to assess the pathogenicity of the EPF, B. bassiana and M. anisopliae, against B. zonata pupae and their extended potential effect on the adult flies under laboratory conditions.
Methods
Target pest
The peach fruit fly, B. zonata, was obtained from the Eradiation of Peach Fruit Fly Laboratory at Damanhour, El-Beheira Governorate, Egypt, which was reared according to El-Gendy (2002).
Fungi used
Two commercial fungus formulations of B. bassiana and M. anisopliae (WP 2.5%, 2.3 × 108 conidia/gm) were tested against B. zonata in the present study. B. bassiana strain was originally isolated from the red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), in Ismailia governorate, while M. anisopliae was isolated from the cotton whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), in Sharkiah governorate, and both were identified in the fungal center of Faculty of Science, Assiut University, Egypt (Ibrahim 2006). Fungal formulations were gained from Bioinsecticides Production Unit, Plant Protection Research Institute, Agricultural Research Center, Giza, Egypt.
Virulence assay of EPF, M. anisopliae and B. bassiana, against B. zonata
Laboratory assay of the EPF, M. anisopliae and B. bassiana, was carried out against the 3rd larval instar of B. zonata (full-grown larvae) under laboratory conditions of 25 ± 2º Ϲ, and 70–80% RH. The experiments were applied in plastic cups (250 cm3) closed at the top with a cloth net (1 mm mesh). The cups contained 75 gm of sterilized sand, autoclaved at 105ºC for 24 h. The fungi were dissolved in tap water. Each fungus was assayed in 5 concentrations, one with a recommended concentration (6 × 105 conidia/ml), and the others were above and below the recommended concentration (2.0 × 105, 4.0 × 105, 1.2 × 106, and 2.3 × 106 conidia/ml), in addition to the control treatment (water only).
One hundred larvae were used per each soil treatment (concentration) in 5 replicates, 20 larvae each. Ten milliliters of fungal suspension per replication was dropped on the soil by the plastic pipette 3 ml. Larvae, full-grown, were transferred to the cups and freely allowed to pupate in the soil. After 2, 3, and 5 days of treatment, the treatments were inspected, and the dead pupae were recorded. On the 7th day, the remained pupae were transferred to Petri dishes until flies' emergence. The newly emerged flies were transferred into plastic cups (250 cm3) covered with a cloth net, supplied with a source of food and water, as previously described. Treatments were checked daily, and the dead flies were removed to Petri dishes containing wet filter papers to confirm that death was caused by the fungal infection.
Determination of the lethal concentration for 50% (LC50) and 90% (LC90) of the individuals
The concentration–mortality relationship, the lethal concentration for 50 and 90% of the fly's individuals (LC50 and LC90), was determined for the tested fungal concentrations (2.0 × 105, 4.0 × 105, 6 × 105, 1.2 × 106, and 2.3 × 106 conidia/ml) of M. anisopliae and B. bassiana against B. zonata pupae 72 h post-treatment.
Determination of the lethal time for 50% (LT50) and 90% (LT90) of the individuals
Time-mortality (median time values associated with 50 and 90% mortality of flies, LT50 and LT90) of B. zonata exposed to the above-mentioned fungal concentrations of M. anisopliae and B. bassiana was determined.
Statistical analysis
Mortality percentages of B. zonata were subjected to two-way analysis of variance (ANOVA), using CoStat Software (2008) Version 6.4. Means were compared by the Tukey–Kramer test at the 5% probability level. LC50 and LC90 and LT50 and LT90 values were determined by probit analysis (Finney 1952), using the Ldp-Line program (Bakr 2007).
Results
Virulence of B. bassiana and M. anisopliae against B. zonata pupae and adult stages
The cumulative mortality of B. zonata pupae of various time intervals
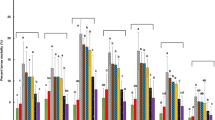
As mentioned above, the EPF, B. bassiana and M. anisopliae, were assayed on the full-grown larvae of B. zonata in 5 fungal concentrations: 2.0 × 105, 4.0 × 105, 6.0 × 105, 1.2 × 106 and 2.3 × 106 conidia/ml, using soil treatment application. It was indicated that no fly’s mortality was recorded during the experimental period, either during the pupal or adult stages in the control treatment. As shown in Fig. 1a, b, pupal mortality was dependent on fungal species and concentration, as well as post-treatment time. No fungal effect was detected on B. zonata pupae after two days of treatment with low concentrations of M. anisopliae and B. bassiana (2.0 × 105 and 4.0 × 105 conidia/ml, respectively). Pupal mortality began at the concentration of 6 × 105 conidia/ml, with 5 and 2.5% mortality, respectively, by M. anisopliae and B. bassiana. Mortality of pupae increased gradually, with increasing the fungal concentration of 1.2 × 106 and 2.3 × 106 conidia/ml, to achieve 7.5 and 12.5% mortality by M. anisopliae and 5 and 7.5% by B. bassiana, respectively. These mortalities varied significantly among fungal concentrations based on fungus species (M. anisopliae: F = 7.68, P = 0.005 and B. bassiana: F = 2.88, P = 0.04).
Accumulated mortality percentages of Bactrocera zonata pupae at three time intervals, treated in the larval stage with different concentrations of Metarhizium anisopliae (A) and Beauveria bassiana (B) fungus under laboratory conditions. Concentrations on x-axis represent Con0 = Control, Con1 = 2.0 × 105-, Con2 = 4.0 × 105-, Con3 = 6.0 × 105-, Con4 = 1.2 × 106-, Con5 = 2.3 × 106-Conidia/ml. Error bars indicate the ± standard error; means sharing similar style letters do not significantly differ at a probability level of 5%
On the 3rd day of pupal age, the treatments exhibited a low mortality percentage (2.5%) at the treatment with 4.0 × 105 conidia/ml of both fungi. Mortality rates increased significantly to 7.5–20 and 7.5–12.5%, respectively, to the fungal concentrations (6.0 × 105–2.3 × 106 conidia/ml) of M. anisopliae (F = 11.2, P = 0.0001) and B. bassiana (F = 9.13, P = 0.0002). On the 5th day, the pupal mortality rate increased significantly as the fungal concentration increased. Pupal mortality rates ranged from 12.5 to 27.5% for M. anisopliae (F = 7.51, P = 0.0006) and from 5 to 17.5% for B. bassiana (F = 8.49, P = 0.0003), with their respective fungal concentrations that ranged from 4.0 × 105 to 2.3 × 106 conidia/ml. In parallel, pupal mortality rates were significantly correlated with fungal species and fungal concentration [M. anisopliae: r = 0.81 (at the 1-day old pupa), 0.87 (3-days) and 0.84 (5-days), while for B. bassiana: r = 0.64 (1-days), 0.86 (3-days) and 0.85 (5-days)].
Pupal mortality was affected by the fungal concentration and post-exposure time by 63.88% with M. anisopliae and 63.59% with B. bassiana of the total factors affected pupal mortality, according to determination coefficient (r2).
Cumulative mortality of adult fly of B. zonata at various time intervals
The effect of EPF continued to the adult fly stage. M. anisopliae was more effective than B. bassiana based on mortality rates that appeared in the adult stage (Fig. 2a, b). On the adult's 5th day, fly mortality rate was 50% (F = 19.11, P = 0.0000) after treating M. anisopliae and 27.5% (F = 14.73, P = 0.0000) after treating with B. bassiana. As both fly age and fungal concentration increased, the fly mortality rate increased. Fungal treatments of M. anisopliae (2.0 × 105–2.3 × 106 conidia/ml) achieved significant mortality effects of 20 to 57.5% (F = 21.15, P = 0.0000), 35–65% (F = 26.93, P = 0.000), 45–82.5% (F = 51.29, P = 0.000), and 62.5–100% (F = 121.8, P = 0.000) with respective of adult fly age of the 7th, 12th, 19th, and 26th, while the treatments by B. bassiana achieved mortalities of 17.5–45.5% (F = 31.75, P = 0.0000), 22.5–57.5% (F = 30.17, P = 0.000), 35–80% (F = 81.71, P = 0.000), and 62.5–97.5% (F = 124.49, P = 0.000) with respective tested days. The mortality rate of the adult fly was significantly different based on the fungal species and fly age [M. anisopliae; r = 0.93 (at 5-days old fly), 0.91 (7-days), 0.89 (10-days), 0.92 (19-days) and 0.95 (26-days), and B. bassiana; r = 0.88 (5-days), 0.93 (7-days), 0.93 (10-days), 0.96 (19-days) and 0.95 (26-days)].
Accumulated mortality percentages of Bactrocera zonata adults at five time intervals, treated in the larval stage with different concentrations of Metarhizium anisopliae (A) and Beauveria bassiana (B) fungus under laboratory conditions. Concentrations on x-axis represent Con0 = Control, Con1 = 2.0 × 105-, Con2 = 4.0 × 105-, Con3 = 6.0 × 105-, Con4 = 1.2 × 106-, Con5 = 2.3 × 106-Conidia/ml. Error bars indicate the ± standard error; means sharing similar style letters do not significantly differ at a probability level of 5%
LC50 and LT50 values of EPF
The concentration–mortality correlation revealed that LC50 and LC90 values were fungus species-dependent, where the fly pupae were more sensitive to B. bassiana than M. anisopliae fungus. The estimated LC50 value for pupal fly, 72 h post-treatment, of B. bassiana was (LC50 = 5.1 × 107 conidia/ml) 5.4-fold higher than that of M. anisopliae (LC50 = 9.5 × 106 conidia/ml), while the LC90 value of B. bassiana was (LC90 = 1.9 × 109 conidia/ml) about 37 times higher than that of M. anisopliae (LC90 = 9.9 × 107conidia/ml) (Table 1).
The LT50 values of B. zonata were fungal species- and concentration-dependent. The fungal impact of M. anisopliae was 1.41 times faster than B. bassiana at the lowest LT50 values, which were 9.48 and 13.33 days for M. anisopliae and B. bassiana fungi, respectively, at 2.3 × 106 conidia/ml. LT50 values were inversely related to the fungal concentration; it reached 28.58 and 30.03 days of treatment at the lowest fungal concentration (2.0 × 105 conidia/ml) of M. anisopliae and B. bassiana, respectively (Table 2).
Discussion
Entomopathogenic fungi (EPF) have received growing interest as microbial agents to control insect pests during the last decades. The pathogenicity of EPF, B. bassiana and M. anisopliae, was assayed on the full-grown larvae of B. zonata in 5 concentrations: 2.0 × 105, 4.0 × 105, 6.0 × 105, 1.2 × 106, and 2.3 × 106 conidia/ml, using soil treatment application. The findings revealed that mortality rates of B. zonata pupae were significantly related to the fungal concentration and fungal species. These findings were confirmed by B. zonata and B. cucurbitae (Coquillett) adult flies, i.e., as the mortality rates of the pests varied based on fungus species and its isolated race (Sookar et al. 2008). The variation in mortality rates of the tested fly stages between both fungal species revealed that B. zonata pupae were much tolerant to the fungal insecticide than in the adult fly stage. They were also more tolerant to B. bassiana than M. anisopliae. In a similar vein, Mahmoud (2009) recorded fairly susceptible of B. zonata pupae by the soil treatment of M. anisopliae than B. bassiana fungus.
On the other hand, fungal mortality rates for B. zonata pupae increased as the fungal concentration and exposure-time interval increased, with higher mortality rates at M. anisopliae than B. bassiana. In parallel with the results of Rashad et al. (2015), the pupal mortality rates of B. zonata increased significantly by increasing the concentration of M. anisopliae and B. bassiana fungi at the same time of exposure. Similar results were recorded on the Medfly, Ceratitis capitata (Wiedemann), as the M. anisopliae fungus was superior pathogenically to the pupae than B. bassiana at the same fungal concentration along with different tested times (Soliman et al. 2020).
Fungal mortality effects of M. anisopliae and B. bassiana on B. zonata pupae appeared on the 2nd day of treatment with little effects to be increased with time post-treatment increased to 27.5% by treatment of M. anisopliae and 17.5% by B. bassiana on the 5th day of pupal age. Obtained results showed differences in pupal mortality rates during the different test periods and between fungal species. However, mortality rates may be different according to the fruit fly species and race. In parallel, Attia (2018) reported 37.75–55.02% mortality in the pupal stage of B. zonata treated with 1–4gm/l of B. bassiana. On C. capitata pupae, M. anisopliae and B. bassiana resulted in about 94% mortality (Ekesi et al. 2002). However, no fungal effect of B. bassiana was detected on Anastrepha ludens (Loew) pupae (Aluja 1993).
Pathogenicity effect of M. anisopliae and B. bassiana extended to the adult fly stage; M. anisopliae fungus was more fatality than B. bassiana. In parallel with the present results, in earlier studies on B. zonata fly, M. anisopliae fungus was superior in the fatality rates on the fly than B. bassiana fungus (Ibrahim et al. 2014). However, Hussein et al. (2018) reported that B. bassiana was more effective than M. anisopliae on the immature stages and adult flies of B. zonata. Furthermore, Rashad et al. (2015) found a significant influence of the soil application of B. Bassiana fungus on mortality rates in adult flies B. zonata, compared to M. anisopliae fungus.
Mortality rates of the adult fly of B. zonata were significantly related to the concentrations of fungi and according to adult stage and fungus species. Similar to the present results, the correlation coefficient values of microbial-insecticide concentrations of B. bassiana and M. anisopliae were positively correlated with mortalities of B. cucurbitae fly (Iqbal et al. 2021).
According to the present study's findings, the median LT50 values on the fly were fungal species- and concentration-dependent. The M. anisopliae fungus affected the fly faster than B. bassiana. Results of LT50 values ranged from 28.57 to 9.475 days by M. anisopliae and from 30.03 to 13.33 days by B. bassiana fungus, respectively, depending on fungal concentration. These results are consistent with the results of Mahmoud (2009), whereby the LT50 values for B. zonata males and females treated with M. anisopliae fungus were less than those with B. bassiana, and LT50 values were 8.93–10.89 days for males and 12.69–14.39 days for females with the respective fungi.
Conclusions
This study concluded that the appreciable lethal effects of M. anisopliae, and B. bassiana fungi were set at the end of the pupal stage of B. zonata with 27.5 and 17.5% mortalities, respectively, which persisted during the adult fly stage, achieving 100 and 97.5% mortality with, respectively, tested fungi at the highest concentration. M. anisopliae fungus was more effective on both pupal and adult stages of B. zonata than B. bassiana. M. anisopliae was more virulent than B. bassiana; M. anisopliae, causing 50% mortality of individuals (LT50 value) in 9.475 and 13.33 days, respectively, at the highest tested fungal concentration. M. anisopliae and B. bassiana had potent effects in B. zonata control under laboratory conditions. This information may help in the pest management of the pest and in integrated and organic production systems.
Availability of data and materials
All data and materials are available.
Abbreviations
- ANOVA:
-
Analysis of variance
- IPM:
-
Integrated pest management
- LC:
-
Lethal concentration
- LT:
-
Lethal time
- P :
-
Probability level
- SE:
-
Standard error
- χ 2 :
-
Chi square
References
Aluja SM (1993) Manejo integrado de la mosca de la fruta , p 251
Attia SHM (2018) Enhancement of the two entopathogenic fungi, as a control measure against the peach fruit fly, Bactrocera zonata (Saunders) (Diptera:Tephritidae) in Egypt. Ph.D., Ain Shams, Ain Shams University, Cairo, Egypt, , p 214
Bakr E (2007) LdP line. http://embakr.tripod.com/ldpline/index.htm
Burges HD (1981) Safety, safety testing and quality control of microbial pesticides. In: Microbial control of pests and plant diseases, 1970–1980
Clarke AR, Armstrong KF, Carmichael AE, Milne JR, Raghu S, Roderick GK, Yeates DK (2005) Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annu Rev Entomol 50:293–319
Delrio G, Cocco A (2012) The peach fruit fly, Bactrocera zonata: a major threat for Mediterranean fruit crops? Acta Hortic 66:557–566
Ekesi S, Maniania NK, Lux SA (2002) Mortality in three African tephritid fruit fly puparia and adults caused by the entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci Techno 12:7–17
El-Gendy IR (2002) Studies on peach fruit fly Bactrocera zonata (Saunders) at El-Bohiera Governorate. M.Sc. Thesis, Alexandria University, Egypt
El-Gendy IR, Abdallah AM (2020) Soil compaction, moisture content and pupal burial depth as a new control strategy of peach fruit fly Bactrocera zonata (Diptera: Tephritidae). Egypt J Plant Prot Res Inst 3:621–635
El-Gendy IR, El-Banobi MI, Villanueva-Jimenez JA (2021) Bio-pesticides alternative diazinon to control peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). Egypt J Biol Pest 31:49
Eppo (2010) Bactrocera zonata: Procedure for official control. OEPP EPPO Bull 40:390–395
Finney DJ (1952) Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge University Press, Cambridge, p 333
Garrido-Jurado I, Torrent J, Barrón V, Corpas A, Quesada-Moraga E (2011) Soil properties affect the availability, movement, and virulence of entomopathogenic fungi conidia against puparia of Ceratitis capitata (Diptera: Tephritidae). Biol Cont 58:277–285
Hussein MA, Khaled AS, Ibrahim AA, Soliman NA, Attia SH (2018) Evaluation of entomopathogenic Fungi, Beauveria bassiana and Metarhizium anisopliae on Peach Fruit Fly, Bactrocera zonata (Saunders)(Diptera: Tephritidae). Egypt Acad J Biolog Sci F Toxicol Pest Cont 10:59–68
Ibrahim AA (2006) Action of certain microbes on cotton leaf worm. Ph.D. Thesis, Faculty of Science, Al-Azhar University, p 171
Ibrahim AA, Soliman NA, El-Deen MM, Ramadan NF, Farag SR (2014) Susceptibility of the peach fruit fly, Bactrocera zonata (Saunders) and the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) adults to the entomopathogenic fungi; Metarhizium anisopliae (Met.) and Beauveria bassiana (Bals.). Egypt J Biol Pest Cont 24:491–495
Iqbal M, Gogi MD, Atta B, Nisar MJ, Arif MJ, Javed N (2021) Assessment of pathogenicity of Beauveria bassiana, Metarhizium anisopliae, Verticillium lecanii and Bacillus thuringiensis var. kurstaki against Bactrocera cucurbitae Coquillett (Diptera: Tephritidae) via diet-bioassay technique under controlled conditions. Int J Trop Insect Sci 41:1129–1145
Lysandrou M (2009) Fruit flies in the mediterranean and Arab world: how serious a threat are they and how can we minimize their impact. Arab J Plant Prot 27:236–239
Magaña C, Hernández-Crespo P, Ortego F, Castañera P (2007) Resistance to malathion in field populations of Ceratitis capitata. J Econ Entomol 100:1836–1843
Mahmoud FM (2009) Susceptibility of the peach fruit fly Bactrocera zonata (Saunders),(Diptera: Tephritidae) to three entomopathogenic fungi. Egypt J Pest Cont 19:169–175
Rashad MM, El-Heneidy AH, Djelouah K, Hassan N, Shaira SA (2015) On the pathogenicity of entomopathogens to the peach fruit fly, Bacterocera zonata (Saunders) (Diptera: Tephritidae). Egypt J Pest Cont 25:649–654
Soliman NA, Al-amin SM, Mesbah AE, Ibrahim AMA, Mahmoud AMA (2020) Pathogenicity of three entomopathogenic fungi against the Mediterranean fruit fly, Ceratitis capitata (Wiedemann)(Diptera: Tephritidae). Egypt J Biol Pest Cont 30:49
Sookar P, Bhagwant S, Awuor Ouna E (2008) Isolation of entomopathogenic fungi from the soil and their pathogenicity to two fruit fly species (Diptera: Tephritidae). J Appl Entomol 132:778–788
Zimmermann G (2007) Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci Technol 17:553–596
Acknowledgements
The authors are grateful to all technical staff and researchers at the Eradiation of the Peach Fruit Fly Laboratory at Damanhour, El-Beheira Governorate.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
IR reared B. zonata, and IR and MFZ put strategy to achieve this work. IR, MFZ, and MI achieved this investigation. IR, MFZ, and MI are the contributors in writing the manuscript. IR and MFZ revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Gendy, I.R., Zawrah, M.F.M. & El-Banobi, M.I. Virulence effect of Metarhizium anisopliae (Met.) and Beauveria bassiana (Bals.) fungi against the peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). Egypt J Biol Pest Control 32, 43 (2022). https://doi.org/10.1186/s41938-022-00545-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00545-3