Abstract
Fusarium oxysporum f.sp. lycopersici (FOL), the incitant of the Fusarium wilt of tomato, is a highly damaging and prevalent disease in the majority of tomato growing areas. Keeping into consideration of high disease occurrence and incidence of FOL in tomato crop, the present investigation was undertaken to develop an effective bio-management approach to combat this disease. Initially, the studies were conducted to evaluate six multi-facial biocontrol isolates of Trichoderma species viz., Trichoderma harzianum AMUTH-1, T. harzianum AMUTH-2, T. harzianum AMUTH-3, T. asperellum (= T. viride) AMUTV-1, T. asperellum AMUTV-3 and T. virens (= Gliocladium virens) AMUTS-1 against FOL in vitro. Among these antagonists, T. harzianum AMUTH-1 and T. asperellum AMUTV-3 exhibited the maximum inhibitory effect while T. virens AMUTS-1 was recorded as the least effective Trichoderma isolate against FOL in vitro. Interestingly, T. harzianum AMUTH-1 and T. asperellum AMUTV-3 were found to produce indole acetic acid, siderophore and possess high enzymatic activities (cellulase, chitinase, ligninase and protease) in vitro. Further, pot trials were conducted and the chemical fungicide, carbendazim was used to compare the effectiveness of Trichoderma isolates. Pot trials also verified the efficacy of T. harzianum AMUTH-1 with 9–28% enhancement in the plant-growth parameters and 15–21% biomass production, and 88% decrease in the soil population of FOL. The effect of T. harzianum AMUTH-1 was also at par with fungicides, carbendazim.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tomato (Solanum lycopersicum L. syn. Lycopesicon esculentum L.) is a widely cultivated vegetable crop and the poor yield of tomato is attributed to its susceptibility to numerous phytopathogens [1]. Among the diseases caused by fungi, Fusarium wilt caused by Fusarium oxysporum f. sp. lycopersici (FOL) is a highly damaging disease under warm soil conditions and causes severe losses to the tomato crop [2, 3]. Infection of FOL results in stunting, wilting and finally, death of the plant. Sometimes, the entire fields are wilted or severely damaged before the crop is harvested [1, 4].
Management of Fusarium wilt can be done in many ways. Crop rotation with non-solanaceous crops (non-host) for four to six years is usually recommended to reduce the inoculum level of FOL in the field [5]. The crop should be rotated with cereals crops wherever possible [6]. Physical methods are quite effective but important hindrance that limits their use is low efficiency and high labour intensive. Synthetic chemicals are commonly used for the management of FOL in tomatoes. Treatment with chemical fungicides especially prochloraz, carbendazim, propiconazole, thiabendazole, benomyl, thiophanate, fuberidazole and benzimidazoles considerably reduce the wilt incidence in tomatoes [7, 8]. Khan and Khan [9] reported that the root-dip treatment of carbendazim on tomato seedlings infected with FOL led to a 24% increase in yield. However, the use of fungicides is a bit expensive as well as environmentally undesirable due to damaging effects on the agroecosystem, human beings and animals [10]. Under this situation, all hopes are set on the exploration of biological management of plant diseases.
Biological control is one of the best and safest methods of disease management and is devoid of residual toxic effects. This method is based on the idea that pathogen’s populations can be decreased by influencing naturally occurring living microbial species, altering the environment, or introducing antagonists. Several antagonists of FOL are known and their utilization resulted in a substantial decrease in the disease severity and correspondingly increase in the crop yield. Trichoderma spp. are well recognized, potential and frequently used biocontrol agents [11,12,13]. Till date, 488 species have been identified and most biocontrol agents are from Hypocrea rufa, Trichoderma asperellum (= T. viride), T. harzianum, T. koeningii, and T. hamatum [14]. Application of these Trichoderma spp. have successfully controlled F. oxysporum f. sp. lycopersici in tomato crop [15,16,17,18].
Generally, Trichoderma isolates/strains are effective antagonists to a particular group of plant pathogens such as true fungi [19], stramenopiles [13] or nematodes [11]. However, their multi-facial potential against different plant pathogens has not been fully explored and it shall be a new and interesting approach to develop effective bio-management strategies to combat many soil-borne pathogens with a single treatment. With these objectives, the present investigations were undertaken to evaluate six multifacial Trichoderma isolates (Trichoderma harzianum AMUTH-1, T. harzianum AMUTH-2, T. harzianum AMUTH-3, T. asperellum AMUTV-1, T. asperellum AMUTV-3 and T. virens AMUTS-1) against Fusarium oxysporum f. sp. lycopersici in vitro and pot conditions. An attempt was also made to comprehend the mechanism of FOL suppression by these Trichoderma isolates.
2 Materials and methods
2.1 Inoculum and mass production of wilt pathogen, Fusarium oxysporum f.sp. lycopersici
A pure culture of Fusarium oxysporum f. sp. lycopersici (FOL) ITCC-1322 was received from the Indian Type Culture Collection, Division of Plant Pathology, Indian Agricultural Research Institute, New Delhi, India. The pure culture was kept in culture tubes on PDA (potato dextrose agar, Himedia™, India) at 5 °C for the study. On sorghum seeds, the pathogen was mass-cultured. For 12 h, the seeds were steeped in a solution of sucrose (5%) and chloramphenicol (0.03%), and transferred in conical flasks of 500 mL capacity. The seeds were autoclaved at 121 °C for 15 kg/cm2 pressure for 15–20 min. The conical flasks were subsequently inoculated with FOL culture and kept in a BOD incubator for ten to fifteen days at 27 °C. To encourage uniform colonisation on seeds, the flasks were manually shaken every day for a short period during incubation. To create the inoculum, double distilled water (DDW) was added with a known weight of FOL-colonized seeds [13]. The mixture was then pulverised in an electric mixer. The DDW was added to the ground sterile mixture, and load of the colony-forming units (CFU) load was estimated and standardized to 3.0 × 106 CFU per gram. In the current investigation, a 2 g inoculum was used per pot.
2.2 Mass culture of Trichoderma isolates
Six indigenous Trichoderma isolates, viz., Trichoderma harzianum AMUTH-1 (NCBI GenBank Accessions no. KM435269), T. harzianum AMUTH-2 (NAIMCC, ICAR-NBAIM, India Accessions no. NAIMCC-F-04335), T. harzianum AMUTH-3 (NCBI accessions no. KY062569), T. asperellum (= T. viride) AMUTV-1 (NAIMCC accessions no. NAIMCC-F-04337), T. asperellum (= T. viride) AMUTV-3 (NCBI accessions no. KY062571) and T. virens (= Gliocladium virens) AMUTS-1 (NAIMCC accessions no. NAIMCC-F-04336) were previously identified and chosen for this study because of their multifacial nature and biocontrol capability [13, 20, 21]. In conical flasks (250 mL), the mass cultures of the aforementioned Trichoderma isolates were prepared using potato dextrose broth (PDB, Himedia™, India). To create mycelial suspension, the mycelial-mats were removed from the conical flasks and processed individually in an electric grinder with 1 L DDW. The hyphae were then removed from the suspension by filtering it through a 0.15 mm mesh sieve [22]. Under a microscope, spores were counted using a hemocytometer, and the spore suspension was standardized to 2.0–3.0 × 106 spores mL−1 using DDW.
2.3 Fungicides
To compare the efficacy of Trichoderma isolates, carbendazim (Rickstin™, 50 WP, Darrick Insecticides Ltd., India) was added to the soil, already inoculated with F. oxysporum f. sp. lycopersici, at 1.5 mg a.i./pot one day before the seedlings (four weeks old) were planted. The accepted dose of 8 kg active ingredient /ha was used to compute the fungicide dose [7].
2.4 In vitro antagonism of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici
The antagonistic potential of six Trichoderma isolates against FOL was estimated by dual culture technique using a PDA medium [23]. In the Petri plate, a mycelial disc (9 mm diameter) of FOL and a Trichoderma isolate were positioned 2.5 cm apart on the solidified PDA and incubated for seven days at 27 °C. Trichoderma isolates were not inoculated in the control plate. The treatments were replicated three times and the whole experiment was repeated for data accuracy. After seven days of incubation, the pathogen's development towards the Trichoderma colony and the inhibitory zone was measured. The pathogen’s radial growth was computed, and the percent mycelial inhibition (PI) was estimated as below
where C is the growth of test the pathogen (mm) in the control. T is the radial growth of FOL in Trichoderma spp. treatment
2.5 In vitro estimation of phosphate solubilization and ammonia, hydrogen cyanide, indole acetic acid, siderophore production and oxalic acid detoxification
All six Trichoderma isolates were tested in culture broth for their ability to solubilize phosphates [24], produce ammonia [25], siderophore [26], indole acetic acid (IAA) [27], hydrogen cyanide (HCN) [28] and detoxify oxalic acid (OA) [29]. The Lowry et al. [30] method was used to measure the activity of the enzymes (cellulase, chitinase, ligninase and protease) in the supernatant of culture medium.
2.6 Plant treatments and cultures
Nursery of tomato cultivar Pusa Ruby was raised independently in 96 well nursery tray with autoclaved cocoa-peat. Earthen pots (15 cm in diameter) filled with 1 kg autoclaved composted soil (farm yard manure and soil in 3:1) were used for the pot trials. The following nine treatments were maintained separately in an open surface receiving uniform sunlight. T1 = Plant + Un-inoculated soil (Control), T2 = Plant + Inoculated soil (Inoculated control), T3 = Plant + Inoculated soil + Trichoderma harzianum AMUTH-1, T4 = Plant + Inoculated soil + T. harzianum AMUTH-2, T5 = Plant + Inoculated soil + T. harzianum AMUTH-3, T6 = Plant + Inoculated soil + T. asperellum AMUTV-1, T7 = Plant + Inoculated soil + T. asperellum AMUTV-3, T8 = Plant + Inoculated soil + T. virens AMUTS-1 and T9 = Plant + Inoculated soil + carbendazim.
One day before transplanting of tomato seedlings, FOL (2 g at 3.0 × 106 CFU g−1) and Trichoderma isolates (2 mL at 2.0–3.0 × 106 spores mL−1) homogenized with 10 mL water separately were mixed to the top-soil of designated pots. On the following day, 3–4 leaf stage (4 weeks old) tomato seedlings cv. Pusa Ruby were planted in the pots (one seedling/pot). Five replicates of each treatment were maintained, and both inoculated and uninoculated controls were kept. The arrangement of the pots was totally randomized. Water (200 mL/pot) was gently given just after planting without overflow. Irrigation was done at one-day breaks and lasted till harvesting. The plants were routinely examined for any obvious disease symptoms. At harvest, plant-length (cm), fresh and dry weight (g), soil population of wilt fungus and Trichoderma isolates were calculated. The disease severity (wilt) was calculated at harvest on a 0–5 scale as determined by Lebeda and Buczkowski [31] with slight modification i.e., 0 = symptomless, no wilt visible; 1 = slight wilting with 5% wilted leaves; 2 = limited wilting with 6–10% wilted leaves; 3 = moderate wilting, 11–20% wilted leaves; 4 = severe wilting, 21–50% wilted leaves; 5 = severe witling and plants dead.
2.7 Soil population of Fusarium oxysporum f.sp. lycopersici and Trichoderma isolates
At harvest, the final soil population of Trichoderma isolates and FOL were estimated. One gram soil sample was taken from the region around the root zone of each infected pot. The sample was processed using serial dilution methods after being diluted to 10–5 with DDW. With a sterile pipette, the final dilution of soil suspension (0.5 mL) was put on solidified potato dextrose agar in petri plates. After that, these petri plates were placed in a BOD and maintained at 27 ± 2 °C for 24 h. A colony counter was used to measure the CFU load after 24-h of incubation period.
2.8 Statistical analysis
Both in vitro and pot experiments were repeated and carried out during 2020–21. The data were pooled because the differences in the data gathered throughout the two repeated studies were not significant at P ≤ 0.05 and were processed to analysis of variance (ANOVA) using MINITAB software for Windows-10. The least significant differences (LSD), degree of freedom, and F values were determined at three levels, P ≤ 0.05, 0.01, and 0.001. The in vitro antagonism and wilt index were examined through single-factor ANOVA.
3 Results
3.1 In vitro studies
3.1.1 Antagonism of Trichoderma spp. against Fusarium oxysporum f.sp. lycopersici
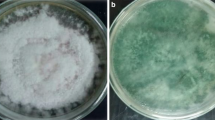
The antagonistic effects of Trichoderma spp. were evaluated by dual culture method. Among the six Trichoderma isolates tested, T. harzianum AMUTH-1 caused maximum inhibition (76.27%) of the mycelial growth of Fusarium oxysporum f.sp. lycopersici (FOL) over inoculated control (Fig. 1). Next in effectiveness were T. asperellum AMUTV-3 (71.34%), T. harzianum AMUTH-3 (68.40%) and T. harzianum AMUTH-2 (53.70%). The isolates, T. asperellum AMUTV-1 (49.45%) and T. virens AMUTS-1 (36.32%) showed lowest mycelial inhibition of FOL (Fig. 1). Overall the order of inhibition was, T. harzianum AMUTH-1 > T. asperellum AMUTV-3 > T. harzianum AMUTH-3 > T. harzianum AMUTH-2 > T. asperellum AMUTV-1 > T. virens AMUTS-1 (Additional file 1: Figure S1).
3.1.2 Solubilization of phosphate and production of HCN, IAA, ammonia, siderophore, detoxification of OA and enzyme activities
All Trichoderma isolates tested for ammonia, hydrogen cyanide, phosphorus and detoxification of oxalic acid exhibited negative responses and did not produce all these compounds in the medium. However, the isolates positively produced indole acetic acid (IAA) and siderophores in the culture medium with varied responses (Table 1). The greatest IAA production was recorded with T. harzianum AMUTH-3 (17.91 µg mL−1) followed by T. harzianum AMUTH-1 (17.4 µg mL−1), and T. asperellum AMUTV-1 (16.02 µg mL−1; Table 1). The lowest IAA was recorded with T. harzianum AMUTH-2 (14.27 µg mL−1) and T. virens AMUTS-1 (14.23 µg mL−1). The isolates T. harzianum AMUTH-3 and T. asperellum AMUTV-3 were recorded as highly positive for siderophore and scored++ (Table 1). The isolate T. harzianum AMUTH-1 was also capable of producing siderophores and scored+. While T. virens AMUTS-1 and T. asperellum AMUTV-1 showed a negative result for siderophores (Table 1).
All isolates of Trichoderma exhibited significant enzymatic activities and synthesis cellulase, chitinase, ligninase and protease (Table 1). Highest cellulase (8.99 µg−1 protein), chitinase (7.12 µg−1 protein), ligninase (6.51 µg−1 protein) and protease (6.02 µg−1 protein) activity was recorded with T. harzianum AMUTH-1. Other Trichoderma isolates also exhibited significant enzymatic activity but relatively lower production of the above enzymes (Table 1).
3.2 Pot experiments
3.2.1 Disease severity and symptoms of Fusarium oxysporum f.sp. lycopersici
Tomato plants inoculated with 2 g FOL displayed stunted growth and mild yellowing in inoculated control pots. The chlorosis progressively became noticeable with plant age and at two months after inoculation, the complete plant became wilted with 4.33 wilt severity at 0–5 scale (Fig. 2). However, Trichoderma isolates and carbendazim as soil application significantly reduced the wilt severity caused by FOL (Fig. 2). The maximum reduction in wilt severity was reported with the treatment of Trichoderma harzianum AMUTH-1 (57%) and T. asperellum AMUTV-3 (45%) over control (Fig. 2). Next in efficacy, was carbendazim and it suppressed the wilt severity by 42% over inoculated control. Treatment with T. virens AMUTS-1 was recorded as least effective in reducing the wilt severity (17%) over inoculated control (Fig. 2).
3.3 Plant growth and biomass
The F. oxysporum f. sp. lycopersici infection in the tomato plants significantly reduced the plant length (16–20%), fresh weight (9–22%) and dry weight (16–24%) over un-inoculated control (Table 2). In contrast to the untreated inoculation control, treatment of Trichoderma isolates (except T. asperellum AMUTV-1 and T. virens AMUTS-1) and carbendazim substantially increased all growth parameters viz., plant length (16–21%), fresh weight (8–26%), and shoot and root dry weight (6–28%; Table 2). The fungus-inoculated tomato plants applied with T. harzianum AMUTH-1 significantly increased plant growth (15–21%) and biomass (9–28%) compared to the untreated inoculated control (Table 2). The plant growth and biomass metrics also improved by 11–20% and 9–18%, respectively, when T. asperellum AMUTV-3 and carbendazim were applied over the inoculated control (Table 2). In contrast to the inoculated control, T. virens AMUTS-1 treatment exhibited lowest increase in plant growth (P ≤ 0.05; Table 2).
3.4 Soil population of Fusarium oxysporum f.sp. lycopersici
The soil population of FOL was amplified over time in untreated pots and it was significantly higher at time of harvesting in comparison to their initial population (Fig. 3). The population was increased up to 380% at harvest in untreated pot over the initial population (Fig. 3). However, application of Trichoderma isolates and carbendazim drastically reduced the soil population of wilt fungus in all treated pots (Fig. 3; P ≤ 0.05). The highest decrease in soil population was recorded with T. harzianum AMUTH-1 (88%), followed by carbendazim (72%), T. asperellum AMUTV-3 (69%) and T. harzianum AMUTH-3 (64%). The isolate T. harzianum AMUTH-2 also significantly declined the soil population of wilt fungus over inoculated control (Fig. 3).
3.5 Soil population of Trichoderma isolates
The soil population of Trichoderma isolates were amplified over time, and they were significantly higher in the Fusarium oxysporum f.sp. lycopersici inoculated pots than their initial population (Fig. 4). Among the six Trichoderma isolates, the highest population increase at the time harvest was recorded with Trichoderma harzianum AMUTH-1 (560–730%) and T. asperellum AMUTV-3 (450–530%) over the initial population at the time of harvesting (Fig. 4). The rhizosphere population of T. harzianum AMUTH-3 and T. harzianum AMUTH-2 also increased in the presence of the wilt pathogen in inoculated pots (Fig. 4; P ≤ 0.05).
4 Discussion
Fusarium-wilt incited by Fusarium oxysporum f.sp. lycopersici (FOL) is highly prevalent and damaging disease of tomatoes [32, 33]. Keeping in view of the high disease occurrence and prevalence of Fusarium wilt, the present study was undertaken to develop an effective biomanagement module, and a series of in vitro and pot trials were conducted. In vitro, six Trichoderma isolates were evaluated against FOL and the isolates, T. harzianum AMUTH-1 and T. asperellum AMUTV-3 exhibited the highest inhibitory effect against FOL while T. virens AMUTS-1 was recorded as the least effective biocontrol. In the current study, the isolate T. harzianum AMUTH-1 showed relatively higher inhibition of FOL than T. asperellum and T. virens. Trichoderma spp. exhibit varied virulence responses to wilt-fungus, that is characterised by the isolate's antagonism and virulence potential, which may be further expressed through different mechanisms such as rapid colonization/competence [34] and greater toxin production [35]. Greater antagonism by T. harzianum than T. asperellum and T. virens against wilt fungus has also been reported in other researches [15, 18]. Singh et al. [36] also recorded the higher production of antifungal metabolites by T. harzianum than T. asperellum.
In pot experiments, FOL-inoculated tomato plants caused 9–24% decline in biomass production of tomato. However, soil treatments with Trichoderma isolates reduced the harmful effects of pathogenic fungus and thus enhanced tomato plant growth and yield. The isolate T. harzianum AMUTH-1 was shown to be the most virulent isolate in antagonizing FOL. The efficacy of this isolate was also better than T. asperellum AMUTV-3 as well as fungicide carbendazim. Trichoderma spp. are the best-known biocontrol agent of a wide range of pathogenic fungi and have proven their potential to suppress the diseases in various crops including tomato [15, 16]. This genus contains numerous species that can be recognized as opportunistic endophytes and avirulent symbionts [11, 14]. Other studies also revealed the antagonistic effect of Trichoderma spp. especially T. asperellum, T. harzianum, T. virens, T. hamatum, etc. and successfully controlled F. oxysporum f. sp. lycopersici on tomato [15, 17, 18].
In this study, an attempt was also made to understand the mechanism of FOL suppression by these Trichoderma isolates. All Trichoderma positively produced IAA and siderophores (except T. asperellum AMUTV-1 and T. virens AMUTS-1) in the culture medium. The greatest IAA and siderophore production was recorded with Trichoderma harzianum AMUTH-3, T. harzianum AMUTH-1 and T. asperellum AMUTV-3, respectively which also exhibited significant mycelial inhibition and disease suppression. However, the lowest IAA and negative siderophores production was recorded with T. asperellum AMUTV-1 and T. virens AMUTS-1, which were least effective Trichoderma isolates in term of mycelial inhibition and wilt disease suppression, respectively. Trichoderma affects phyto-pathogens via various mechanisms, such as enzymatic hydrolysis, direct-parasitism, nutrient competition, antibiosis and induced resistance [11, 37] as recorded in the present study.
In our study, all Trichoderma isolates showed significant enzymatic activities and synthesis chitinase, ligninase, protease and cellulase with the overall highest activity recorded with T. asperellum AMUTV-3. The hydrolytic enzymes like chitinases, xylanases, cellulases, glucanases, and proteases which break down the fungal cell wall, are produced by Trichoderma species in ample amounts [37]. Among these, chitinases, which are released as secondary metabolites, are thought to be of utmost significance against plant pathogens [38]. T. asperellum AMUTV-3 and T. harzianum AMUTH-1 successfully parasitized FOL possibly due to the action of chitinases, glucanases, and proteases. The fungal cell wall is degraded by these chitinolytic enzymes, as their high activity was observed in our study.
The biocontrol applied in the soil to suppress wilt fungus first needs to multiply in the rhizosphere and then colonize the root to become systemic. Most of the Trichoderma species generally grow in their natural habitat and colonize root surfaces or become endophytes [39]. In the present study, the Trichoderma isolates, particularly T. harzianum AMUTH-1, T. asperellum AMUTV-3 and T. harzianum AMUTH-3 multiplied well in the rhizpsphere as showed by their increased population at harvest.
The study has demonstrated that Fusarium oxysporum f.sp. lycopersici is a devastating pathogen of tomatoes and inflicted growth and biomass production by 9–24%. Application Trichoderma harzianum AMUTH-1 drastically reduced the wilt severity and improved the plant-growth parameters by 9–28% and tomato biomass by 15–21%. The effect of this multifacial isolate was also at par with fungicides, carbendazim. Hence, T. harzianum AMUTH-1 may provide an alternative control of tomato diseases in the scenario of multipathogenic attack. This finding could also be used to device suitable integrated management practices to safeguard tomato from the FOL. However, field experiments are required to verify the effectiveness of the above Trichoderma isolates before recommending the treatment to the farmers/growers.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hassan HA. Biology and integrated control of tomato wilt caused by Fusarium oxysporum Lycopersici: a comprehensive review under the light of recent advancements. J Bot Res. 2020;3(1):84–99.
Sudhamoy M, Nitupama M, Adinpunya M. Salicylic acid-induced resistance to Fusarium oxysporum f. sp. lycopersici in tomato. Plant Physiol Biochem. 2009;4:161–89.
Chaudhary N, Singh C, Pathak P, Rathi A, Vyas D. Evaluation of the impact of pathogenic fungi on the growth of Pisum sativum L. a review article. Int J Agric Technol. 2021;17(2):443–64.
Kirankumar R, Jagadeesh KS, Krishnraj PU, Patil MS. Enhanced growth promotion of tomato and nutrient uptake by plant growth promoting rhizobacterial isolates in presence of tobacco mosaic virus pathogen. J Agric Sci. 2008;21(2):309–11.
Maurya S, Dubey S, Kumari R, Verma R. Management tactics for fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici (Sacc.): a review. Management. 2019;4(5):1–7.
Sally AM, Randal CR, Richard MR. Fusarium verticillium wilts tomato, potato, pepper, and eggplant. Ohio State University Press; 2006.
Amini J, Sidovich D. The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J Plant Protect Res. 2010. https://doi.org/10.2478/v10045-010-0029-x.
Singh AK, Kamal S. Chemical control of wilt in tomato (Lycopersicon esculentum L.). Int J Horticult. 2012;2(2):5–6.
Khan MR, Khan SM. Effects of root-dip treatment with certain phosphate solubilizing microorganisms on the fusarium wilt of tomato. Biores Technol. 2002;85(2):213–5.
Haque Z, Khan MR. Optimization of different application methods of multi-facial bacterial and fungal antagonists against sheath blight pathogen of rice, Rhizoctonia solani AG1-IA. J Phytopathol. 2023;171(1):23–35. https://doi.org/10.1111/jph.13151.
Khan MR, Mohiddin FA. Trichoderma: Its multifarious utility in crop improvement. In: Prasad R, Gill SS, Tuteja N, editors. New and future developments in microbial biotechnology and bioengineering: Crop improvement through microbial biotechnology. Elsevier Publications; 2018. p. 263–91.
Mukhopadhyay R, Kumar D. Trichoderma: a beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt J Biol Pest Control. 2020;30(1):133. https://doi.org/10.1186/s41938-020-00333-x.
Haque Z, Khan MR. Host resistance and bio-management of tobacco root-rot caused by Pythium aphanidermatum. Indian Phytopathol. 2022;75(3):703–12. https://doi.org/10.1007/s42360-022-00491-y.
Moo-Koh FA, Cristóbal-Alejo J, Andrés MF, Martín J, Reyes F, Tun-Suárez JM, Gamboa-Angulo M. In vitro assessment of organic and residual fractions of nematicidal culture filtrates from thirteen tropical Trichoderma strains and metabolic profiles of most-active. J Fungi. 2022. https://doi.org/10.3390/jof8010082.
Akrami M, Yousefi Z. Biological control of Fusarium wilt of tomato (Solanum lycopersicum) by Trichoderma spp. as antagonist fungi. Biol Forum. 2015;7:887.
Christopher DJ, Raj TS, Rani SU, Udhayakumar R. Role of defense enzymes activity in tomato as induced by Trichoderma virens against Fusarium wilt caused by Fusarium oxysporum f sp. lycopersici. J Biopest. 2010;3(1):158.
Ramezani H. Antagonistic effects of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici causal agent of tomato wilt. J Novel Res Plant Protect. 2010;2(2):173–167.
Sundaramoorthy S, Balabaskar P. Biocontrol efficacy of Trichoderma spp. against wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici. J Appl Biol Biotechnol. 2013;1(3):1–4.
Ghazanfar MU, Raza M, Raza W, Qamar MI. Trichoderma as potential biocontrol agent, its exploitation in agriculture: a review. Plant Protect. 2018;2(3):109–35.
Haque Z, Khan MR, Ahamad F. Relative antagonistic potential of some rhizosphere biocontrol agent for the management of rice root-knot nematode, Meloidogyne graminicola. Biol Control. 2018;126:109–16. https://doi.org/10.1016/j.biocontrol.2018.07.018.
Haque Z, Khan MR. Identification of multifacial microbial isolates from the rice rhizosphere and their biocontrol activity against Rhizoctonia solani AG1-IA. Biol Control. 2021;161: 104640. https://doi.org/10.1016/j.biocontrol.2021.104640.
Ben-David A, Davidson CE. Estimation method for serial dilution experiments. J Microbiol Methods. 2014;107:214–21. https://doi.org/10.1016/j.mimet.2014.08.023.
Zivkovic S, Stojanovic S, Ivanovic Z, Gavrilovic V, Oro V, Balaz J. Screening of antagonistic activity of microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Arch Biol Sci Belgrade. 2010;62(3):611–23.
Jackson ML. Soil chemical analysis. Prentice Hall of India Pvt Ltd; 1973.
Dye DW. The inadequacy of the usual determinative tests for identification of Xanthomonas spp. NZT Sci. 1962;5:69–71.
Santoyo G, Orozco-Mosqueda MD, Govindappa M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci Tech. 2012;22(8):855–72.
Bric JM, Bostock RM, Silverstone SE. Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol. 1991;57(2):535–8. https://doi.org/10.1128/aem.57.2.535-538.1991.
Bakker AW, Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biol Biochem. 1987;19(4):451–7. https://doi.org/10.1016/0038-0717(87)90037-X.
Nagarajkumar M, Jayaraj J, Muthukrishnan S, Bhaskaran R, Velazhahan R. Detoxification of oxalic acid by Pseudomonas fluorescens strain PfMDU2: implications for the biological control of rice sheath blight caused by Rhizoctonia solani. Microbiol Res. 2005;160(3):291–8. https://doi.org/10.1016/j.micres.2005.02.002.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. https://doi.org/10.1016/S0021-9258(19)52451-6.
Lebeda A, Buczkowski J. Fusarium oxysporum, Fusarium solani—Tubetest. In: Lebeda A, editor. Methods of testing vegetable crops for resistance to plant pathogens. VHJ Sempra, VŠÚZ Olomouc; 1986. p. 247–9.
Sheu ZM, Wang TC. First report of Race 2 of Fusarium oxysporum f. sp. lycopersici, the causal agent of fusarium wilt on tomato in Taiwan. Plant Dis. 2006;90(1):111. https://doi.org/10.1094/PD-90-0111C.
Abdel-Monaim MF. Induced systemic resistance in tomato plants against Fusarium wilt disease. Int Res J Microbiol. 2012;3(1):14–23.
Mohiddin FA, Khan MR, Khan SM, Bhat BH. Why Trichoderma is considered super hero (super fungus) against the evil parasites? Plant Pathol J. 2010;9(3):92–102. https://doi.org/10.3923/ppj.2010.92.102.
Harman GE. Trichoderma—not just for biocontrol anymore. Phytoparasitica. 2011;39(2):103–8. https://doi.org/10.1007/s12600-011-0151-y.
Singh R, Maurya S, Upadhyay RS. Antifungal potential of Trichoderma species against Macrophomina phaseolina. Agric Technol. 2012;8:1925–33.
Bae H, Roberts DP, Lim HS, Strem MD, Park SC, Ryu CM, Melnick RL, Bailey BA. Endophytic Trichoderma isolates from tropical environments delay disease and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms. Mol Plant Microbe Interact. 2011;24(3):336–51. https://doi.org/10.1094/MPMI-09-10-0221.
Khan RAA, Najeeb S, Hussain S, Xie B, Li Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms. 2020;8(6):817. https://doi.org/10.3390/microorganisms8060817.
Ruano-Rosa D, Prieto P, Rincón AM, Gómez-Rodríguez MV, Valderrama R, Barroso JB, Mercado-Blanco J. Fate of Trichoderma harzianum in the olive rhizosphere: Time course of the root colonization process and interaction with the fungal pathogen Verticillium dahliae. Biocontrol. 2016;61(3):269–82. https://doi.org/10.1007/s10526-015-9706-z.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZH conceived the idea, prepared the original draft, developed the research facility, supervised the execution and data recording, reviewed the manuscript. KP and SZ executed the experiments, performed the word processing, data analysis and prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Statement on guidelines
The study followed the institutional guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Additional file 1: Figure S1.
Antagonistic effects of Trichoderma isolates on mycelial growth of Fusarium oxysporum f.sp. lycopersici on potato dextrose agar medium in vitro.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haque, Z., Pandey, K. & Zamir, S. Bio-management of Fusarium wilt of tomato (Fusarium oxysporum f.sp. lycopersici) with multifacial Trichoderma species. Discov Agric 1, 7 (2023). https://doi.org/10.1007/s44279-023-00007-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-023-00007-w