Abstract
Background
A lot of factors contribute to the breeding of Anopheles mosquitoes, especially vectors of malaria parasites. This indirectly contributes to the transmission of these parasites. The physicochemical parameters associated with the population of Anopheles larvae were studied for the period of twelve months in five selected communities in Akure North Local Government area of Ondo State. This study was carried out to understand the relationship between selected physicochemical parameters and the population of Anopheles larvae in the study area.
Results
Electrical conductivity was significantly correlated with the abundance of An. gambiae and An. funestus in the area (r = 0.840 and 0.843, respectively). Abundance of Anopheles larvae follows a positive linear regression with electrical conductivity (R2 = 0.691). The pH was not significantly different in all the communities (P > 0.05); pH was negatively correlated with the abundance of An. gambiae and An. funestus larvae, r = − 0.530 and − 0.470, respectively. Anopheles larvae population decreases as pH increases (R2 = 0.292). Total dissolved solid was positively correlated with the abundance of Anopheles larvae, though the correlation was weak (r = 0.21). There was slightly increase in Anopheles larvae population as total dissolved solid increases (R2 = 0.048). The abundance of Anopheles larvae increases as the dissolved oxygen, R2 = 0.552. Dissolved oxygen was not significantly correlated with Anopheles larvae population (r = − 0.734 and − 0.789 , respectively), there was no significant difference across the study area (P > 0.05). Temperature was significantly correlated with the abundance of Anopheles larvae (r = 0.74). Anopheles larvae increase as the temperature increases (R2 = 0.582).
Conclusions
The study revealed the important physicochemical parameters that influence the abundance of Anopheles larvae in the study area. Manipulation of these important parameters could help in reducing the population of the immature stages of this vector.
Similar content being viewed by others
Background
Malaria accounts for more cases of illness and death in Nigeria more than any other country of the world (Oladepo et al., 2019). Schantz-Dunn and Nour (2009) reported that malaria infection was responsible for underweight infants at birth and anaemia in the mother and women. A lot of factors contribute to the epidemy of this disease, such as the lack of knowledge about malaria, poverty, and chronic nature of most cases of the disease all of which together form a vicious circle, which is difficult to break. Attempts have been made in controlling this disease through the use of insecticide-treated bed nets, quick diagnosis and appropriate treatment. These have resulted in significant drop in mortality and morbidity in some countries, while the disease continues to be the primary cause of illness and deaths in other countries, most especially Afro-tropical countries (Lindsay et al., 2000; White, 2018). The control of malaria involves the management of three living entities and their environment; Man (the secondary host), a moving target and can take the disease very far and wide; Anopheles mosquitoes (the primary host), which are mobile, highly adaptable and have shown resistance to insecticides; and the third entity are the aquatic stages of the primary host known as vector (eggs, larval, and pupa stages), which are restricted to an identified habitat. Therefore, it is important to target the larval and pupa stages of this vector.
The main factors responsible for the burden of malaria disease are the climatic and ecological factors that favour the survival and developmental stages of malaria vectors (MARA, 1998; Kibret et al., 2019). The knowledge of the ecological features of the breeding habitats such as electrical conductivity, dissolved oxygen, hydrogen concentration (pH), salinity, CO2, total dissolved solid, turbidity, temperature, and other relevant environmental factors that have a direct influence on the abundance of mosquito can help in designing optimal vector control strategies (Elmalih et al., 2018; Overgaard et al., 2001; Yee et. al., 2010). These factors must also successfully support the development of the immature stages, from the first-instar larvae to the emergency of the adult stage of the mosquitoes. The immature stages of Anopheles mosquitoes have body temperature that varies depending on the outside temperature; this makes them to depend on temperature of the aquatic habitat (Abiodun et al., 2016). Mosquito habitats are characterised with different ranges of electrical conductivity; the habitat types are also a major factor that can make the electrical conductivity differ (Obi et al., 2019). The electrical conductivity is an important parameter used to estimate the level of dissolved salts in water and soil (Corwin & Yemoto, 2017). An increase in conductivity of aquatic environment may be an indicator that pollutants have been discharge into the habitat (Emidi et al., 2017; Obi et al., 2019).
Mosquito larvae have been found to adapt to ranges of ambient pH that are higher than those tolerated by other aquatic organisms (Ukubuiwe et al., 2020). Mosquito larvae depend mostly on atmospheric oxygen of the environment, although some mosquito larvae have been reported to use dissolved oxygen to complement the atmospheric oxygen (Clements, 1992; Dida et al., 2015). Larvae of mosquitoes are not affected by reduction of dissolved oxygen since atmospheric oxygen is readily accessible (Dale et al., 2007; Lancaster & Downes, 2012). In the absence of atmospheric oxygen, mosquito larvae use dissolved oxygen. It has been reported that mosquito survives for some days when deny access to atmospheric oxygen (Silberbush et al., 2015) and that the survival rate decreases as dissolved oxygen in the concealed habitat decreases. This research aimed to study the relationship between immature stages of malaria vector and some selected physicochemical parameters such as temperature, pH, total dissolved solid, dissolved oxygen and electrical conductivity of breeding habitats of malaria vectors (Anopheles mosquitoes) in Akure North Local Government Area. This could help in planning for habitat-based control of the vector in the study area, which might result to reduction in vector-biting rates and disease transmission.
Methods
Study area
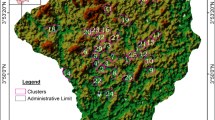
Akure North Local Government Area is located in the Northern part of Akure, the State capital of Ondo State Nigeria. The local government covered an area of 660km2 with more than 131,587 human population (NPC, 2009). The five locations where Anopheles mosquito habitats were sampled include Oba-Ile, Igoba, Isinigbo, Ita-Ogbolu and Iju. The selected locations within the study area are popular towns with large populations. Anopheles mosquito larvae and pupa were collected from identified breeding sites within the study area. The breeding sites were identified by random sampling of stagnant water in drainages, ponds, river edges and open soil surfaces. The geographical positioning system (GPS) coordinate of the Anopheles mosquito habitats was taken using GPS device (Fig. 1).
Collection of Anopheles mosquito
The collection of Anopheles larvae was carried out between 8 and 11am in the morning from 360 breeding sites. The breeding sites of Anopheles mosquitoes were identified by random sampling of stagnant water in the study area. Anopheles mosquito larvae were collected from pond bed, river bed, spoors, river edges, rain pool, pot hole and canal in the study area. Larvae were collected thrice monthly from different breeding sites; 10–15 dips were taken from Anopheles mosquito habitat in selected localities from the study area between October 2018 and September 2019. Anopheles larvae were identified by their characteristic horizontal positioning on the surface of the water. The larvae were carefully collected into small plastic containers by scooping gently to avoid injuring the larvae. Each sample was labelled indicating date, site and locality of collection. The containers were loosely capped to avoid suffocation and immediately transported to the laboratory.
Morphological identification of mosquitoes
Larvae of Anopheles mosquitoes were identified under dissecting microscope using standard morphological characters keys provided by Gillies and Coetzee (1987) and Nagpal and Sharma (1995).
Determination of the physicochemical parameters of Anopheles mosquito habitats
After the identification of Anopheles mosquito habitat, selected physicochemical parameters such as pH, temperature, electrical conductivity, dissolved oxygen and total dissolved solid were determined in situ before Anopheles mosquito larvae and pupae samples were collected. Electrical conductivity and temperature were determined using Aqua-pro water tester (model-AP2). The hydrogen ion concentration (pH) was determined using Aquarium pool IA digital meter (model-PH 009, range 0.0–14.0pH). AMTAST DO meter (model-AMT08) was used in determining dissolved oxygen. The total dissolved solids of the habitats were determined using Hanna instrument TDS meter with ATC (model-DIST 1, range 10–1990 ppm). All studied parameters were measured and recorded in four replicate, three times per month for a period of 12 months (between October 2018 and September 2019).
Data analysis
All collected data were analysed using Statistical Package for Social Sciences (SPSS Version 26). One-way ANOVA was used to determine significance between the mean across different locations, and Duncan’s new multiple range test (DNMRT) was used to measure specific differences. Pearson correlation analysis (r) was used to study the relationship between the physicochemical parameters and distribution of the malaria vectors. Regression analysis was also used to determine the linear regression (R2) value between the study parameters and abundance of Anopheles mosquitoes in the study areas.
Results
Distribution of Anopheles mosquitoes immature stages in the study area
The monthly distribution of immature stages of Anopheles mosquitoes collected from the study area is presented in Fig. 2. An. gambiae s. l. is the most abundant throughout the period of study, the highest percentage was recorded in the month of November (12.78%) followed by month of December (12.54%), and the lowest percentage was collected in the month of May (4.35%). An. funestus group was encountered in the month of October (0.62%), November (0.79%), December (0.62%), January (0.98%), February (0.88%), March (0.20%), and September (0.13%).
Monthly variation and correlation of physicochemical parameters with Anopheles larvae in the study area
The result of physicochemical parameters from five locations selected for sample collection in the study area is presented in Table 1. All the parameters’ study was not significantly different across the study areas. The range of pH recorded from Anopheles mosquito’s habitats was between 6.05 and 8.23 with the mean value of 6.92 ± 0.06 (P > 0.05). The range of electrical conductivity (E.C.) of habitat was found to be between 140 and 557 μS/cm with the mean value of 250.52 ± 14.52 (P > 0.05). The temperature was found to be between 23.1 and 28.7 °C. The mean value of the habitats’ temperature in the study area was 26.04 ± 0.19 (P > 0.05). Dissolved oxygen range was between 7.67 and 8.56 mg/L with an average mean value of 8.09 ± 0.03 (P > 0.05). The range of total dissolved solid was between 10 and 27 ppm, with total mean value of 16.60 ± 0.06 (P > 0.05).
Correlation between the physicochemical parameters and distribution of Anopheles mosquitoes is represented in Table 2. The result shows that the relationship between electrical conductivity and abundance of An. gambiae s. l. and An. funestus was significantly and positively strong (r = 0.840 and 0.843, respectively). The abundance of An. gambiae s. l. and An. funestus was positively and significantly related to the habitat temperature, r = 0.737 and 0.759, respectively, and this shows that the relationship between these two variables was fairly strong (P < 0.05). The abundance of An. gambiae s. l. and An. funestus is negatively related to the dissolved oxygen (r = − 0.73 and − 0.79 , respectively) and pH (r = − 0.53 and r = − 0.47 , respectively) recorded from the habitat. The monthly mean of physicochemical parameter and the monthly percentage of Anopheles mosquito larvae are represented in Table 3. The highest percentage of Anopheles mosquitoes larvae was collected in the month of November (13.25%), with the highest mean of electrical conductivity (443.80 µS/cm) and total dissolved solid of 23.40 ppm.
Monthly proportion of Anopheles mosquitoes and physicochemical parameters
Monthly proportion of Anopheles mosquitoes larva alongside with the average mean of some selected physicochemical parameters are represented in Table 3. The highest mean of electrical conductivity (E. C.) and highest number of Anopheles gambiae larva were recorded in the month of November and December; with 954 larvae of Anopheles gambiae collected in the month of November with 443.80 μS/cm electrical conductivity and 936 larvae collected in the month of December with 37.8.40 μS/cm electrical conductivity, while the highest number of 73 larvae representing 23.18% of Anopheles funestus larvae was collected in the month of January with 290.60 μS/cm electrical conductivity. There was no significant difference (P > 0.05) in the average mean of electrical conductivity (μS/cm), temperature (°C), and dissolved oxygen (mg/L) recorded from the month of April (199.80 μS/cm, 24.76 °C and 8.28 mg/L), May (183.80 μS/cm, 24.60 °C and 8.31 mg/L), June (146 μS/cm, 24.98 °C. and 8.27 mg/L), July (152.20 μS/cm, 25 °C and 8.24 mg/L), August (147 μS/cm, 25.08 °C, and 8.24 mg/L) and September (145.60 μS/cm, 25.38 °C, and 8.19 mg/L); 694, 325, 445, 349, 405 and 462 Anopheles mosquitoes larvae were collected from all those months, respectively.
Regression relationship between malaria vectors population and physicochemical parameters
The regression relationship (R2) between the population of Anopheles mosquitoes and five selected physicochemical parameters are shown by scatter plot in Figs. 3, 4, 5, 6 and 7. The population of the Anopheles mosquito larvae increased as the electrical conductivity (EC) of the larval habitat increased (R2 = 0.69). In this same way, the population of this vector increases as the temperature of the habitat increases (R2 = 0.58). The population of Anopheles mosquitoes increases as the level of hydrogen population (pH) decreases (R2 = 0.29). Likewise, the population of the aquatic stages (larval and pupa) of the vector decrease as the dissolved oxygen increases (R2 = 0.55). Total dissolved solid of the larval habitat was very low, and the population of vector slightly increased as the total dissolved solid also increases (R2 = 0.047).
Discussion
All the physicochemical parameters considered in this study have a significant influence on the abundance of malaria vector in the study area. Among the studied physicochemical parameters, only pH and dissolved oxygen were found not to be significantly correlated with the abundance of Anopheles mosquitoes in the study area. The result of this research shows that pH is negatively correlated with habitat preference of Anopheles mosquitoes in the study area. This conformed to the findings of Chaiphongpachara et al. (2018) and Obi et al. (2019); they both reported that pH of the habitat of immature stages of Anopheles mosquito was not significantly correlated with the density of Anopheles larvae in their respective location of study, though some other species of mosquito larvae have been reported to show a significant correlation with pH (Dejenie et al., 2011; Nikookar et al., 2017). The findings of Getachew et al. (2020) reported that the pH of Anopheles mosquito larval habitat could be slightly acidic or slightly alkaline. This was in agreement with the report of the present study, where the pH of the larvae habitat was found between the range of 6.05 (slightly acidic) and 8.23 (slightly alkaline). This shows that the adult Anopheles mosquitoes have the ability of selecting a suitable pH environment for their oviposition, which is less acidic and less basic.
Abundance of Anopheles mosquito larvae in this research was not significantly correlated with the habitat dissolved oxygen. In the study carried out by Kenawy et al. (2013), the results of the multiple regression show that dissolved oxygen is negatively associated with the development of immature stage of the malaria vectors. This is not in agreement with the findings of Bashar et al. (2016), which reported dissolved oxygen as a predictor for the abundance of Anopheles mosquito larvae. In some specific area where Kenawy et al. (2013) carried out the study some species of mosquitoes other than Anopheles spp were positively correlated with dissolved oxygen. In another study, it was reported that mosquito breeding is negatively associated with dissolved oxygen (Adebote et al., 2008; Alkhayat et al., 2020). Dida et al. (2015) reported the collection of more Anopheles mosquito larvae in a habitat with low mean of dissolve oxygen. This supported the result of this current study. The range of dissolved oxygen collected from Anopheles larvae habitats in this study was between 7.67 and 8.56 mg/L. Garba et al. (2018) had reported that Anopheles larvae can survive in habitats with 5.57–8.60 mg/L dissolved oxygen; this range was recorded in all the habitats of anopheles larva sampled on the highlands of Mambilla Plateau in Taraba State North East Nigeria. The report of Dida et al. (2015) recorded 6.4 mg/L in a habitat populated by Anopheles mosquito larvae. Clements (1992) and Silberbush et al. (2015) have reported that mosquito larvae primarily depend on atmospheric oxygen, while dissolved oxygen serves as an additional source of oxygen. Thus, as far as atmospheric oxygen is available and accessible, mosquito larvae are not affected by the depletion of dissolved oxygen (Lancaster & Downes, 2012; Yamada et al., 2020).
The habitat’s electrical conductivity, temperature and total dissolved solid were significantly and positively correlated with the abundance of immature stages of Anopheles mosquitoes in the study area, though the correlation between TDS and abundance of immature stages of Anopheles mosquitoes was found to be very weak compared to the relationship between electrical conductivity and immature stages of Anopheles mosquitoes which was positively strong, and also the correlation between the temperature and the immature stages of the Anopheles mosquitoes showed positively strong. This indicates the important of these two physicochemical parameters (electrical conductivity and temperature) in preference and development of vector of malaria diseases. Getachew et al. (2020) reported the density of Anopheles mosquito larvae not significantly associated with temperature; this was not in agreement with the result of the present study. The report of the current research work indicated that temperature has a positive correlation with the abundance of Anopheles mosquitoes in the study area. Asare et al. (2016) reported that temperature of larval habitat has a significant influence on the development of the aquatic stages of Anopheles mosquito. It was also reported that the development of many mosquito species critically decreases when the temperature of the larval habitat goes below 14–16 °C (Afrane et al., 2012). Gillooly et al. (2001) reported that temperature mediates mosquito physiology and metabolic rate, because metabolic rate increases exponentially rather than linearly with temperature in ectotherms. The multiple regression carried out by Kenawy et al. (2013) suggested that water temperature is positively associated with the development of immature stage of the vector. This was in agreement with the findings of Bashar et al. (2016), which reported that temperature was positively associated with the breeding of the Anopheles mosquito larvae. Different species of mosquito larvae have been found to develop and survive in different ranges of temperature. Those mosquitoes found at edges or banks of moving water are found to develop under 16–19 °C (Dida et al., 2018). In this study, the temperature range of Anopheles mosquito larval habitat was found between 23.1 and 28.7 °C. Some other research works carried out in Nigeria reported anopheline vector larval habitat temperature from 21.39 to 31.90 °C (Garba et al., 2018) and 26.5–29.3 °C (Afolabi et al., 2013); these are in agreement with the result of this study.
Electrical conductivity is one of the important parameters used to determine the quality of water. This study reports a fairly strong positive relationship between Anopheles mosquito larvae and electrical conductivity with range between 140 and 557 μS/cm. The electrical conductivity associated with mosquito breeding habitat reported in Ghana by Tiimub et al. (2012) was lower compared to what was reported with Anopheles mosquito habitat in this study. This may be due to differences in study location. In an experiment carried out by Othaman et al. (2020), the result shows that the electrical conductivity is directly proportional to the nutrient concentration. The nutrient concentration level is significant to the survival and growth of the immature stages of Anopheles mosquitoes; this could be the main reason for the positive correlation between the abundance of the immature stages of Anopheles mosquitoes and the electrical conductivity. The range of total dissolved solid at which Anopheles mosquito larvae occur in their natural habitat in the study area was between 10 and 27 ppm. Dida et al. (2015) had earlier reported that mosquitoes larvae survive in habitat with total dissolved solids of 8–87 ppm. The total dissolved solid reported in this study fall between the ranges that were reported by Dida et al. (2015). The report by Garba et al. (2018) suggested that Anopheles mosquito thrived in average total dissolved solid of 21.85–50.45 ppm; this was different compared to the range of total dissolved solid reported in this study. Abai et al. (2015) reported a very high total dissolved solid (1261.40 ± 1214.31) associated with Anopheles mosquito; the report disagreed with the result of this study. The differences in total dissolved solid may be due to difference in study area and adaption of the Anopheles mosquitoes to difference level of total dissolved solid in the study location. Vanlalhruaia et al. (2014) reported a positive association between total dissolved solid and abundance of Anopheles mosquito. Vanlalhruaia et al. (2014) also reported that Anopheles mosquitoes have a fairly weak association with total dissolved solid compared to other genus of mosquitoes, most especially Culex mosquito which prefer habitats with high dissolved matter and high total dissolved solid. Significant positive relationship between total dissolved solids and abundance of Anopheles mosquito has been reported by Musonda and Sichilima (2019), which supported the result of this study.
Conclusions
The findings of this study have revealed physicochemical factors that contributed to the abundance of larvae and pupae of Anopheles mosquitoes in the studied area. The physicochemical factors that influence the abundance of immature stages include electrical conductivity, temperature and total dissolved solids. The stability of pH of the oviposition habitats also contributes to the abundance of non-flying stages of this vector. Manipulation or alteration of these factors could help in managing the non-flying stages of malaria vectors.
Availability of data and materials
All analysed data involved in this study are included in this manuscript.
References
Abai, M. R., Saghafipour, A., Ladonni, H., Jesri, N., Omidi, S., & Azari-Hamidian, S. (2015). Physicochemical characteristics of larval habitat waters of mosquitoes (Diptera: Culicidae) in Qom Province, Central Iran. Journal of Arthropod Borne Disease, 10, 65–77.
Abiodun, G. J., Maharaj, R., Witbooi, P., & Okosun, K. O. (2016). Modelling the influence of temperature and rainfall on the population dynamics of Anopheles arabiensis. Malaria Journal, 15, 364.
Adebote, D. A., Oniye, S. J., & Muhammed, Y. A. (2008). Studies on mosquitoes breeding in rock pools on inselbergs around Zaria, Northern Nigeria. Journal of Vector Borne Disease, 45, 21–28.
Afolabi, O. J., Simon-Oke, I. A., & Osomo, B. O. (2013). Distribution, abundance and diversity of mosquitoes in Akure, Ondo State, Nigeria. Journal of Parasitology and Vector Biology, 5(10), 132–136.
Afrane, Y. A., Githeko, A. K., & Yan, G. (2012). The ecology of Anopheles mosquitoes under climate change: Case studies from the effects of deforestation in East African highlands. Annals of the New York Academy of Sciences, 1249, 204–210.
Alkhayat, F. A., Ahmad, A. H., Rahim, J., Dieng, H., Ismail, B. A., Imran, M., Sheikh, U. A. A., Shahzad, M. S. S., Abid, A. D., & Munawar, K. (2020). Characterization of mosquito larval habitats in Qatar. Saudi Journal of Biological Sciences, 27, 2358–2365.
Asare, E. O., Tompkins, A. M., Amekudzi, L. K., Ermert, V., & Redl, R. (2016). Mosquito breeding site water temperature observations and simulations towards improved vector-borne disease models for Africa. Geospatial Health, 11, 391.
Bashar, K., Rahman, M. S., Nodi, I. J., & Howlader, A. J. (2016). Species composition and habitat characterization of mosquito (Diptera: Culicidae) larvae in semi-urban areas of Dhaka, Bangladesh. Pathogens and Global Health, 110, 2.
Chaiphongpachara, T., Yusuk, P., Laojun, S., & Kunphichayadecha, C. (2018). Environmental factors associated with mosquito vector larvae in a malaria-endemic area in Ratchaburi Province, Thailand. The Scientific World Journal, 2018, 1–8.
Clements, A. N. (1992). The Biology of Mosquitoes: Development, Nutrition, and Reproduction (pp. 118–123). Chapman and Hall.
Corwin, D. L., & Yemoto, K. (2017). Salinity: Electrical conductivity and total dissolved solids. Soil Science of America, 2, 2558.
Dale, P. E., Greenway, M., Chapman, H., & Breitfuss, M. J. (2007). Constructed wetlands for sewage effluent treatment and mosquito larvae at two sites in subtropical Australia. Journal of the American Mosquito Control Association, 23, 109–116.
Dejenie, T., Yohannes, M., & Assmelash, T. (2011). Characterization of mosquito breeding sites in and in the vicinity of tigray microdams. Ethiopian Journal of Health Sciences, 21, 57–66.
Dida, G. O., Anyona, D. N., Abuom, P. O., Akoko, D., Adoka, S. O., Matano, A., Owuor, P. O., & Ouma, C. (2018). Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infectious Diseases of Poverty, 7, 2.
Dida, G. O., Gelder, F. B., Anyona, D. N., Abuom, P. O., Onyuka, J. O., Matano, A., Adoka, S. O., Kanangire, C. K., Owuor, P. O., Ouma, C., & Ofulla, A. V. O. (2015). Presence and distribution of mosquito larvae predators and factors influencing their abundance along the Mara River, Kenya and Tanzania. Springerplus, 4, 136.
Elmalih, E. B. I., Almugadam, B. S., Tamomh, A. G., & Hassan, S. M. (2018). Influence of Environmental factors on breeding habitats of mosquito species in Kosti City, White Nile State, Sudan. Health Science Journal, 12(4), 577.
Emidi, B., Kisinza, W. N., Mmbando, B. P., Malima, R., & Mosha, F. W. (2017). Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasites and Vectors, 10, 304.
Garba, L. C., Oyieke, F. A., Owino, E. A., Mwansat, G. S., Houmsou, R. S., Chintem, D. G. W., & Wama, B. E. (2018). Larval habitats of anopheline vectors of malaria on the highlands of Mambilla Plateau Taraba State North East Nigeria. International Journal of Mosquito Research, 5, 96–100.
Getachew, D., Balkew, M., & Tekie, H. (2020). Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malaria Journal, 19, 65.
Gillies, M. T., & Coetzee, M. (1987). A supplement to the Anophelinae of Africa, South of the Sahara. Publication of the South African Institute of International Medicine and Research, 55, 1–143.
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M., & Charnov, E. L. (2001). Effects of size and temperature on metabolic rate. Science, 293, 2248–2251.
Kenawy, M. A., Ammar, S. E., & Abdel-Rahman, H. A. (2013). Physicochemical characteristics of the mosquito breeding water in two urban areas of Cairo Governorate, Egypt. Journal of Entomological and Acarological Research, 45, 96.
Kibret, S. G., Wilson, G., Ryder, D., Tekie, H., & Petros, B. (2019). Environmental and meteorological factors linked to malaria transmission around large dams at three ecological settings in Ethiopia. Malaria Journal, 18, 54.
Lancaster, J., & Downes, B. J. (2012). Aquatic entomology (pp. 41–47). Oxford University Press.
Lindsay, S., Ansell, J., Selman, C., Cox, V., Hamilton, K., & Walraven, G. (2000). Effect of pregnancy on exposure to malaria mosquitoes. The Lancet, 335, 1972.
Mapping Malaria Risk in Africa (MARA) (1998). Towards an Atlas of malaria risk in Africa. Medical Research Council, South Africa.
Musonda, M., & Sichilima, A. (2019). The effect of total dissolved solids, salinity and electrical conductivity parameters of water on abundance of anopheles mosquito larvae in different breeding sites of Kapiri Mposhi district of Zambia. International Journal of Scientific & Technology Research, 8, 70.
Nagpal, B. N., & Sharma, V. P. (1995). Mosquito Taxonomic Inventory (p. 416). Sci Pub Inc.
National Population Commission (NPC). (2009). 2006 Population and Housing Census of the Federal Republic of Nigeria: National and State Population and Housing Tables, Priority Tables (Volume I) National Population Commission, Abuja, Nigeria.
Nikookar, S. H., Fazeli-Dinan, M., Azari-Hamidian, S., Mousavinasab, S. N., Aarabi, M., Ziapour, S. P., Esfandyari, Y., & Enayati, A. (2017). Correlation between mosquito larval density and their habitat physicochemical characteristics in Mazandaran Province, northern Iran. PLOS Neglected Tropical Diseases, 11, e0005835.
Obi, O. A., Nock, I. H., & Adebote, D. A. (2019). Biodiversity of Microinvertebrates coinhabiting mosquitoes habitats in patchy rock pools on inselbergs within Kaduna State, Nigeria. The Journal of Basic and Applied Zoology, 80, 57.
Oladepo, O., Oyeyemi, A. S., Titiloye, M. A., Adeyemi, A. O., Burnett, S. M., Apera, I., Oladunni, O., & Alliu, M. (2019). Malaria testing and treatment knowledge among selected rural patent and proprietary medicine vendors (PPMV) in Nigeria. Malaria Journal, 18, 103.
Othaman, N. N. C., Isa, M. N. M., Ismail, R. C., Ahmad, M. I., & Hui, C. K. (2020). Factors that affect soil electrical conductivity (EC) based system for smart farming application. AIP Conference Proceedings., 2203, 020055.
Overgaard, H. J., Tsuda, Y., Suwonkerd, W., & Takagi, M. (2001). Characteristics of Anopheles minimus (Diptera: Culicidae) larval habitats in northern Thailand. Environmental Entomology, 10, 134–141.
Schantz-Dunn, J., & Nour, N. M. (2009). Malaria and pregnancy: A global health perspective. Reviews in Obstetrics and Gynecology, 2, 186.
Silberbush, A., Abramsky, Z., & Tsurim, I. (2015). Scientific Note: Dissolved oxygen levels affect the survival and developmental period of the mosquito Culex pipiens. Journal of Vector Ecology, 40, 425.
Tiimub, B. M., Adu, B. K., & Obiri-Danso, K. (2012). Physico–chemical assessment of mosquito breeding sites from selected mining communities at the Obuasi municipality in Ghana. Journal of Environment and Earth Science, 2, 10.
Ukubuiwe, A. C., Ojianwuna, C. C., Olayemi, I. K., Arimoro, F. O., & Ukubuiwe, C. C. (2020). Quantifying the roles of water pH and hardness levels in development and biological fitness indices of Culex quinquefasciatus Say (Diptera: Culicidae). The Journal of Basic and Applied Zoology, 81, 5.
Vanlalhruaia, K., Gurusubramanian, G., & Kumar, N. S. (2014). Effect of environmental parameters on the relative abundance of immature stages of mosquitoes in Mizoram, India. Environmental Science: an Indian Journal, 9, 255–261.
White, N. J. (2018). Anaemia and malaria. Malaria Journal, 17, 371.
Yamada, H., Maiga, H., Bimbile-Somda, N. S., Carvalho, D. O., Mamai, W., Kraupa, C., Parker, A. G., Abrahim, A., Weltin, G., Wallner, T., Schetelig, M. F., Caceres, C., & Bouyer, J. (2020). The role of oxygen depletion and subsequent radioprotective effects during irradiation of mosquito pupae in water. Parasites and Vectors, 13, 198.
Yee, D. A., Kneitel, J. M., & Juliano, S. A. (2010). Environmental correlates of abundances of mosquito species and stages in discarded vehicle tires. Journal of Medical Entomology, 47, 53.
Acknowledgements
The authors appreciate the Chief Laboratory Technologist of the Department of Biology, Federal University of Technology, Akure for her support. We appreciate the support of Isaac Olabimi and Kolade Orimolade for their assistance during data collection.
Funding
This research work did not receive external funding.
Author information
Authors and Affiliations
Contributions
AVA, IAS-O and TAO, contributed to the research design and involved in field and laboratory work. AVA carried out statistical analysis and interpreted the result of the study. AVA wrote the first draft of the manuscript. TAO and IAS-O reviewed the manuscript. All author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akeju, A.V., Olusi, T.A. & Simon-Oke, I.A. Effect of physicochemical parameters on Anopheles mosquitoes larval composition in Akure North Local Government area of Ondo State, Nigeria. JoBAZ 83, 34 (2022). https://doi.org/10.1186/s41936-022-00298-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-022-00298-3