Abstract
Background
Early blight disease of tomato caused by pathogenic fungi Alternaria solani is the most significant and common disease throughout the world as well as in Kingdom of Saudi Arabia. The aim of this study was to isolate and identify native Trichoderma species from the Jeddah region in Saudi Arabia; evaluate their antagonistic potential against A. solani; and study their influence early blight disease severity in greenhouse and in open field.
Results
The present study focused to explore the biocontrolling potential of native Trichoderma spp. against A. solani strain to compare with a conventional fungicide. Out of 21, 3 Trichoderma isolates showed an antifungal activity and significantly inhibited the mycelial growth of pathogen that were identified as Trichoderma atroviride, T. harzianum and T. longibrachiatum by their ITS region sequence analysis. Strong in vitro mycelial growth suppression (70.66%) was also recorded at 400 ppm Mancozeb (90%WP®) fungicide. Further, these Trichoderma bioagents and fungicide were further evaluated in greenhouse (artificially inoculated) and in field on naturally infected tomato plants. In greenhouse, (13.74%) disease severity after T. harzianum treatment was recorded, followed by T. longibrachiatum (25.83%) and T. atroviride (21.67%). The disease severity after fungicide (50 mg/L; 10 ml per plant) application was (7.91%). Further, positive impact on the plant biomarkers was demonstrated by all selected Trichoderma isolates in greenhouse. Under natural infection in season I, the disease severity (%) after T. longibrachiatum, T. atroviride and T. harzianum treatments was 11.5, 13.26 and 16.81%, respectively, followed by control (32.12%), whereas 7.18% disease severity was recorded after fungicide application.
Conclusions
The results revealed that native Trichoderma of this region had potential to mitigate the early blight disease intensity in field.
Similar content being viewed by others
Background
Tomato (Solanum lycopersicum L.) is susceptible to different pests present in the environment such as nematodes, viruses, bacteria and fungus. Early blight disease of tomato caused by pathogenic fungi Alternaria solani is the most significant and common disease throughout the world as well as in Kingdom of Saudi Arabia (Imran et al. 2021). This pathogen infects various solanaceous crops including eggplant, tomato, potato and pepper (Sallam and Abo-Elyousr, 2012). Numerous fungicides such as carbendazim, captan, mancozeb, propiconazole, copper oxychloride, propineb and tebuconazole are wildly used to fight against this pathogen (Deshmukh et al. 2020). However, among all fungicides Mancozeb fungicide with different formulations has widely been used to control the pathogen of early blight disease in tomato (Sowmya and Ram, 2021). Various studies have reported the significant growth suppression of fungal pathogens in field conditions (Yang et al. 2019). The successful control of this pathogen by fungicides clearly demonstrated the sensitivity of this pathogen toward this fungicide. Although the development of resistance risk of Mancozeb in A. solani is low (Yang et al. 2019), the frequent use of fungicide can lead to the development of resistance in pathogen. Agricultural practices and agro-chemical applications can control early blight disease and are generally considered effective (Adhikari et al. 2017). Inadequate utilization of the fungicides, however, implies serious environmental and human health hazards (Nishant et al. 2021). Furthermore, toxicity of fungicides confines applicability during fruiting period and multiple fungicide application may lead to resistance development in pathogens (Zhang et al. 2021). Therefore, biological control of plant diseases is considered a safe and eco-friendly alternative approach (Imran et al. 2022). In nature, some bacterial and fungal species are found harmless, protecting the roots of plants from rhizospheric pathogens. Trichoderma spp. are pre-eminent antagonists against various plants pathogens including nematodes, bacteria, and particularly fungi. They inhibit the growth of pathogens either directly as competitors for space and nutrients or by hyper-parasitism or indirectly by improving plant vigor, escalating stress lenience, promoting active uptake of nutrients and/or producing numerous secondary metabolites and pathogenesis-related (PR) proteins to plants (Zhang et al. 2017). Many soil borne pathogens are controlled by T. harzianum triggering-induced systemic resistance by rapid root colonization (Kamala and Devi, 2012). Secondary metabolites of T. harzianum such as pyrone, harzianopyridone, furanone, palmitic acid, stigmasterol and anthraquinone have antimicrobial effects (Ahluwalia et al. 2015). T. viride is also a significant bioagent and, due to its antagonistic activity, it has been widely used against many fungal plant pathogens (Mohamed and Gomaa, 2019). T. longibrachiatum as a promising biocontrol agent has been widely used because it secrets huge amount of peptaibols, small peptides approx. 5–20 amino acid residues in length, with a wide spectra of biological activities (Elegbede and Lateef, 2019).
Considering the importance of bio-control agents and rising fungicides resistance problems, the present study aimed to: obtain and identify native Trichoderma species from the Jeddah region in Saudi Arabia; evaluate their antagonistic potential against A. solani; study their influence early blight disease severity in greenhouse and in open field; and compare bio-agents with a conventional fungicide with respect to fruit yield and disease control in open field under natural infection conditions. This is the first study on the isolation and evaluation of Trichoderma and their utilization against early blight disease in fields of the Kingdom of Saudi Arabia (KSA).
Methods
Fungal strain and growth conditions
Highly virulent strain of A. solani “9013” was previously isolated by Imran et al. (2022). This fungal strain was tested for pathogenicity and found highly virulent on tomato plants causing early blight disease. The strain was collected from the fungal stock culture of the laboratory. Strain was sub-cultured on PDA medium plates incubated at 27 °C for 7 days. Culture was maintained at 4 °C for further use.
Isolation and field-testing of Trichoderma as potential bioagents
For the isolation of naturally existing potential bioagents against fungal pathogens, soil samples were collected from the rhizosphere of healthy tomato plants. Then, 5 ml of sterilized double distilled water was added to each 2 g of soil sample, followed by mixing by vortexing. Dilutions 10–4, 10–5, 10–6, 10–7, 10–8 and 10–9 were prepared from supernatant from each sample (Bin et al. 2020) and inoculated on rose Bengal (RB) medium plates and incubated at 27 °C for 48 h. Germinating fungal colonies were further purified by single spore transfer (Dou et al. 2019). Colonies derived from single spores were further transferred to new PDA plates, and 3-mm-diameter mycelial disks from 3-day-old culture were transferred to PDA glass tubes. Fungal cultures were maintained in glycerol tubes and stored at 4 °C for further use.
In vitro screening and selection of antagonists
All isolates were tested for their antagonistic potential against the highly virulent A. solani strain. Antagonistic activity was determined on PDA plates. Bioagent isolates grown on PDA for 3 days were used to determine the antagonistic effect by dual culture assay against A. solani strain. Briefly, 5-mm-diameter mycelial disk from pathogen and identical diameter disks from each bioagent (priorly grown on PDA) were placed face to face on new PDA plates at equal distance from edges PDA plates containing pathogen disks were used as controls. Three replicates (each comprising 5 plates) were prepared for each potential bioagent isolate, and plates were incubated at 27 °C for 7 days. At this stage, in control plates the selected highly virulent A. solani strain would typically cover the whole plate. Diameters of pathogen isolate colonies on test plates were measured, and mean values were calculated to quantify the inhibitory effect of each potential bioagent isolate. The experiment was repeated twice. Based on pathogen colony mean diameters, bioagents showing best antagonistic activity were selected for further experiments.
Morphological and molecular identification of bioagents
All potential bioagent isolates were classified morphologically based on colony color, length and width of conidia, hyphae length, and shape and diameter of conidia (Kumar et al. 2012). Selected bioagent isolates were classified by amplification and Sanger sequencing of internal transcribed spacer (ITS) regions using generic primer pair ITS5 (5′GGAAGTAAAAGTCGTAACAAGG3′) and ITS4 (5′TCCTCCGCTTATTGATATGC3′) (White et al. 1990). DNA of the selected isolates was extracted by CTAB as of pathogen. PCR was performed in a thermal cycler with the final volume 50 µl of reaction mixture with this primer pair as described above for pathogen isolates. PCR was performed as following: initial denaturation at 94 °C for 3 min followed by 30 cycles, denaturation at 94 °C for 1 min, 56 °C for 30 s, 72 °C for 30 s, and final incubation at 72 °C for 10 min. Reaction products were cooled to 4 °C for 10 min. PCR products were then separated on 2% agarose gel in 1× Tris–acetate (TAE) buffer and stained with 0.5 μg ethidium bromide solution for 10 min, and an Alpha Imager™ gel imager system was used to record fluorescence images. PCR products were submitted to Sanger sequencing by Macrogen Company, Seoul, South Korea. Obtained sequences of PCR products were compared ITS sequences available in public domain of National Center for Biotechnology Information (NCBI) library using Basic Local Alignment Search Tool (BLAST). Respective potential bioagent isolates were identified on the basis of their similarity to published reference sequences, and obtained new ITS sequences were submitted to NCBI under specific accession numbers. Phylogenetic trees were constructed from ITS1 sequence data with the help of the neighbor joining algorithm in MEGA 6X software package (Tamura et al. 2013).
Establishment of conventional control of A. solani with chemical fungicides
For in vitro mycelial inhibition virulent A. solani strain, different concentrations of a commercial fungicide Mancozeb (90% WP) [ethylenebisdicarbamates] were used that is recommended to control early blight disease in local commercial farming. Stock solution (0.5 g/L) of the fungicide was prepared in sterile double distilled water that was further used to prepare the tested concentrations, viz. 50, 100, 200 and 400 ppm. These concentrations were dissolved in PDA, and 5-mm mycelial disks from 7-day-old A. solani culture were placed face-down in the middle of fungicide-amended plates. PDA plate lacking fungicide but the pathogen was subjected as control. Plates were incubated at 27 °C for 7 days, and colony diameter was measured. Percent mycelial growth inhibition was calculated according to Bekker et al. (2006) as: Percent inhibition = [(C − T)/C]*100, where, C representing colony diameter (mm) observed in controls, and T colony diameter (mm) observed in treatments. Experiments were performed in triplicates, with each replicate consisting of five plates. The experiment was repeated twice for the consistency of results.
Effect of potential Trchicoderma bioagents early blight disease severity
Under greenhouse condition
To test the efficacy of selected potential Trichoderma against early blight disease under greenhouse conditions relevant to horticultural praxis, experiments were conducted (in 2020) in greenhouses of the Department of Arid Land Agriculture, King Abdulaziz University Jeddah, Saudi Arabia, with tomato variety “Doucen.” Briefly, tomato seeds were germinated and seedlings were grown in 18 cm plastic pots containing peat moss (1:3). At 3–4 leaf stage, seedlings were singled out to new pots. Selected potential Trichoderma isolates to be tested were grown on PDA for 5 days at 27 °C. Growing mycelia were scraped with a sterilized scraper and crushed in 20 ml of sterilized double distilled water, and resulting debris was filtered through 3 layers of cheese cloth to remove fragmented mycelium from spores. Twenty-days-old plants were sprayed with the resulting spore suspension (10 mL/plant) with a compression sprayer (Blue Stallion Co., Ltd, India) (Singh et al. 2019). Spore suspension of highly virulent A. solani strain priorly grown on PDA was prepared identical methods used for the preparation of Trichoderma suspension. Two days of spraying Trichoderma, pathogen spore suspension (104 spores mL−1 adjusted by hemo-cytometer) 5 mL/plant was sprayed with a compression sprayer (Blue Stallion Co., Ltd, India). Plants sprayed first with potential bio-agent, but then only with sterilized distilled water at the time of pathogen spraying served as “healthy” control. In contrast, “diseased” control plants were sprayed only with pathogen. Mancozeb fungicide as “conventional treatment” was sprayed at a concentration of 50 mg L−1 (25 mL per plant) was sprayed at the same time of pathogen inoculation (Gondal et al. 2012). After pathogen inoculation, plants were covered with sterile polythene bags for 3 days. Standard agronomic practices were carried out in greenhouse, and experiment was performed with 4 replicates for each treatment, with 3 plants for each replicate. The experiment was performed twice and disease severity was estimated with a grade 0–5 disease rating scale as: 0 = leaves free from leaf spots; 1 = 0–5% of leaf area infected; 2 = 6–20% of leaf area infected; 3 = 21–40% leaf area infected; 4 = 41–70% leaf area infected; 5 = more than 70% of leaf area infected (Gondal et al. 2012).
Prior to disease severity, plant height was calculated, and after this, fresh and dry weight of roots and shoots were measured to determine the dry weight; plants were placed in moisture dryer chamber at 60 °C for 3 days. Means of parameters were calculated and compared for different treatments.
Open field trials
The study was conducted for 2 consecutive seasons early (season I: Jan-Mar) and late (season II: Sep-Dec) 2020 to monitor the efficacy of potential Trichoderma under open field conditions. In season I, seedlings of the variety “Doucen” were grown in plastic seedling trays (50 holes) containing peat moss (1:3). At 3–4 leaf stage, tomato seedlings were transplanted to the field, maintaining 60 cm distance between rows and 45 cm within plants in a row. Suspensions of fungal Trichoderma were prepared as described above for greenhouse inoculation. Two weeks after transplanting, Trichoderma suspensions were applied as foliar spray (100 mL per plant) on tomato plants with a garden sprayer (Skybird Agro Ind. Amritsar, Punjab, India), while Mancozeb fungicide as 50 mg L−1 was sprayed (50 mL/plant) (Majumder et al. 2020) with hand sprayer (Taizhou Jiolong Machinery Co., Ltd, Zhejiang, China). Control plants were sprayed with sterile distilled water only. All treatments were applied in the evening hours, and plants were left for natural pathogen infection under open field conditions. After applying treatments, weather conditions were monitored to exclude washout of pesticides as well as Trichoderma suspensions due to rain. Standard agronomic practices were carried out in field. Disease severity was recorded based on a grade 0–9 disease rating scale as: 0 = no infection, 1 = 0–10%, 2 = 10–20%, 3 = 20–30%, 4 = 30–40%, 5 = 40–50%, 6 = 50–60%, 7 = 60–70%, 8 = 70–80%, 9 = 80–90% or more leaf area infected (Singh et al. 2014) and percent disease severity was calculated by above mentioned formula. Ripened tomato fruits were harvested regularly from all replicates in all treatments and fruit yield per treatment was calculated.
The experiments were conducted under complete randomized block design with 4 replicates, each carrying 4 plants. All recommended agronomic practices were adapted in experimental zone. Plants were randomly sprayed for each treatment. Experiment with same parameters was repeated in season II. Percent disease severity and fruit yield were calculated and compared among treatments.
Data analysis
All in vitro experiments were conducted in triplicates, while field experiment was conducted with 4 replicates. Field experiments were performed in a complete randomized design, and all collected data were analyzed by using statistix 8.1 (Analytical software, statistix; Tallahassee, FL, USA, 1985–2003) software. The data for disease severity were transformed into arcsine values, and a one-way analysis of variance (ANOVA) was performed. Means of replicates in all treatments were compared using Fisher’s least significant difference test at p = 0.05 (Steel et al. 1996).
Results
Isolation and identification of potential Trichoderma
A total of 21 fungal isolates were collected from rhizosphere of healthy tomato fields as starting material to identify potentially beneficial bioagents. Morphological characterization like mycelial growth rate, colony facade, conidial shape, structure of conidiophores and branching pattern of phialides was consistent with species of genus Trichoderma. Colony color was green/yellow to dark green/green; conidia were green to dark green. Most of the isolates showed green conidia color with 2.5–5.3 µm size having globose to subglobose/obovoid conidial shape, while the phialide were slender, hooked, and flask shaped, as well as elongated with size 3.0–11.5 µm × 2–6.2 µm (data not shown). Therefore, all isolates were identified as members of genus Trichoderma. Based on morphological identification, isolates were used to study their antagonistic behavior against highly virulent A. solani strain.
In vitro application of Mancozeb fungicide
All the tested concentrations of Mancozeb fungicide effectively inhibited the mycelial growth of A. solani. However, increase in the concentration of fungicide suppressed the mycelial growth of A. solani, and at 400 ppm concentration, the mycelial growth was significantly inhibited (70.66%) than the control. In these results, all the used concentrations remained effective against the mycelial growth suppression of A. solani. Results showed that an increase in fungicide concentration reduced the growth of A. solani (Fig. 1).
Screening of antagonists and molecular characterization
All 21 potential bioagent isolates showed antagonistic activity against A. solani strain 9013 in vitro. Pathogen mycelial growth inhibited by the isolates 1006, 1007 and 1013 was significant (30.6, 27.3nd 23.3 mm), respectively, as compared to all other fungal bioagents. The colony diameter of pathogen growth in the dual culture plates by the isolates 1018, 1021, 1003, 1011 and 1015 was 73.5, 46.5, 45.6 44.6 and 46.3 mm, respectively, over control (82.66 mm) and other isolates (Fig. 2).
Potential bioagent isolates 1006, 1007 and 1013 identified by PCR amplification of internal transcribed spacer (ITS) regions showed approximately 560–680 bp length. Sequences were submitted to NCBI database under accession numbers MW590689, MW590687 and MW590688, respectively. Comparison of obtained ITS sequences to NCBI database entries by basic local alignment search tool (BLAST) indicated the isolate 1007 into the species Trichoderma harzianum (KR868283.1), 1013 into T. longibrachiatum (MT409889.1), and the isolate 1006 into T. atroviride (MT604177.1).
Phylogenetic analysis
The phylogenetic tree of 3 selected Trichoderma bioagents (1006, 1007 and 1013) is represented in Fig. 3a–c constructed with 80–90% nucleotide sequences similarity from NCBI. In Fig. 3a, red dot demonstrated the identified isolate T. atroviride supported with a bootstrap value of 100% (Fig. 3b) T. harzianum was supported by a bootstrap value of 97% and Fig. 3c T. longibrachiatum is supported by a bootstrap value of 99%.
Phylogenetic trees of identified isolates of Trichoderma, constructed by the analysis of ITS1 sequences with neighbor joining algorithm in MEGA 6X package. The numbers over branches represent the coefficient of bootstrap, tested by 100 replications. a Isolate 1006, identified as Trichoderma atroviride (accession no. MW590689); b Isolate 1007, identified as Trichoderma harzianum (accession no. MW590687); c Isolate 1013, identified as Trichoderma longibrachiatum (accession no. MW590688). A red dot in each figure represents the highest similarity of identified isolates based on the sequences available in NCBI
Efficacy of Trichoderma spp. on disease severity under greenhouse conditions
Application of three Trichoderma isolates effectively reduced in early blight disease severity in greenhouse. Symptoms appeared after 15 days of inoculation as browning of tissues followed by necrosis. Earlier, oval shape spots were observed which extended and converted to concentric rings and changed to dark brown lesions. Disease severity of infected control was significantly higher (77.91%) than other treatments, which conferred that the A. solani isolate “9013” was highly virulent (Fig. 4). Plants treated with Mancozeb fungicide showed lowest disease severity (7.91%). In comparison, the disease severity in plants treated with T. atroviride isolate 1006 was 21.67%, while disease severity of plants treated with T. harzianum isolate 1007 and T. longibrachiatum isolate 1013 was 13.71and 25.83%, respectively.
Efficacy of bioagents on disease severity (%) of artificially inoculated virulent isolate of Alternaria solani on tomato plants in greenhouse. Values followed by different letters indicate that means are significantly different from one another according to Fisher’s least significant difference at p = 0.05. Error bars on graphs represents the Mean ± SE
Plant biomarkers
Results demonstrated that T. harzianum isolate 1007 and T. longibrachiatum isolate 1013 considerably increased the fresh and dry weight over infected plants that where otherwise untreated, indicating the efficacy of them as bio-control agents. Plants treated with Mancozeb fungicide and bioagents also sustained the height of plants over infected plants that where otherwise untreated. The results (Table 1) showed that the inoculation of tomato with Trichoderma bioagents has an invigorating effect on plants. All treatments also improved the root weight as fresh and dry weight was higher than infected control.
Open field trials
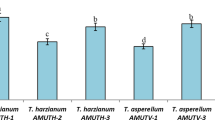
In the field, percent disease severity was almost constant in differing between seasons (Fig. 5) among all treatments after 15 days of transplanting. Application of Mancozeb in field showed lower severity disease (%) in both seasons (7.18 and 6.31%). Treatments with Trichoderma spp. also showed promising and comparable effects for the reduction in disease severity over naturally infected control. However, in season I, treatment with T. atroviride isolate 1006, T. harzianum isolate 1007 and T. longibrachiatum isolate 1013 showed promising potential in the reduction in disease severity as 13.26, 16.81% and 11.5%, respectively, recorded. While in season II, disease severity after applying T. harzianum slightly reduced to 16.5%, disease severity percent after the application of T. atroviride and T. longibrachiatum was 16.12 and 16.5% that was lower than control (31.68%) but higher than fungicide treatment (6.31%) (Fig. 5). Alternatively, under field experiments, plant height was higher after treatment with Trichoderma bioagents compared to fungicide and water treatment which indicated that Trichoderma bioagents might have acted as plant growth promoters.
Open field efficacy of Trichoderma bioagents against natural infection of Alternaria solani on tomato plants in two different seasons. Values followed by different letters indicates that means are significantly different from one another, while values followed by same letter indicate no significant difference in means according to Fisher’s least significant difference at p = 0.05. Error bars on graphs represent the Mean ± SE
In field conditions, the efficacy of Trichoderma bioagents was assessed and compared with conventional fungicide treatment. Fruits were randomly harvested for at least 3–4 times in each season. Fruit yield after application of Mancozeb fungicide was 19.69 kg per plant in season I and 18.31 kg in season II, compared to 8.67 kg in season I and 8.08 kg in season II for untreated control plants. Thus, after fungicide application fruit yield was consistently higher in both seasons. Application of T. harzianum isolate 1007, T. atroviride isolate 1006 and T. longibrachiatum isolate 1013 also effectively increased the fruit yield in both seasons (Fig. 6). In season I, fruit yield after the application of T. harzianum isolate 1007, and T. longibrachiatum isolate 1013 was 13.92 kg and 12.1 kg, respectively, followed by T. atroviride isolate 1006 (10.91 kg). In season II, fruit yield after the application of T. harzianum was 12.78 kg compared to T. atroviride (9.85 kg), and T. longibrachiatum (9.43 kg). Results showed that Trichoderma bioagents indeed protected fruit yield under field conditions, but not too the same extent as conventional fungicide treatment.
Open field efficacy of Trichoderma bioagents on yield of tomato after natural infection of Alternaria solani in two different seasons. Values followed by different letters indicate that means are significantly different from one another, while values followed by same letter indicate no significant difference in means according to Fisher’s least significant difference at p = 0.05. Error bars on graphs represent the Mean ± SE
Discussion
Early blight disease of tomato caused by a fungal pathogen A. solani is the most significant disease (Rahmatzai et al. 2017). Significant mycelial growth inhibition was recorded with mancozeb fungicide at 400 ppm over control. The fungicide concentrations used herein this study showed that the increase in the concentration of fungicide significantly suppressed the mycelial growth of A. solani, and most probably at 2000 ppm the mycelial growth might be completely inhibited because the previous studies reported 86.4–100% mycelial growth inhibition of A. solani with the 0.2% concentration of mancozeb (Adhikari et al. 2017). Further, the significant reduction in the mycelial growth of Fusarium oxysporum was obtained at 10, and 100 and 1000 ppm (Shah et al. 2006). More, the highest reduction in the growth (86.4%) of A. solani was achieved at 1500 ppm concentration of identical fungicide (Sadana and Didwania, 2015). A study by Vanitha et al. (2013) revealed that increase in the concentration significantly suppresses the A. solani growth these previously reported studies strongly support present results.
Recent approaches have been made to use more eco-friendly bioagents to control early blight disease (Behiry et al. 2020) because biological control is considered an important approach over chemical control with fungal and bacterial antagonists to early blight pathogen. In the present study, 21 isolates of Trichoderma spp. were obtained from field sites with healthy tomato plants. Former studies have reported the antagonistic behavior of Trichoderma strains as effective bio-control agents against different disease such as T. harzianum against cucumber Rhizoctonia solani (Srivastava, 2021) and blight late disease in potato (Purwantisari et al. 2021). Many Trichoderma, such as T. atroviride, T. virens and T. harzianum as bio-control can inhibit the pathogen growth (Kumar et al. 2012). Trichoderma spp., showed significant mycelial growth suppression against many fungal pathogens by exhibiting the capability to contend for space and nutrients (Hirpara et al. 2017) and ability for rapid growth was significant advantage for nutrient and space competition (Amira et al. 2017). These findings evidently and sturdily support these results.
Considerable increase in plant height, fresh and dry weight of roots and shoots was recorded over infected control. Trichoderma spp. as potential bio-control agents was reported with beneficial effects on the repression of pathogen by producing antifungal metabolites and antibiotic promoted the plant growth and caused significant yield improvement under greenhouse and field conditions (Koley et al. 2015). This may be due to the synergetic mode of action of Trichoderma which hindered the phytopathogen development (Alabouvette et al. 2006). Further, similar findings were recorded when T. viride was used against A. solani of potato in greenhouse and significant reduction in disease severity was observed (Udhav, 2013). Similar results reported by various researchers strongly and clearly supported our findings and our finding corroborated that previous.
The present results showed remarkable difference (25.83, 21.67 and 13.74% disease severity after T. longibrachiatum, T. atroviride and T. harzianum,, respectively, treatment, followed by infected control (77.91%). Between all treatments, disease severity (%) after the application of mancozeb 90% WP (7.91%) in greenhouse was significant in comparison with all selected microbial antagonists. Similar findings have been reported when Ridomil (Mancozeb 64% + Metalaxyl 4%) along with selected bioagents was used against A. solani on tomato plants in greenhouse over treated control and recorded significant reduction in the intensity of early blight (Ngoc, 2013). Trichoderma spp. have also been reported as plant growth promoters (Sánchez-Montesinos et al. 2020). Field application of selected Trichoderma bioagents showed significant reduction in disease severity in both seasons. The results here in showed that disease severity in both seasons was reduced after the application of Trichoderma bioagents than naturally infected control. Similar results were recorded by Kulimushi et al. (2021) upon field application of Trichoderma against early blight which caused significant reduction in disease severity (%), which strongly supported the present results. As in the results, fruit yield after treatments with T. harzianum was higher in both seasons than the control and followed by T. atroviride and T. longibrachiatum. However, studies reported the difference in yield with same antagonists (Ngoc 2013) and these variations might be due to the type of antagonists used, varieties and environmental conditions because antagonists could be influenced by diverse environmental factors like relative humidity temperature and pH (Benítez et al. 2004).
Conclusion
The results presented in this study showed that native Trichoderma spp. could reduce the severity of early blight of tomato under the conditions in the Kingdom of Saudi Arabia. Further, these isolates may promote the growth and development of plants and ultimately increase the fruit yield. These isolates might be integrated with other management strategies to reduce the disease losses and sustainable tomato production.
Availability of data and materials
Data for sequence of Trichoderma spp )Trichoderma atroviride (accession no. MW590689); Trichoderma harzianum (accession no. MW590687) and Trichoderma longibrachiatum (accession no. MW590688) are available at website https://www.ncbi.nlm.nih.gov.
Abbreviations
- PDA:
-
Potato dextrose agar
- PCR:
-
Polymerase chain reaction
- ITS:
-
Internal transcribed spacer
- ANOVA:
-
Analysis of variance
References
Adhikari P, Oh Y, Panthee DR (2017) Current status of early blight resistance in tomato: An update. Int J Sci 18:10. https://doi.org/10.3390/ijms18102019
Ahluwalia V, Kumar J, Rana VS, Sati OP, Walia S (2015) Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat Prod Res 29(10):914–920. https://doi.org/10.1080/14786419.2014.958739
Alabouvette C, Olivain C, Steinberg C (2006) Biological control of plant diseases: the European situation. Eur J Plant Pathol 114(3):329–341. https://doi.org/10.1007/s10658-005-0233-0
Amira MB, Lopez D, Mohamed AT, Khouaja A, Chaar H, Fumanal B, Gousset DA, Bonhomme L, Label P, Goupil P, Ribeiro S, Pujade-Renaud V, Julien J, Auguin D, Venisse J (2017) Beneficial effect of Trichoderma harzianum strain Ths97 in bio controlling Fusarium solani causal agent of root rot disease in olive trees. Biol Control 110:70–78. https://doi.org/10.1016/j.biocontrol.2017.04.008
Behiry SI, EL-Hefny M, Salem MZM, (2020) Toxicity effects of Eriocephalus africanus L. leaf essential oil against some molecularly identified phytopathogenic bacterial strains. Nat Prod Res 34:3394–3398. https://doi.org/10.1080/14786419.2019.1566824
Bekker TF, Kaiser C, Merwe RVD, Labuschagne N (2006) In-vitro inhibition of mycelial growth of several phytopathogenic fungi by soluble potassium silicate. South African J Plant Soil 23(3):169–172. https://doi.org/10.1080/02571862.2006.10634750
Benítez T, Rincon AM, Limon MC, Codon AC (2004) Biocontrol mechanism of Trichoderma strains. Int Microbiol 7(4):249–260
Bin L, Shida J, Huifang Z, Yucheng W, Zhihua L (2020) Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol Res 235:126445. https://doi.org/10.1016/j.micres.2020.126445
Deshmukh HV, Deokar CD, Raghuwanshi KS, Khaire PB, Brahmane PR (2020) Efficacy of different fungicides against the Alternaria solani under in vitro conditions. J Pharm Phyto 9(6):1957–1960
Dou K, Gao JX, Zhang CL, Yang HT, Jiang XL, Li JS, Li YQ, Wang W, Xian HQ, Li SG, Liu Y, Hu JD, Chen J (2019) Trichoderma biodiversity in major ecological systems of China. J Microbiol 57:668–675. https://doi.org/10.1007/s12275-019-8357-7
Elegbede JA, Lateef A (2019) Optimization of the production of xylanases in corncob-based media by Aspergillus niger and Trichoderma longibrachiatum using Taguchi approach. Acta Biol Szeg 63(1):51–58. https://doi.org/10.14232/abs.2019.1.51-58
Gondal AS, Ijaz M, Riaz K, Khan AR (2012) Effect of different doses of fungicide (Mancozeb) against Alternaria leaf blight of tomato in tunnel. J Plant Pathol Microb 3:3. https://doi.org/10.4172/2157-7471.1000125
Imran M, Esmat FA, Sabry H, Kamal AMA, Nashwa MAS, Muhammad MMK, Muhammad WY (2021) Characterization and sensitivity of Botrytis cinerea to benzimidazole and succinate dehydrogenase inhibitors fungicides, and illustration of the resistance profile. Aus Plant Pathol. https://doi.org/10.1007/s13313-021-00803-2
Imran M, Abo-Elyousr KAM, Magdi M, Maged MS (2022) A study on the synergetic effect of Bacillus amyloliquefaciens and dipotassium phosphate on Alternaria solani causing early blight disease of tomato. Eur J Plant Pathol. https://doi.org/10.1007/s10658-021-02384-8
Kamala T, Devi IS (2012) Biocontrol properties of indigenous Trichoderma isolates from north-east India against Fusarium oxysporum and Rhizoctonia solani. African J Biotech 11(34):8491–8499. https://doi.org/10.5897/AJB11.1938
Koley S, Mahapatra SS, Kole PC (2015) In vitro efficacy of bio-control agents and botanicals on the growth inhibition of Alternaria solani causing early leaf blight of tomato. Int J Bio-Resour Environ Agric Sci 1:114–118
Kulimushi SM, William MM, Eunice WM (2021) Potential of Trichoderma spp. Bacillus subtilis and Pseudomonas fluorescens in the management of early blight in tomato. Bio Sci Technol 31:912–923
Kumar K, Amaresan N, Bhagat S, Madhuri K, Srivastava RC (2012) Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J Microbiol 52(2):137–144. https://doi.org/10.1007/s12088-011-0205-3
Majumder S, Parshant K, Virendra SR, Parimal S, Najam AS (2020) Amphiphilic polymer based nanoformulations of mancozeb for management of early blight in tomato. J Env Sci Health 55(5):501–507. https://doi.org/10.1080/03601234.2020.1724750
Mohamed AA, Gomaa FH (2019) Molecular characterization and biological control of some rice seed-borne fungal pathogens. J Phytopathol Pest Manag 6:40–53
Ngoc NK (2013) Management of early blight of tomato caused by Alternaria solani (Ellis and Martin) Jones and Grout (MSc thesis). University of Agricultural Sciences. Bengaluru
Nishant P, Karuna V, Gohar TK, Pramod P (2021) SA, ABA and Pseudomonas fluorescens elicit defense responses in tomato against Alternaria blight. J Plant Biochem Biotech 30(1):13–25. https://doi.org/10.1007/s13562-020-00564-x
Purwantisari S, Harum S, Isworo R, Arina TL, Kadarwati B (2021) Indigenous Trichoderma harzianum as biocontrol toward blight late disease and biomodulator in potato plant productivity. Biosaint 13(1):26–33. https://doi.org/10.15294/biosaintifika.v13i1.26706
Rahmatzai N, Ahmed AZ, Mohamed HM, Abdullah A, Zainullah H, Magdi AAM (2017) In vitro and in vivo antifungal activity of botanical oils against Alternaria solani causing early blight of tomato. Int J Biosci 10(1):91–99. https://doi.org/10.12692/ijb/10.1.91-99
Sadana D, Didwania N (2015) Bio efficacy of fungicides and plant extracts against Alternaria solani causing early blight of Tomato. J Agro Ecol 2(3):181–186
Sallam MAN, Abo-Elyousr KAM (2012) Evaluation of Various plant extracts against the early blight disease of tomato plants under greenhouse and field conditions. Plant Protect. Sci 48(2):75–80. https://doi.org/10.17221/14/2011-PPS
Sánchez-Montesinos B, Fernando D, Alejandro MG, Francisco JG, Mila S (2020) Role of Trichoderma aggressivum f. europaeum as plant-growth promoter in horticulture. Agronomy 10:1004. https://doi.org/10.3390/agronomy10071004
Shah MI, Sultan P, Nasier A (2006) In vitro study on effect of some fungicides viz., Carbendazim, Mancozeb, conjoint Carbendazim Mancozeb and sulphur against F. oxysporum. Res J Microbiol 1(4):360–365. https://doi.org/10.3923/jm.2006.360.365
Singh A, Vineeta S, Yadav SM (2014) Cultural, morphological and pathogenic variability of Alternaria solani causing early blight in tomato. Plant Pathol J 13:167–172. https://doi.org/10.3923/ppj.2014.167.172
Singh S, Arpita T, Deepamala M, Ashutosh A, Poornima V, Alok K (2019) Evaluating the potential of combined inoculation of Trichoderma harzianum and Brevibacterium halotolerans for increased growth and oil yield in Mentha arvensis under greenhouse and field conditions. Indus Crops Prod. https://doi.org/10.1016/j.indcrop.2019.01.039
Sowmya V, Ram C (2021) In vitro and in vivo efficacy of chemical fungicides against early blight of tomato (Solanum lycopersicum L.) incited by Alternaria solani (Ell. & Mart.). J Pharm Phytol 10(1):833–837
Srivastava A (2021) Studies the effect of Trichoderma harzianum for biocontrol of cucumber (Cucumis sativus L.) and suppression of Rhizoctonia solani. Plant Arch. 21(1):2059–2062. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.338
Steel RGD, Torrie JH, Dickey DA (1996) Principles and procedures of statistics: a biometric approach, 3rd edn. McGraw Hill Book Co., Inc., New York
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(2):2725–2729. https://doi.org/10.1093/molbev/mst197
Udhav BS (2013) Studies on epidemiology and integrated management of early blight of potato caused by Alternaria solani (Ellis and Mart.) Jones and Grout (MSc thesis). Rheinischen Friendrich-Welhems University of Bonn, Bonn, Germany
Vanitha LS, Jayappa J, Mallu G, Manjunath L, Chandrashekar SC (2013) Determination of medium inhibitory concentration of carbendazim against fungus Alternaria solani associated with early blight of potato. Envir Ecol 31(1A):270–272
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Yang LN, He MH, Ouyang HB, Zhu W, Pan ZC, Sui QJ, Shang LP, Zhan J (2019) Cross-resistance of the pathogenic fungus Alternaria alternata to fungicides with different modes of action. BMC Microbiol 19(1):1–10. https://doi.org/10.1186/s12866-019-1574-8
Zhang J, Chen GY, Li XZ, Hu M, Wang BY, Ruan BH, Zhou H, Zhao LX, Zhou J, Ding ZT, Yang YB (2017) Phytotoxic, antibacterial, and antioxidant activities of mycotoxins and other metabolites from Trichoderma sp. Nat Prod Res 31:2745–2752. https://doi.org/10.1080/14786419.2017.1295235
Zhang C, Imran M, Xiao L, Hu Z, Li G, Zhang F, Liu X (2021) Difenoconazole resistance shift in Botrytis cinerea from tomato in China associated with inducible expression of CYP51. Plant Dis 105(2):400–407. https://doi.org/10.1094/PDIS-03-20-0508-RE
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King AbdulAziz University, Jeddah, Saudi Arabia under grant no (DG: 085-155-1443). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King AbdulAziz University, Jeddah, Saudi Arabia under grant no (DG: 085-155-1443)
Author information
Authors and Affiliations
Contributions
MI involved in conceptualization, methodology, formal analysis, writing—original draft. KAMA-E took part in supervision, review and editing. MM involved in conceptualization, formal analysis review and editing. MMS took part in formal analysis, review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. This manuscript is in accordance with the guide for authors available on the journal’s website. Also, this work has not been published previously and is approved by all authors and host authorities.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imran, M., Abo-Elyousr, K.A.M., Mousa, M.A. et al. Screening and biocontrol evaluation of indigenous native Trichoderma spp. against early blight disease and their field assessment to alleviate natural infection. Egypt J Biol Pest Control 32, 40 (2022). https://doi.org/10.1186/s41938-022-00544-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00544-4