Abstract
Background
Energy drinks prevent fatigue and increase physical and cognitive performance; however, they also inflict toxic effects on the body. Blueberry (BB) possesses antioxidant and anti-inflammatory properties. The present study aimed to assess the possible the therapeutic effects of BB on testicular damage in adult male Wistar albino rats induced by administering the energy drink Code Red.
Results
Thirty adult rats were used in the study, divided into five groups; Group 1 (Control), where rats were fed on distilled water and basal rodent diet only. The other four groups received different energy drink Code Red doses for 8 weeks and BB supplementation for another 6 weeks. Administration of low and high doses of Code Red induced a decline in serum levels of testosterone and antioxidant enzymes like superoxide dismutase (SOD), and glutathione (GSH), while malondialdehyde (MDA) was significantly increased relative to controls. A low dose of Code Red led to sporadic and scattered appearance of seminiferous tubules with loss of spermatogenic germ cells and marked degeneration of interstitial cells. A high dose of Cod Red exhibited increased degenerative changes in the tubules with highly congested thick vessels in the interstitial tissue. Also, testis from rats consuming either low or high doses of Code Red showed increased caspase-3 immunostaining in seminiferous tubules with early degeneration features. However, the deleterious effect of the administration of Code Red was remarkably ameliorated with the supplementation of BB. A reversal in the mutilative effect of Code Red was observed where with BB supplementation, the histopathology of the testis displayed recovery of most of the seminiferous tubules to normal structure. BB administration in both groups also showed negative or mild immunostaining for caspase-3.
Conclusions
Oral exposure of rats to Code Red produced noticeable testicular damage, especially in high doses, probably due to increased oxidative stress and inflammation. Blueberry administration exhibited therapeutic effects through its anti-inflammatory and antioxidative properties.
Similar content being viewed by others

Background
Energy drinks (EDs) are non-alcoholic drinks composed of high concentrations of caffeine, sugar, amino acids, vitamins, and plant or herbal derivatives, ultimately enhancing the consumer's energy levels (Nadeem et al., 2021). The first beverage of this kind was produced in Austria in 1987, and 10 years later, comparable drinks with high amounts of caffeine and taurine were introduced in the United States of America (Malinauskas et al., 2007). Energy drink consumption is primarily prevalent among young people and adolescents (Heckman et al., 2010). Al-Hazzaa et al. (2011), in a study, showed that 16.3% of male teenagers (14–19 years) in the three major Saudi cities, viz. Riyadh, Jeddah, and Al Khobar consumed energy drinks more than three times a week (Al‑Hazzaa et al., 2011). Another study from Saudi Arabia established that 40% of consumers consumed more than three cans per week, with more than half of consumers being youngsters (13–35 years) (Elsoadaa et al., 2016). Local energy drink manufacturers in Saudi Arabia include Boom, Bugzi, and Code Red. However, Code Red was the most preferred energy drink among the participating youngsters (Alrasheedi, 2016).
Although energy drinks proclaim to boost physical and mental performance, they have questionable and transient benefits. Caffeine (1,3,7-trimethylxanthine) is a major ingredient in energy drinks that stimulates cardiovascular and neurological systems (Burke, 2008). In addition to poisoning by caffeine, energy drink consumption can cause seizures, strokes, and gonadotoxic effects (Dias et al., 2015; Subaiea et al., 2019; Worrall et al., 2005). ED consumption can also lead to epithelial hydrops, intertubular bleeding, and inflammatory alterations in the renal tubules (Jahedi et al., 2014). Rats administered with ED exhibited severe necrotic alterations, vacuolization, and nuclear karyolysis, in addition to clogged blood arteries and perivascular infiltration in the pancreatic cells. In another study, ED-treated rats showed extensive glandular atrophy, gastric ulcers, and vascular congestion, accompanied by injury to the physiological barrier separating the gastric lumen and underlying mucosa (Khayyat et al., 2014). A significant rise in the incidence of male infertility has been observed worldwide (Okonofua et al., 2022), and thus necessitates the need to study this issue. Earlier reports demonstrate that ingested caffeine can cross the blood-testis barrier, yet our knowledge about the inimical effect of ED on the reproductive system is limited. Data from in vitro experiments demonstrate that the effect of caffeine on human sperm motility, number, and shape varied with dosage. Males who consumed one or two cups of coffee each day had more active and dense sperms compared to those who did not. However, sperm motility and density decreased in males whose consumption was more than two cups of coffee daily (Dlugosz & Bracken, 1992).
Blueberries (BB) plants belong to the genus Vaccinium (Vaccinium corymbosum) and Ericaceae family (Nardi et al., 2016). Proanthocyanidins, flavonols, anthocyanins, and phenolic acids like ferulic, p-Coumaric, chlorogenic, and caffeic acid are the antioxidant that are found to be abundant in blueberries (Yuan et al., 2016). Due to the presence of several antioxidant molecules, particularly anthocyanins, blueberries possess several therapeutic health benefits (Zhou et al., 2020). Zepeda et al. reported that the administration of blueberries resulted in lowered lipid peroxidation, reduced apoptosis, and elevated glutathione reductase (GR) and superoxide dismutase (SOD) levels in the testis of the rats under hypobaric hypoxia conditions (Zepeda et al., 2012). The widespread high consumption level of energy drinks by people necessitates a detailed investigation regarding the harmful impact on health and their potential side effects. Here, in this study, we aimed to assess the possible therapeutic and ameliorating effects of blueberry administration on the Code Red energy drink-induced toxic and harmful effects on the testis of Wistar albino rats.
Methods
Preparation of blueberry extract
Blueberry powder was purchased from Xi’an Pincredit Bio-tech Co., Ltd, Xian, China. The blueberry extract was prepared using the reported protocol (Zheng et al., 2013), which was used to administer the experimental Wistar rats. Briefly, the blueberry powder was frozen at 5–10 °C for 4 h and then sliced and loaded onto a plate. Then it was subsequently frozen at − 30 °C for 4 h and freeze-dried at 65–85 °C for 20 h. The foreign body was sorted from the material, followed by grinding at 20 °C, with a relative humidity of 35%. The resultant dry extract was stored at 4 °C until further use.
Code Red energy drink
The energy drink, brand name “Code Red” was used in this study, and it was obtained from a local store in Jeddah, Saudi Arabia.
Experimental animals
Thirty adult male Wistar albino rats aged 90–120 days and weighing 200–250 g were used in this study. The rats were maintained in the animal house facility of the King Fahd Medical Research Center, King Abdulaziz University (KAU), Jeddah, Saudi Arabia. The rats were maintained in the facility following the recommendations laid by the International Laboratory Animal Use and Care throughout the experiment [National Research Council (US), 2011]. King Abdulaziz University Research Ethics Committee approved the study protocols and ethics. All the experiments were performed at the King Fahd Medical Research Center, KAU, Jeddah, Saudi Arabia. The animals were kept in a standard cage (6 rats per cage) at an ambient temperature of 21 ± 1 °C with a 12 h light–dark cycle. The animals were provided unrestricted access to water ad labium and a chow diet.
Study design
The rats were housed under standard laboratory conditions. They were fed a normal commercial chow diet and water ad labium for 1 week for acclimatization before the start of the experiment. Any rats that showed abnormal behaviors were excluded from the study. Later, the rats were randomly divided into 5 groups (6 rats per group), and each group was housed in separate cages. Group 1 (Control) rats were only fed with distilled water and a basal diet. Group 2 (Low dose Code Red) received a small dose of Code Red (0.72 ml/100 g/day) for 8 weeks. Group 3 (High dose Code Red) received a high dose of Code Red (1.44 ml/100 g/day) for 8 weeks (Backer & Baeissa, 2014). Group 4 (Low dose Code Red + Blueberry) received a low dose of Code Red for 8 weeks, followed by blueberry (250 mg/kg/day) extracts for 6 weeks. Group 5 (High dose Code Red + Blueberry) received a high dose of Code Red for 8 weeks, followed by blueberry (250 mg/kg/day) extracts for 6 weeks (Larrosa et al., 2010). All treatments were given orally via gastric gavage. The low dose of energy drink (0.72 ml/100 g/day) was determined considering that an adult intakes two cans of energy drink, or 500 ml, each day with the assumption that the average weight of an adult is 70 kg. A high dose (1.44 ml/100 g/day) for the treatment was determined based on the consideration that the maximum quantity of energy drink allowed for intake by an adult is four cans, or 1000 ml each day, with an average weight of 70 kg (Backer & Baeissa, 2014). The total body weights of rats were recorded weekly, while the testis weight was determined at the end of the experiment.
Sample collection
At the end of experiment, experimental rats were subjected to overnight fasting (12 h), and their blood samples were obtained from the retro-orbital veins into plain dry tubes. The samples were stored at room temperature for 15 min and then centrifuged for 10 min at 3000×g to separate serum. The serum was aspirated and transferred into dry tubes using a Pasteur pipette and then frozen at − 20 °C until further analysis. Testosterone hormone and oxidative stress markers such as superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) were estimated in the central laboratory using commercially available kits according to the manufacturer's protocol.
Histology examination
After the withdrawal of blood samples, rats of all groups were euthanized by cervical dislocation under deep ether anesthesia. The abdomen and pelvis were opened, then both testes were excised and washed with saline. The testes were weighed, opened, fixed immediately in 10% formalin, and prepared for microscopic study in the histopathology laboratory in KAUH. For microscopic evaluation, paraffin sections of 5 µm thickness were stained with hematoxylin and eosin (H&E).
Caspase-3 staining
The testes sections mounted on positive slides were immersed in 10 mM sodium citrate buffer. The endogenous peroxidase activity in the tissue samples was blocked by treatment with hydrogen peroxide, followed by the addition of an anti-caspase 3 blocking solution to the testis tissues, respectively.
Statistical analysis
The results obtained from the experiments were analyzed using IBM SPSS Statistics software for Windows, version 23 (IBM SPSS, IBM Corp., Armonk, N.Y., USA). The data obtained in the study are presented as mean ± standard deviation (SD). One-way ANOVA (analysis of variance) was performed for the statistical comparisons, and Tukey’s test was used to calculate the significance between groups. The P-value < 0.05 was considered statistically significant.
Results
Effect of ED on testosterone levels and oxidative stress
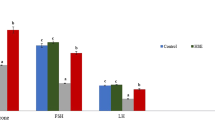
The data from Table 1 showed that the serum testosterone levels were significantly decreased in experimental group 2 (Low dose Code Red), group 3 (High dose Code Red), and group 4 (Low dose Code Red + Blueberry) compared to the control group (P < 0.0001, P < 0.0001 and P = 0.013, respectively) (Table 1). Also, the levels of serum testosterone dropped significantly further in group 3 (High dose Code Red) compared to group 2 (Low dose Code Red) (P = 0.005), depicting that the increased consumption of ED can have a more devastating effect on the testes (Table 1). Overall, the results indicated the adverse effects of continued consumption of low and high doses of ED for 8 weeks on the testis of rats. Thus, regular consumption of either low or high amounts of ED can have detrimental effects on the testes, thereby increasing the risk of male fertility.
Meanwhile, with the administration of blueberry for 6 weeks, serum levels of testosterone were found to be significantly increased in rats from group 4 (Low dose Code Red + Blueberry) compared to group 2 (Low dose Code Red) (P < 0.0001) and also in group 5 (High dose Code Red + Blueberry) compared to group 3 (High dose Code Red) (P < 0.0001) (Table 1). The BB administration in groups 4 and 5 showed restoration of the normal serum testosterone levels as in group 1 (Table 1). These results suggest the ameliorating and therapeutic effects of blueberry on the testicular functions against the damaging effects of energy drinks.
Further, to assess the impact of energy drinks on the reactive oxygen species (ROS) levels of the experimental rats, we measured the levels of oxidative stress markers (MDA) and antioxidants (SOD and GSH) in the blood serum, as shown in Table 2. Serum levels of MDA were significantly increased in groups 2, 3, 4, and 5 compared with control (P < 0.0001, P < 0.0001, P < 0.0001, and P = 0.027, respectively); in group 3 (High dose Code Red) compared with group 2 (Low dose Code Red) (P < 0.0001); meanwhile, it was significantly decreased in group 5 (High dose Code Red + Blueberry) compared with groups 3 and 4 (P < 0.0001 for both) (Table 2). Serum levels of SOD were significantly decreased in groups 2 and 3 versus controls (P < 0.0001, for both); in group 3 compared with group 2 (P < 0.0001); meanwhile, it was significantly increased in group 4 compared with groups 2 (P < 0.0001) and in group 5 compared with group 3 (P < 0.0001) (Table 2). Serum levels of GSH were significantly decreased in groups 2 and 3 as compared to control (P < 0.0001, for both); in group 3 compared with group 2 (P < 0.0001); meanwhile, it was significantly increased in group 4 compared with control and group 2 (P = 0.023; P < 0.0001, respectively) and in group 5 as compared to control and group 3 (P = 0.008; P < 0.0001, respectively) (Table 2). These results indicated that the administration of low and high doses of energy drinks for 8 weeks increased oxidative stress and decreased anti-oxidants and that improvement was observed after the administration of blueberry for a period of 6 weeks (Table 2).
Histological monitoring of the effect of ED on testes
H&E stain
In the present study, it was observed that consuming energy drinks (Code Red) by albino rats resulted in drastic changes in their testis compared to that of the control animals, whose testis showed normal seminiferous tubules with regular outlines, intact complete germ cell layers thickness, with most showing mature spermatozoa, besides the appearance of interstitial cells with its normal population and histological features and appearance (Fig. 1). Meanwhile, the rats consuming energy drinks (Code Red) showed marked histological alterations that were dose-dependent. Low dosage Code Red treatment led to the sporadic scattered appearance of seminiferous tubules with marked degeneration and loss of spermatogenic germ cells series (dividing stages), sparing only basal spermatogonia (stem precursor cells) (Fig. 1). Interstitial cells looked sparse and degenerated. High dose Code Red group showed increase in the number of tubules exhibiting similar degenerative changes in addition to highly congested thick vessels in the interstitial tissue.
Paraffin sections from adult Wistar albino rat testis stained by H&E and photographed at low and high powers (× 100 bar = 200 µm and × 400 bar = 50 µm, respectively). Group 1 (Control): Normal control with seminiferous tubules (ST) showing regular outlines and fully-thickened intact germ cell layers (double heads arrow) with mature sperm tails extending to the lumen (star). Interstitial cells show normal morphology (white arrows). Group 2 (Low dose Code Red): shows sporadically scattered tubules with marked degeneration and loss of germ cell layers (thin black arrows). Interstitial cells appear sparse and degenerated (thick black arrow). Group 3 (High dose Code Red): shows marked interstitial vascular congestion (white arrows), numerous tubules with degenerated germ cells (black arrows), and thickened fibrous interstitial tissue (white stars). Group 4 (Low dose Code Red + Blueberry): shows marked protection against Code Red-induced trophic changes on spermatogenic cells. Group 5 (High dose Code Red + Blueberry): shows a decrease in the degeneration of seminiferous tubules (black arrows), with most of the tubules having near fully-thickened germ cell layers (black arrows and double heads arrows). A few tubules show degenerated cells (dotted arrows) with nearby congested blood vessels (thick white arrow). Also, the interstitial tissue shows thickened blood vessels and degenerated cells (thick black arrow)
Administration of Code Red, followed by blueberry (Vaccinium corymbosum L.) extract for 6 weeks, showed markedly amelioration effects, such as histological changes where most seminiferous tubules showed nearly normal appearance with more impact on low energy drink group. Interstitial tissue (cells and blood vessels) of the treated group restored their normal appearance compared to the non-treated groups (Fig. 1).
Immunohistochemistry staining for the apoptotic marker (caspase-3)
Figure 2 showed negative or sporadic positive immunostaining for caspase-3 (the apoptotic marker) in the control testis (in normally degenerated cells). On the other hand, testis from rats consuming either low or high doses of Code Red energy drink showed increased caspase-3 immunostaining in seminiferous tubules with early degeneration features, while negative impact in the seminiferous tubules exhibited loss (necrosis) of germ cells (Fig. 2). Administration of blueberry extract to both groups showed negative or mild immunostaining for caspase-3, which was similar to the control group (Fig. 2).
Sections from rat testis immunostained for caspase-3 (apoptotic marker) and photographed at × 100 bar = 200 µm and × 200 bars = 100 µm. Group 1 (Control): with negative immunostaining for caspase-3 in seminiferous tubules (ST) except for mild reaction in interstitial tissue (white arrows). Group 2 (Low dose Code Red): showing desquamated germ cells within ST lumina (thin black arrows) and increased immunostaining for caspase-3 in interstitial cells (thick black arrow). Group 3 (High dose Code Red): showing marked degenerative changes in most ST (Black arrows) and a marked increase in immunostaining for caspase-3 in the degenerated germ cells (black arrows) as well as the interstitial cells (thick black arrows). Group 4 (Low dose Code Red + Blueberry): showing a marked decrease in caspase-3 immunostaining in the interstitial cells (arrows) as well as germ cells (white arrows). Group 5 (High dose Code Red + Blueberry) showed decreased caspase- 3 immunoexpression, although some seminiferous tubules still showed deformity or germ cell desquamation (white arrows)
Discussion
The testosterone hormone secreted by testicular interstitial Leydig cells regulates gene expression related to sexual function, which plays a vital role in male reproductive ability. This study demonstrated that oral administration of the energy drink Code Red in low and high doses to rats for 8 weeks led to a considerable decline in serum testosterone levels versus the control group that was dose-dependent. These results indicated the harmful effects of consumption of low as well as a high dose of energy drinks on rat testis. Meanwhile, the administration of blueberry to Code Red treated groups led to an increase in serum testosterone levels versus untreated groups, indicating the beneficial effects of blueberry on the testicular functions against the damaging impact of the energy drink. The decrease in testosterone production in rats receiving energy drinks could be explained by energy drink-induced damage of the interstitial cells of Leydig that produce testosterone. Al‑Eryani et al. (2018) reported a reduction in serum testosterone levels in rats treated with ED for 7 weeks. Male reproductive function is affected by various factors in vivo and in vitro, and the hypothalamic-pituitary-gonad axis is the key in vivo regulatory pathway (Drobnis & Nangia, 2017; Liu et al., 2016; Nabi et al., 2020). Testosterone cooperates with luteinizing hormone and follicle-stimulating hormone to enhance the development of spermatogenic tubules, spermatogenesis, and maturation, thus affecting male reproductive capacity (Drobnis & Nangia, 2017; Kaplan et al., 2016). Meanwhile, administration of BB leads to an improvement in stromal interstitial Leydig cell structure and function, which in turn results in improvement of testosterone serum levels as is observed in this study.
The present study revealed that the administration of low and high doses of Code Red energy drinks for 8 weeks led to elevated oxidative stress markers. The results indicate that Code Red intake led to a notable reduction in serum activities of SOD and GSH and a significant elevation in MDA levels. Meanwhile, the administration of blueberry to these groups led to a marked reduction in the MDA levels and elevated SOD and GSH levels; however, these levels were still significantly changed versus the control group. In this respect, Al-Eryani (2018) revealed a significantly higher concentration of lipid peroxides in the testis of male rats that were administered energy drinks for 7 weeks (Al‑Eryani et al., 2018). Also, Abdollahi et al. (2004) reported that energy drink administration induced a significant reduction in the antioxidant activity of SOD, catalase (CAT), and glutathione peroxidase (GPx) enzymes (Abdollahi et al., 2004). Together with the non-enzymatic antioxidant system, these enzymes guard cells from free radical oxidative damage. The highly reactive superoxide anions are neutralized by SOD by converting to hydrogen peroxide, which GPx and CAT subsequently break down into water (Sharma & Sangha, 2014). The considerable decrease in enzyme levels in the blood, particularly in rats given medium, low, and high doses of ED, may be caused by an ED-induced increase in superoxide radicals, which exceeds enzymes' antioxidant ability to neutralize these radicals. Studies have also shown that higher caffeine exposure causes a pro-oxidant environment in human cells, which promotes protein oxidation, whereas low amounts of caffeine do not affect the antioxidant capacity of the cells (Dias et al., 2015). Caffeine causes a decrease in the antioxidant defense system (SOD, GPx, and CAT), resulting in elevated free radical activities and subsequently leading to oxidative stress, as evident by increased MDA concentration (Ekaluo et al., 2016). When blood urea nitrogen levels are dramatically increased by caffeine, xanthine oxidase is activated, stimulating the conversion of xanthine to uric acid and the production of superoxide anion and H2O2. On the other hand, several studies have shown that many ED ingredients, including taurine, ginseng, caffeine, and guarana, have antioxidant qualities (Obochi et al., 2010). Blueberries are abundant in antioxidants that possess anti-inflammatory properties. Anthocyanins, flavanols, and phenolic acid are among the antioxidant polyphenols that are known to be present in BB. Studies have shown that eating a diet rich in anthocyanins lowers the incidence of myocardial infarction in women and suggests that anthocyanins may act as an anti-inflammatory agent (Cassidy et al., 2013; Johnson et al., 2013).
In the present study, it was observed that consuming energy drinks (Code Red) by albino rats resulted in drastic changes in their testis compared to the control testis. The rats consuming energy drinks showed marked histological alterations, which were dose-dependent. Low-dose cod red treatment led to the sporadic scattered appearance of seminiferous tubules with marked degeneration and loss of spermatogenic germ cells series sparing only basal spermatogonia as well as degeneration of interstitial cells. Meanwhile, the high-dose cod red group showed more tubules exhibiting similar degenerative changes in addition to highly congested thick wall vessels in the interstitial tissue. Administration of Code Red and then blueberry extract for 6 weeks markedly ameliorated such histological changes, where most seminiferous tubules showed a nearly normal appearance with more effect on low energy drink group. These findings were in agreement with the findings of a similar study (Elshennawy & Elwafa, 2011). A recent study showed that male adult rats' testicles had serious histopathological alterations after consuming Red Bull energy drinks in excess and for an extended period of time (Ahmed, 2016). According to Al-Eryani (2018), male rats exposed to Red Bull and Power Horse energy drinks continuously for 7 weeks reported several histological alterations, including epithelial cell sloughing, atrophic changes, and a decrease in germ cell count as a result of cytotoxicity (Al‑Eryani et al., 2018). Degenerative alterations in the seminiferous tubules and a decline in spermatozoa in the testis showed genotoxicity. Previous research showed that the consumption of energy drinks caused histological alterations in many organs, such as the testis (Dias et al., 2015), pancreas, fundus of the stomach (Ayuob & ElBeshbeishy, 2016), and liver (Khayyat et al., 2012). These lesions may be caused by an unbalanced oxidant/antioxidant environment in these tissues, which results in increased oxidative stress due to the generation of tumor necrosis factor-alpha (TNF-alpha) and inducible nitric oxide synthase (iNOS). These mechanisms are triggered as a result of high content of caffeine in energy drinks (Ayuob & ElBeshbeishy, 2016). According to Ali (2015), a safe amount of caffeine is 200 to 300 mg; however, the caffeine concentration in popular energy drinks is in the 500 mg range (Ali et al., 2015). Numerous epidemiologic studies have linked caffeine to an increased incidence of infertility (Wilcox et al., 1988). A study in Sprague–Dawley rats found that administering a high dosage of caffeine-200 mg/kg of body weight adversely alters the histoarchitecture of the testis' seminiferous tubules, resulting in a significant loss of spermatogenic cells (Bassey et al., 2011). Another research using the same coffee dosage also noted histological changes such as atrophic cells with necrosis, increased spermatid degeneration, and nearly no spermatozoa (Ekaluo et al., 2014). Adult albino rats who received 30 mg/kg/day of caffeine administered by gavage for 15–38 days in a row experienced a breakdown of the germinal epithelium (Pollard & Smallshaw, 1988). Caffeine, given daily to adult male rabbits (30–60 mg/kg) for 4 weeks in a row, resulted in a reduction in the size of the seminiferous tubules and an inhibition of spermatogenesis (Ezzat & El‑Gohary, 1994). In another study, 85–100% of 4–6-week-old male rats administered caffeine for 14–75 weeks showed severe bilateral testicular atrophy with aspermiogenesis or oligospermatogenesis. Reduced spermatogenesis caused by coffee in mature animals resulted in testicular atrophy (Friedman et al., 1979). Additionally, one of the effects caused was that the testicles shrink and lighten, and Leydig cells produce less testosterone (Park et al., 2015).
In our study, the expression of the cytoplasmic immunoreactivity caspase-3 was highly positive in testicular tissues of Code Red-treated groups. Administration of blueberry extract to low and high doses ED groups showed negative or mild immunostaining for caspase-3. This was in harmony with the results of a similar study (Ayuob & ElBeshbeishy, 2016), where it was reported that the administration of power horse, one of the energy drinks, to rats resulted in a strong cytoplasmic caspase-3 reaction in the pancreas and stomach.
Conclusions
The findings of this study showed that 8 weeks of exposure to low and high doses of Code Red causes damage to the testicles and decreases blood testosterone levels in rats. A significant decrease in the levels of key antioxidant enzymes (SOD and GSH) in blood and elevation of MDA with increased caspase-3 expression in testicular tissue clearly indicate that the deleterious effects of Code Red occur as a result of enhanced ROS generation, oxidative stress, and inflammation. Blueberries had a marked therapeutic effect against Code Red-induced testicular damage via their anti-inflammatory and antioxidant effects. Therefore, the use of Code Red with BB is recommended. These findings urge moderation and caution in the intake of Code Red and other energy drinks if animal-to-man extrapolation is considered. Therefore, the need for having appropriate public information regarding energy drinks cannot be understated.
Availability of data and materials
This published paper contains all of the data collected throughout the research.
Abbreviations
- ED:
-
Energy drink
- BB:
-
Blueberry
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- GR:
-
Glutathione reductase
- SD:
-
Standard deviation
- ROS:
-
Reactive oxygen species
- ST:
-
Seminiferous tubules
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- TNF-alpha:
-
Tumor necrosis factor-alpha
- iNOS:
-
Inducible nitric oxide synthase
- KAU:
-
King Abdulaziz University
- ST:
-
Seminiferous tubules
- H&E:
-
Hematoxylin and eosin
References
Abdollahi, M., Ranjbar, A., Shadnia, S., Nikfar, S., & Rezaie, A. (2004). Pesticides and oxidative stress: A review. Medical Science Monitor, 10(6), RA141-147.
Ahmed, A. M. (2016). Expression of transcription factor NF-KAPPA B/P65 and cyclooxygenase-2 (COX-2) in testicular damage induced by Red Bull energy drink in rat. International Journal of Advanced and Applied Sciences, 3(10), 49–56. https://doi.org/10.21833/ijaas.2016.10.009
Al-Eryani, F. S., Kelany, A. M., Amin, H. A., & Shazly, H. F. (2018). Histological and physiological studies on the effects of some energy drinks on male rats. International Journal of Pharmaceutical Research and Allied Sciences, 7(1), 165–176.
Al-Hazzaa, H. M., Abahussain, N. A., Al-Sobayel, H. I., Qahwaji, D. M., & Musaiger, A. O. (2011). Physical activity, sedentary behaviors and dietary habits among Saudi adolescents relative to age, gender and region. The International Journal of Behavioral Nutrition and Physical Activity, 8, 140. https://doi.org/10.1186/1479-5868-8-140
Ali, F., Rehman, H., Babayan, Z., Stapleton, D., & Joshi, D.-D. (2015). Energy drinks and their adverse health effects: A systematic review of the current evidence. Postgraduate Medicine, 127(3), 308–322. https://doi.org/10.1080/00325481.2015.1001712
Alrasheedi, A. A. (2016). Prevalence and reasons for consumption of energy drinks among adolescents and young adults in Jeddah, Saudi Arabia. Global Journal of Health Science, 9(2), 56709. https://doi.org/10.5539/gjhs.v9n2p23
Ayuob, N., & ElBeshbeishy, R. (2016). Impact of an energy drink on the structure of stomach and pancreas of Albino Rat: Can omega-3 provide a protection? PLoS ONE, 11(2), e0149191. https://doi.org/10.1371/journal.pone.0149191
Backer, W. S., & Baeissa, H. M. (2014). Effect of different energy drinks on liver and heart enzymes in rats. International Journal of Biotechnology, 3(1), 1–11.
Bassey, R. B., Yama, O. E., Osinubi, A. A., Noronha, C. C., & Okanlawon, A. (2011). Effects of Tahitian Noni dietary supplement on caffeine-induced testicular histo-pathological alterations in adult Sprague-Dawley rats. Middle East Fertility Society Journal, 16(1), 61–66. https://doi.org/10.1016/j.mefs.2010.11.003
Burke, L. M. (2008). Caffeine and sports performance. Applied Physiology, Nutrition, and Metabolism, 33(6), 1319–1334. https://doi.org/10.1139/H08-130
Cassidy, A., Mukamal, K. J., Liu, L., Franz, M., Eliassen, A. H., & Rimm, E. B. (2013). High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation, 127(2), 188–196. https://doi.org/10.1161/CIRCULATIONAHA.112.122408
Dias, T. R., Alves, M. G., Bernardino, R. L., Martins, A. D., Moreira, A. C., Silva, J., et al. (2015). Dose-dependent effects of caffeine in human Sertoli cells metabolism and oxidative profile: Relevance for male fertility. Toxicology, 328, 12–20. https://doi.org/10.1016/j.tox.2014.12.003
Dlugosz, L., & Bracken, M. B. (1992). Reproductive effects of caffeine: A review and theoretical analysis. Epidemiologic Reviews, 14, 83–100. https://doi.org/10.1093/oxfordjournals.epirev.a036093
Drobnis, E. Z., & Nangia, A. K. (2017). Male reproductive functions disrupted by pharmacological agents. Advances in Experimental Medicine and Biology, 1034, 13–24. https://doi.org/10.1007/978-3-319-69535-8_3
Ekaluo, U. B., Ikpeme, E. V., Etta, S. E., Erem, F. A., & Daniel, I. O. (2014). Protective role of soursop (Annona muricata L.) fruit on testicular toxicity induced by caffeine in albino rats. Journal of Life Sciences Research, 1, 26–30.
Ekaluo, U. B., Uno, U. U., Edu, N. E., Ekpo, P. B., & Etta, S. E. (2016). Effect of Trévo dietary supplement on caffeine induced oxidative stress in albino rat models. The Pharmaceutical and Chemical Journal, 3(2), 92–97.
Elshennawy, W. W., & Elwafa, H. R. A. (2011). Histological and ultrastructural changes in mammalian testis under the effect of hydrocortisone. Journal of American Science, 7(9), 38–48.
Elsoadaa, S. S., Hejazi, H. H., Sonbul, A. A., Fayyadhah, S. A., Al-Ahdal, S. E., Al-Turkistani, S. A., et al. (2016). Prevalence of energy drinks consumption among adolescents and young adults in Makkah, KSA. Journal of Health, Medicine and Nursing, 33, 79–90.
Ezzat, A. R., & El-Gohary, Z. M. (1994). Hormonal and histological effects of chronic caffeine administration on the pituitary-gonadal and pituitary-adrenocortical axes in male rabbits. Functional and Developmental Morphology, 4(1), 45–50.
Friedman, L., Weinberger, M. A., Farber, T. M., Moreland, F. M., Peters, E. L., Gilmore, C. E., et al. (1979). Testicular atrophy and impaired spermatogenesis in rats fed high levels of the methylxanthines caffeine, theobromine, or theophylline. Journal of Environmental Pathology and Toxicology, 2(3), 687–706.
Heckman, M. A., Sherry, K., & De Mejia, E. G. (2010). Energy drinks: An assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the united states. Comprehensive Reviews in Food Science and Food Safety, 9(3), 303–317. https://doi.org/10.1111/j.1541-4337.2010.00111.x
Jahedi, M., Shamsasenjan, K., Sanaat, Z., Aliparasti, M., Almasi, S., Mohamadian, M., et al. (2014). Aberrant phenotype in Iranian patients with acute myeloid leukemia. Advanced Pharmaceutical Bulletin, 4(1), 43–47. https://doi.org/10.5681/apb.2014.007
Johnson, M. H., de Mejia, E. G., Fan, J., Lila, M. A., & Yousef, G. G. (2013). Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Molecular Nutrition & Food Research, 57(7), 1182–1197. https://doi.org/10.1002/mnfr.201200678
Kaplan, A. L., Hu, J. C., Morgentaler, A., Mulhall, J. P., Schulman, C. C., & Montorsi, F. (2016). Testosterone therapy in men with prostate cancer. European Urology, 69(5), 894–903. https://doi.org/10.1016/j.eururo.2015.12.005
Khayyat, L., Essawy, A., Sorour, J., & Rawi, M. A. (2014). Impact of some energy drinks on the structure and function of the kidney in Wistar Albino Rats. Life Science Journal, 11(10), 1131–1138.
Khayyat, L., Sorour, J., Rawi, M. A., & Essawy, A. (2012). Histological, ultrastructural and physiological studies on the effect of different kinds of energy drinks on the liver of Wistar albino Rat. Journal of American Science, 8(8), 688–697.
Larrosa, M., González-Sarrías, A., Yáñez-Gascón, M. J., Selma, M. V., Azorín-Ortuño, M., Toti, S., et al. (2010). Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. The Journal of Nutritional Biochemistry, 21(8), 717–725. https://doi.org/10.1016/j.jnutbio.2009.04.012
Liu, W., Chen, C., Chen, L., Wang, L., Li, J., Chen, Y., et al. (2016). Sex-dependent effects of microcystin-LR on hypothalamic-pituitary-gonad axis and gametogenesis of adult zebrafish. Scientific Reports, 6, 22819. https://doi.org/10.1038/srep22819
Malinauskas, B. M., Aeby, V. G., Overton, R. F., Carpenter-Aeby, T., & Barber-Heidal, K. (2007). A survey of energy drink consumption patterns among college students. Nutrition Journal, 6, 35. https://doi.org/10.1186/1475-2891-6-35
Nabi, G., Hao, Y., Liu, X., Sun, Y., Wang, Y., Jiang, C., et al. (2020). Hypothalamic-pituitary-thyroid axis crosstalk with the hypothalamic-pituitary-gonadal axis and metabolic regulation in the eurasian tree sparrow during mating and non-mating periods. Frontiers in Endocrinology, 11, 303. https://doi.org/10.3389/fendo.2020.00303
Nadeem, I. M., Shanmugaraj, A., Sakha, S., Horner, N. S., Ayeni, O. R., & Khan, M. (2021). Energy drinks and their adverse health effects: A systematic review and meta-analysis. Sports Health, 13(3), 265–277. https://doi.org/10.1177/1941738120949181
Nardi, G. M., Farias Januario, A. G., Freire, C. G., Megiolaro, F., Schneider, K., Perazzoli, M. R. A., et al. (2016). Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacognosy Research, 8(Suppl 1), S42–S49. https://doi.org/10.4103/0974-8490.178642
National Research Council (US). (2011). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the care and use of laboratory animals (8th ed.). National Academies Press.
Obochi, G. O., Amali, O. O. E., & Ochalefu, D. O. (2010). Effect of melatonin and caffeine interaction on caffeine induced oxidative stress and sleep disorders. Nigerian Journal of Physiological Sciences, 25(1), 17–24.
Okonofua, F. E., Ntoimo, L. F. C., Omonkhua, A., Ayodeji, O., Olafusi, C., Unuabonah, E., et al. (2022). Causes and risk factors for male infertility: A scoping review of published studies. International Journal of General Medicine, 15, 5985–5997. https://doi.org/10.2147/IJGM.S363959
Park, M., Choi, Y., Choi, H., Yim, J.-Y., & Roh, J. (2015). High doses of caffeine during the peripubertal period in the rat impair the growth and function of the testis. International Journal of Endocrinology, 2015, 368475. https://doi.org/10.1155/2015/368475
Pollard, I., & Smallshaw, J. (1988). Male mediated caffeine effects over two generations of rats. Journal of Developmental Physiology, 10(3), 271–281.
Sharma, D., & Sangha, G. K. (2014). Triazophos induced oxidative stress and histomorphological changes in liver and kidney of female albino rats. Pesticide Biochemistry and Physiology, 110, 71–80. https://doi.org/10.1016/j.pestbp.2014.03.003
Subaiea, G. M., Altebainawi, A. F., & Alshammari, T. M. (2019). Energy drinks and population health: Consumption pattern and adverse effects among Saudi population. BMC Public Health, 19(1), 1539. https://doi.org/10.1186/s12889-019-7731-z
Wilcox, A., Weinberg, C., & Baird, D. (1988). Caffeinated beverages and decreased fertility. The Lancet, 2(8626–8627), 1453–1456. https://doi.org/10.1016/s0140-6736(88)90933-6
Worrall, B. B., Phillips, C. D., & Henderson, K. K. (2005). Herbal energy drinks, phenylpropanoid compounds, and cerebral vasculopathy. Neurology, 65(7), 1137–1138. https://doi.org/10.1212/01.wnl.0000178985.35765.e0
Yuan, Z., Zhang, J., Tu, C., Wang, Z., & Xin, W. (2016). The protective effect of blueberry anthocyanins against perfluorooctanoic acid-induced disturbance in planarian (Dugesia japonica). Ecotoxicology and Environmental Safety, 127, 170–174. https://doi.org/10.1016/j.ecoenv.2016.01.019
Zepeda, A., Aguayo, L. G., Fuentealba, J., Figueroa, C., Acevedo, A., Salgado, P., et al. (2012). Blueberry extracts protect testis from hypobaric hypoxia induced oxidative stress in rats. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2012/975870
Zheng, X., Xu, X., Liu, C., Sun, Y., Lin, Z., & Liu, H. (2013). Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Separation and Purification Technology, 104, 17–25. https://doi.org/10.1016/j.seppur.2012.11.011
Zhou, L., Xie, M., Yang, F., & Liu, J. (2020). Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT, 117, 108621. https://doi.org/10.1016/j.lwt.2019.108621
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TMA conceived the idea, TMA and NAR performed the experiments, and TMA and NAR wrote the manuscript. Both authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the experiments and animal care were carried out under the guidelines of King Abdulaziz University Research Ethics Committee that approved the study protocols and ethics.
Consent for publication
Not applicable.
Competing interests
All the authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Shaikh, T.M., Rajeh, N.A. Ameliorating effect of blueberry consumption on energy drink-induced testicular damage in rats: histological and immunohistochemical study. JoBAZ 84, 9 (2023). https://doi.org/10.1186/s41936-023-00330-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-023-00330-0