Abstract
Background
Documentation of the species composition of Anopheles mosquitoes and characterization of larval breeding sites is of major importance for the implementation of larval control as part of malaria vector control interventions in Ethiopia. The aims of this study were to determine the Anopheles larval species composition, larval density, available habitat types and the effects of related environmental and physico-chemical parameters of habitats in the Ghibe River basin of southwestern Ethiopia.
Methods
Anopheles larvae were sampled from November 2014 to October 2016 on a monthly basis and 3rd and 4th instars were identified microscopically to species. The larval habitats were characterized based on habitat perimeter, water depth, intensity of light, water current, water temperature, water pH, water turbidity, distance to the nearest house, vegetation coverage, permanence of the habitat, surface debris coverage, emergent plant coverage, habitat type and substrate type.
Results
In total, 9277 larvae of Anopheles mosquitoes and 494 pupae were sampled from borrow pits, hoof prints, rain pools, pools at river edges, pools in drying river beds, rock pools, tire tracks and swamps. Anopheles larval density was highest in pools in drying river beds (35.2 larvae per dip) and lowest in swamps (2.1 larvae per dip) at Darge, but highest in rain pools (11.9 larvae per dip), borrow pits (11.2 larvae per dip) and pools at river edges (7.9 larvae per dip), and lowest in swamps (0.5 larvae per dip) at Ghibe. A total of 3485 late instar Anopheles mosquito larvae were morphologically identified. Anopheles gambiae sensu lato was the primary Anopheles mosquito found in all larval habitats except in swamps. Temperature at the time of sampling and emergent vegetation, were the most important variables for Anopheles mosquito larval density. Anopheles gambiae density was significantly associated with habitats that had smaller perimeters, were sunlit, had low vegetation cover, and a lack of emergent plants. Generally, Anopheles mosquito larval density was not significantly associated with water pH, water temperature, water turbidity, algal content, and larval habitat depth.
Conclusion
Different species of Anopheles larvae were identified including An. gambiae s.l., the main malaria vector in Ethiopia. Anopheles gambiae s.l. is the most abundant species that bred in most of the larval habitat types identified in the study area. The density of this species was high in sunlit habitat, absence of emergent plants, lack of vegetation near habitat and habitats closer to human habitation. Rainfall plays a great role in determining the availability of breeding habitats. The presence of rain enable to create some of the habitat types, but alter the habitats formed at the edge of the rivers due to over flooding. Controlling the occurrence of mosquito larvae through larval source management during the dry season, targeting the pools in drying river bed and pools formed at the edge of the rivers as the water receded can be very crucial to interrupt the re-emergence of malaria vectors on the onset of rainy season.
Similar content being viewed by others
Background
In 2017, malaria affected 219 million globally and killed 435,000 people. Most cases and deaths occurred in the WHO African Region in children under 5 years of age [1]. In Ethiopia it is estimated that about 75% of the total area of the country is malarious [2]. About 58.3 million people lived in areas at risk of malaria in 2013 [3] and in 2015 it was estimated that malaria infected 2.8 million and resulted in 4900 deaths in Ethiopia [4]. Malaria transmission is seasonal and mostly occurs at the end of the main rainy season from June to August and during the small rains from March to April [2, 5].
As in most malaria endemic countries, the most commonly used malaria vector control interventions in Ethiopia are the application of indoor residual insecticide spraying (IRS) and utilization of long-lasting insecticide-treated nets (LLINs), which target the adult stages of malaria vectors [4]. Due to the development of insecticide resistance by the major malaria vector, Anopheles arabiensis [5, 6] and drug resistance by the Plasmodium species causing malaria [7, 8], implementation of integrated control interventions that target the larval stage could become very important. In addition to adult vector control, malaria transmission can be reduced by suppressing larval densities using appropriate methods based on the type of their breeding habitat [9, 10].
Anopheles gambiae sensu lato larvae are more abundant in sunlit, small and temporary habitats with low emergent plants and canopy cover [11]. These habitats may not favour the development of predators and competitors which may feed upon the mosquito larvae [12, 13]. Such habitats may also dry out due to evaporation before the larvae complete their development [14].
For the implementation of malaria vector control interventions, understanding the distribution patterns of Anopheles species in specified area is very important [9, 10]. This can be done through identification of larval habitat ecology and larval population dynamics particularly for the control of immature stages [15]. Control of immature stages of malaria vectors can be advantageous because the larvae are concentrated in specific habitats, relatively immobile, and occupy minimal habitat areas compared with adults that can rapidly disperse over large areas [16]. Hence, knowledge of vector distribution and species composition is very important to design effective malaria vector control programmes [17,18,19]. In addition, understanding the characteristics of larval habitats is also helpful [17, 20]. Thus, the main objective of this study was to determine the species composition and abundance of Anopheles larvae and to describe mosquito larval habitats at Ghibe and Darge study sites in Ghibe River basin, southwestern Ethiopia.
Methods
Study area
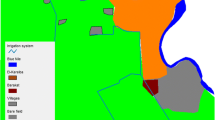
The study was conducted at Ghibe and Darge study sites located along the Ghibe River basin in southwestern Ethiopia in Abeshge district, Guraghe Zone, Southern Nations Nationalities and Peoples Regional State (Fig. 1 ). The zonal and Abeshge district town (Wolkite) is located 158 km southwest of Addis Ababa. Ghibe study site [8°14′ N, 37°33′ E, altitude 1080–1134 m above sea level (masl)] is located 30 km south of Wolkite near the Ghibe River. The area has an annual average rainfall of 625 mm (National Meteorological Agency, unpublished report). Acacia trees and savannah grassland dominate the vegetation of the area. In 2016, the study site had 420 households with 2167 total inhabitants of whom 1105 were male and 1062 were female (Abeshge district health office, unpublished report). There is a government-owned health post and one clinic owned by a local Ethiopian seed enterprise.
Darge study site (8°24′ N, 37°31′ E, altitude 1500–1800 masl) is located 42 km west of Wolkite and 52 km from Ghibe study site at the outskirts of Darge town. The Darge River crosses Darge town and serves as one of the tributaries of the Ghibe River. There is a health post and a health center in the study site. In 2016, Darge study site had 731 households with 3518 inhabitants, of whom 1724 were male and 1794 were female (Abeshge district health office, unpublished report). The study site has an annual average rainfall of 1022 mm (National Meteorological Agency, unpublished report). In both study sites, short (March and April) and long (June to August) rainy seasons are important for agricultural activities. The study area was selected due to its malaria endemicity and presence of perennial rivers near the study sites.
Mosquito larval sampling
Longitudinal larval collections were carried out in each of the study sites every month over a 24-month period (November 2014–October 2016). In each month, mosquito larvae were surveyed in each natural habitat that contained water. During sampling, 3–15 dips were taken using a standard dipper (350 ml capacity, BioQuip Products, Inc. California, USA) depending on the size of each larval habitat at intervals along the edge, with a greater sampling effort in areas of low mosquito density. For small habitats like hoof prints, several sites were pooled to get the required sample volume [13]. The water was collected in a white plastic tray and carefully observed for the presence of Anopheles larvae. Sampling was always done in the morning (09:00–12:00) or in the afternoon (14:00–17:00) for about 30 min by the same individual (DG) at each larval habitat.
All Anopheles larvae were sorted from culicine larvae and counted. Larval density was determined by taking the average number of mosquito larvae from the total dips taken at specific habitat. Anopheles larvae were then sorted into early stages (1st and 2nd instars) and late stages (3rd and 4th instars) and counted and recorded. Early stages were discarded but late stage larvae were killed in hot water (48 to 50 °C for 2 min) and immediately preserved in vials containing 70% ethanol and transported to laboratory for species identification.
Identification of Anopheles mosquito larvae
Third and fourth instar larvae which were collected from different habitats were transported to the Insect Vectors and Entomopathogen Research Laboratory, Department of Zoological Sciences, Addis Ababa University. A drop of Hoyer’s mounting medium was placed on a clean microscopic glass slide. Each larval specimen was mounted on a slide, covered with cover slip and allowed to dry and identified morphologically using the identification key of Gillies and Coetzee [21] under a compound microscope.
Characterization of larval habitats
Habitats containing Anopheles larvae were identified. Environmental variables including habitat perimeter, water depth, direct sunlight, presence of water flow, water temperature at the time of sampling, water pH, water turbidity, distance to the nearest house, vegetation coverage, permanence of the habitat, presence of algae, surface debris coverage, emergent plant coverage, habitat type and substrate type were recorded for each habitat containing Anopheles larvae with excluding habitats without larvae.
The depth of water of a habitat was measured from different places depending on size of the habitat using a meter stick and the average depth was taken. The distance to the nearest homestead was measured using a tape measure for less than 100 m and estimated if more than 100 m. Distance was then categorized into four classes: (1) ≤ 100 m, (2) 101 to 200 m, (3) 201 to 300 m, (4) 301 to 400 m. Surface debris, presence of algae and emergent plant coverage were determined based on visual observation. Vegetation cover was visually observed and expressed as open (no vegetation), tree (for the presence of large tree within a range of 10–15 m where shade and foliages could reach), and shrub (woody plants smaller than a tree within 10–15 meters). Habitat perimeter was measured using a tape measure and classified as < 10 m, 10–100 m and > 100 m. Habitat stability was expressed in terms of the length of time the habitat contained water after the rain. A habitat was considered temporary if it held water for 2 weeks or less and permanent if it held water for more than 2 weeks after rain [22]. Though larval sampling was taken on monthly bases, the area was inspected for the presence or absence of rain continuously. Turbidity was measured by placing water samples in glass test tubes and holding them against a white background, and categorized into three levels: low, medium, and highly turbid [23]. Light intensity was visually categorized as sunlit if the habitat received full sunlight that could occur throughout the day, otherwise the site was described as shaded. The substrate type was categorized as mud, stone if the pool was lined with stones that were large in size (rocks generally larger than 10 cm in diameter) and gravel when the stones were small in size but larger than sand. Water temperature was recorded using water thermometer at the time of collection and pH was measured using pH indicator paper [24]. Rainfall of the study area during the study period was obtained from National Meteorological Agency (unpublished report).
Data analysis
Larval breeding habitats and number of immature Anopheles mosquitoes sampled were described using tables. Correlation analysis was used to investigate the relationship between pH, temperature and water depth to the Anopheles larval density. Anopheles larval density was determined as the number of Anopheles larvae (early or late) divided by the number of dips taken from each larval habitat. Larval density was log transformed log10 (x + 1) to improve the normality of distribution. Multiple regression analysis was used to identify the environmental variables associated with the occurrence of Anopheles larvae. Mann–Whitney U test was used to compare samples with two variables; presence of algae (presence or absence), habitat permanency (temporary or permanent), surface debris (present or absent), intensity of light (sunlit or shaded) and water movement (still or flowing). Kruskal–Wallis H test was used to compare samples with more than two groups: water turbidity, water perimeter, distance to the nearest house, canopy cover, emergent plant coverage, habitat type and substrate type. These non-parametric tests were used to compare larval densities from sites with different habitat characteristics.
Data were analysed using IBM SPSS statistical for Windows (IBM corp., Armonk, NY), version 20.0. Values were considered significantly different if p < 0.05 for all the tests.
Results
Anopheles larvae species composition
Anopheles mosquitoes identified from each study site is shown in Table 1. In total, 3485 late instar Anopheles mosquito larvae were morphologically identified belonging to 10 species. From the total Anopheles larval species, An. gambiae sensu lato (s.l.), An. christyi, An. pharoensis and An. pretoriensis were identified from Darge and Ghibe study sites. Anopheles gambiae s.l. constituted 97.8% and An. pharoensis 1.3% of all identified larvae in Darge. In Ghibe, 90.6% were An. gambiae s.l. (henceforth referred to as An. gambiae) and 7.9% were An. christyi.
Anopheles larval productivity in different habitat types
The results of larval sampling and the types of larval habitats that were productive in the study area are presented in Table 2. Eight habitat types were identified in Darge, including borrow pits, hoof prints, rain pools, pools at river edges, pools in the bed of drying river, rock pools, tire tracks and swamps. All these types of habitats were also identified in Ghibe except pools in the bed of drying river. In both study sites, the most frequently encountered larval habitats were pools at river edges (Darge, n = 38 and Ghibe, n = 24), rain pools (Darge, n = 24), and tire tracks (Ghibe, n = 17).
In total, 9277 larvae and 494 pupae of Anopheles mosquitoes were sampled and the result was shown in Table 2. At Darge, large densities of immature Anopheles mosquitoes were collected from pools in the drying river beds (mean density of 35.2 larvae per dip and 3.5 pupae per dip). At Ghibe, larvae were densely populated in rain pools, borrow pits and pools at river edges with 11.9, 11.2 and 7.9 larvae per dip and pupae were from borrow pits (0.5 pupae per dip).
Abundance of Anopheles species larvae in breeding sites
Anopheles species composition and their preferred habitats in each study site are depicted in Table 3. Anopheles gambiae was identified from all types of larval habitats, but Anopheles christyi were from pools at river edge, rain pools, rock pools and tire tracks, and Anopheles pharoensis were from swamps, borrow pits, pools at river edge and rain pools. In Darge study site, except swamps which had the largest proportion of An. pharoensis (52.6%), in all other larval habitats almost all the identified larvae were An. gambiae. In Ghibe, almost all the identified larvae from hoof prints and tire tracks were An. gambiae.
Anopheles species composition and larval habitats
In total, 3485 late instar Anopheles mosquito larvae were morphologically identified belonging to 10 species which included An. gambiae (95.5%), An. christyi (2.9%), An. pharoensis (1.1%), Anopheles rivulorum (0.2%), Anopheles coustani (0.1%), Anopheles pretoriensis (0.1%), Anopheles concolor (0.1%), Anopheles demeilloni (< 0.1%), Anopheles nili (< 0.1%) and Anopheles ardensis (< 0.1%) (Table 4). In swamps the most abundant species was An. pharoensis (51.7%) followed by An. gambiae (36.7%) and An. coustani (5.0%). From borrow pits An. gambiae (98.8%) and An. pharoensis (1.2%) were identified.
Anopheles gambiae was the only species found in hoof prints and pools in drying river beds and the remaining habitats were also identified with large proportions of An. gambiae larvae. Anopheles christyi was identified from pools at river edges, rain pools, rock pools and tire tracks. Anopheles pharoensis was collected from swamps, borrow pits, pools at river edges and rain pools (Table 4).
Association between larval density and habitat variables
The multiple regression model showed that temperature at the time of collection (p = 0.03), and emergent vegetation (p = 0.003) were the best predictors of Anopheles larval density in the habitats (Table 5). The F-ratio in the ANOVA table showed that, the larval densities were statistically significantly associated with the physico-chemical parameters recorded during the larval sampling, F15, 151 = 3.7, p < 0.001, R2 = 0.266.
In Ghibe study site, the largest proportion of Anopheles larvae were sampled during June–August 2015 and the least were between October 2015–January 2016 and July–August 2016 (Fig. 2). In Darge, during the months of February to May 2015, September to October 2015 and January to March 2016 Anopheles larvae were high in number, but they were not collected in December 2015 and between the months of April–July 2016. The study further showed that in Ghibe, early instar Anopheles larvae monthly counts were significantly positively correlated with rainfall (r = 0.516, p = 0.01) while late instars were not significantly correlated with rainfall (r = 0.114, p = 0.595). At Darge, rainfall was not significantly correlated with early (r = − 0.012, p = 0.954) or late (r = − 0.104, p = 0.629) instar larvae.
There was slight correlation between water pH and that of Anopheles larval density (r = 0.187, p = 0.016) (Table 6). Water temperature was also positively correlated with the Anopheles larval density (r = 0.163, p = 0.035). However, there was no association between the depth of the larval habitat and Anopheles larval density (r = 0.069, p = 0.376).
Adequate numbers of late instar larvae for the analysis of environmental variables were available for An. gambiae, An. christyi and An. pharoensis. Mann–Whitney U tests and Kruskal–Wallis H test showed that (Table 7), An. gambiae larval density showed no significant difference between permanent and temporary habitats (U = 3137, p = 0.455), habitats with or without algae (p > 0.05), between habitats of small and large perimeter (p > 0.05), and between clean, moderately turbid, or turbid water (p > 0.05). However, it was significantly higher in habitats without surface debris (U = 2117, p = 0.009), sunlit habitats (U = 228, p = 0.028), closer (< 100 m) to human habitations (χ2 = 10.6, df = 3, p = 0.014), without vegetation (trees or bushes) near larval habitat (χ2 = 8.0, df = 2, p = 0.019), and in habitats without emergent plants within the larval habitat (χ2 = 8.7, df = 3, p = 0.034).
Anopheles pharoensis larval density showed no significant difference in habitats with or without algae (p > 0.05), in sunlit and shaded habitats (p > 0.05), and between habitats of clean, moderately turbid, or turbid water (p > 0.05). However, larval density was high significantly in permanent than temporary habitats (U = 3115, p = 0.047), in habitats with perimeter greater than one hundred meter (χ2 = 68.7, df = 2, p < 0.001), in habitats with the presence of vegetation (χ2 = 9.5, df = 2, p = 0.009), and in habitats with grass and weeds available together (χ2 = 11.8, df = 3, p = 0.008).
Anopheles christyi larval density showed no significant difference in habitats with or without algae (p > 0.05), in sunlit or shaded habitats (p > 0.05), between habitats of small and large perimeters (p > 0.05), between habitats of clean, moderately turbid, or turbid water (p > 0.05), and in habitats with or without vegetation (p > 0.05). However, larval density was significantly high in permanent habitats than temporary habitat (U = 2960.5, p = 0.019), with surface debris than without surface debris (U = 2267, p = 0.00), in habitats that were further (between 301 and 400 m) from houses than closer to houses (χ2 = 24.9, df = 3, p < 0.001), and in habitats with the substrates that were lined with large stones (χ2 = 21.1, df = 2, p = 0.00).
Discussion
This study examined the species composition and identified and characterized the larval habitats of Anopheles in two localities in the Ghibe River basin, southwestern Ethiopia. The most commonly encountered larval habitats were pools at river edges, rain pools and tire tracks. During the rainy season water accumulated in sites such as rain pools and tire tracks which served as larval sites. However, these small water bodies do not persist for long if there is no rain [25]. On the other hand, during the rainy season, the rivers increase in size and larval sites may not be formed at the edges of these rivers [26,27,28]. In line with our study, stream edges served as Anopheles breeding habitats during the dry seasons in the central Rift Valley of Ethiopia [24] and in the Butajira area [29].
In Darge, during an extended period without rain, pools of stagnant water formed in the river beds that served as larval breeding habitats. This corroborates with other studies that showed that drying streams [24, 30], drying river beds [25] and habitats at river fringes [27] supported the greatest numbers of mosquito larvae during the dry season. During the dry season, areas which were flooded during the rainy season are very important for the quick re-colonization of the larval habitats shortly after the rainfall [25, 31]. Generally, Anopheles larval distribution and abundance are affected by hydrological processes that govern the formation and persistence of different habitat types. Larval productivity depends on rainfall and subsequent changes in water table and river levels [32].
The highest proportions of Anopheles larvae were collected from borrow pits, pools in drying river beds and pools at river edges. Larval survival and development depends on biological and physicochemical properties of the habitats [33] including the stability of habitats for longer periods [15], oviposition behavior of gravid females [34], cannibalism and predation by late instars [35, 36], and increased dispersal of early instars by the flow of rivers and streams [37].
Larval breeding habitats such as hoof prints, rain pools, rock pools and tire tracks had lower larval densities than other sites. This corroborates a study conducted in the Butajira area where Anopheles larvae were not available in hoof-prints and most temporary rain pools [29]. This could be due to less stable types of habitats (hoof prints, rain pools, and tire tracks) which may dry rapidly after rains [38, 39], and such habitats rarely lasted more than 5 days to enable the larval stages to complete their development to emerge into an adult [15]. It may also have resulted from the infrequent larval sampling which was done on a monthly basis and larvae might not have been missed if it was done on a weekly or fortnightly basis [38]. On the other hand, temporary water bodies such as drainage canals, hoof prints, rain water pools and tire tracks were identified as the most productive habitats in two studies in Kenya [15, 27] but erosion pits and habitats along the river fringe were identified as less important mosquito breeding habitats [27]. This might be related with frequent occurrence of rain in study areas of those reports which support a longer duration of water in these temporary habitats [15].
Anopheles gambiae was the primary Anopheles mosquito found in all larval habitats except in swamps. In line with this study, An. gambiae was found to be the most abundant species in a wide variety of sites in Mbita, western Kenya [40], in stream edges in Eritrea [9] and southern Ethiopia [41] and along river edges during the dry season and short rainy season in the Rift Valley in central Ethiopia [24].
In swamps, the smallest densities of identified Anopheles larvae were collected compared to other larval habitats. Sattler et al. [42] stated that Anopheles larvae were less likely to be present in swamps and if present they were present in low densities. This type of habitats might support large number of macroinvertebrate predators and competitors which may affect the development of Anopheles. The longer development time of Anopheles larvae to complete its larval stage in such type of habitats increases their chance to be preyed upon [13, 36]. In swamps, though An. gambiae were identified, An. pharoensis was the predominant species identified as has been observed elsewhere [24].
Habitats near the edges of rivers were not available when the river level rose as a result of rainfall in upstream areas and, in the case of the Ghibe River, when there was a release of water from Gilgel Gibe I reservoir. When there was intense rainfall the densities of larvae collected was reduced. A study in Eritrea showed that Anopheles larval densities were negatively correlated with rainfall [43]. This might happen as the result of the flushing of larvae from habitats and mortality due to heavy rainfall [18, 27, 38].
The study showed that mosquito larvae were abundant after rainy seasons due to the formation of larval habitats, particularly at the edges of rivers which served to sustain Anopheles populations during the dry seasons. A study by Gimnig et al. [34] also showed that, the proportion of Anopheles mosquito larvae were higher during and immediately after rains. The ability of larval habitats to retain water and the presence of other sources of water during the dry season determines seasonal distribution of Anopheles larval development [27].
Temperature at the time of sampling and emergent vegetation, were the most important variables for Anopheles larval density. Anopheles gambiae density was significantly associated with habitats that had smaller perimeters, were sunlit, had low vegetation cover, and a lack of emergent plants. This is in line with studies conducted elsewhere [24, 34]. Habitats without shade have a higher average daily water temperature than shaded habitats. In habitats with lower water temperature, Anopheles larval development period can become elongated and increases the chance of larvae to be predated [44]. In contrast to our study, it was observed that habitats that contained growing grass and other vegetation had more Anopheles larvae than habitats without vegetation in study conducted in the Lake Victoria basin, western Kenya [27].
Anopheles pharoensis was sampled more from habitats that were permanent with large perimeter, had presence of trees and emergent plants that contain weeds and grasses. A study by Kenea et al. [24] showed that higher densities of An. pharoensis larvae were sampled from permanent lakeshore habitats with vegetation and algal mats.
Generally, Anopheles larval density was not significantly associated with water pH, water temperature, water turbidity, algal content, and larval habitat depth. The mean water temperature was 28.5 ± 3.2 °C which may have at a range of suitable water temperature for the Anopheles larvae to survive and develop into an adult as was stated by Bayoh and Lindsay [45]. Larval habitat depth was measured only from those habitats which contained Anopheles larvae so that most of the habitats were not more than 65 cm deep with few exceptions and we did not find that water depth was significantly associated with larval densities. Turbidity was associated with eroded soils that accumulated after rains or when the habitats with muddy substrates became disturbed by animals drinking water from stagnant water particularly at Darge. It has been suggested that turbid habitats attract ovipositing female Anopheles [22]. Anopheles gambiae larvae were more abundant in habitats closer to human habitation as compared to those habitats far from houses. It was stated that gravid mosquitoes prefer to lay eggs in habitats closer to human habitation to conserve energy lost flying long distances in search of oviposition sites [22].
There were a few limitations to this study. Larval samplings were conducted once each month so the presence of mosquito larvae might be missed during intervening periods which may have affected species abundance and larval habitat productivity. Water temperature of the larval habitat was recorded only during the larval sampling time and was not measured at different times of the day. Characterizations of larval habitats were performed only for those habitats with Anopheles larvae. In addition, other environmental variables including a detailed analysis of water chemistry and quantification of mosquito predators and competitors were not done. The important of these factors in mosquito presence and habitat productivity remains unknown.
Conclusion
Anopheles gambiae, which is considered the main malaria vector in Ethiopia was the most abundant species in larval habitats in the study area. Larvae of this species were present during both the dry and rainy seasons. Understanding the breeding habitats of malaria vectors and reducing their availability is important for the control and elimination of malaria [46, 47]. The rivers in the study area served as refugia during the dry seasons, enabling malaria vectors to persist throughout the year. While planning for malaria control programmes that incorporate larval control interventions, both dry and rainy seasons should be considered. As habitats became more limited, application of control interventions during the dry season might be more effective. However, due to the availability of different types of Anopheles mosquitoes breeding habitats in the study area, environmental management interventions can be significantly lower mosquito productivity [47].
Availability of data and materials
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IRS:
-
Indoor residual insecticide spraying
- LLINs:
-
Long-lasting insecticide-treated nets
- ITNs:
-
Insecticide-treated mosquito nets
- Masl:
-
Metres above sea level
- ANOVA:
-
Analysis of variance
References
WHO. World Malaria Report 2018. Geneva: World Health Organization; 2018.
Federal Ministry of Health. Entomological profile of malaria in Ethiopia. Federal Ministry of Health: Addis Ababa; 2007.
Federal Ministry of Health. Health and health related indicators 2005 E.C (2012/2013). Policy planning directorate. Addis Ababa, 2014.
WHO. World Malaria Report 2016. Geneva: World Health Organization; 2016.
Yeshiwondim AK, Gopal S, Hailemariam AT, Dengela DO, Patel HP. Spatial analysis of malaria incidence at the village level in areas with unstable transmission in Ethiopia. Int J Health Geogr. 2009;8:5.
Balkew M, Ibrahim M, Koekemoer LL, Brooke BD, Engers H, Aseffa A, et al. Insecticide resistance in Anopheles arabiensis (Diptera: Culicidae) from villages in central, northern and south west Ethiopia and detection of kdr mutation. Parasit Vectors. 2010;3:40.
Schunk M, Kumma WP, Miranda IB, Osman ME, Roewer S, Alano A, et al. High prevalence of drug-resistance mutations in Plasmodium falciparum and Plasmodium vivax in southern Ethiopia. Malar J. 2006;5:54.
Ketema T, Getahun K, Bacha K. Therapeutic efficacy of chloroquine for treatment of Plasmodium vivax malaria cases in Halaba district South Ethiopia. Parasit Vectors. 2011;4:46.
Shililu J, Ghebremeskel T, Seulu F, Mengistu S, Fekadu H, Zerom M, et al. Larval habitat diversity and ecology of anopheline larvae in Eritrea. J Med Entomol. 2003;40:921–9.
Liu XB, Liu QY, Guo YH, Jiang JY, Ren DS, Zhou GC, et al. Random repeated cross sectional study on breeding site characterization of Anopheles sinensis larvae in distinct villages of Yongcheng City, People’s Republic of China. Parasit Vectors. 2012;5:58.
Kweka EJ, Zhou G, Munga S, Lee M-C, Atieli HE, Nyindo M, et al. Anopheline larval habitats seasonality and species distribution: a prerequisite for effective targeted larval habitats control programmes. PLoS ONE. 2012;7:e52084.
Mwangangi J, Shililu J, Muturi E, Gu W, Mbogo C, Jacob B, et al. Dynamics of immature stages of Anopheles arabiensis and other mosquito species (Diptera: Culicidae) in relation to rice cropping in a rice agro-ecosystem in Kenya. J Vector Ecol. 2006;31:245–51.
Mereta ST, Yewhalaw D, Boets P, Ahmed A, Duchateau L, Speybroeck N, et al. Physico-chemical and biological characterization of anopheline mosquito larval habitats (Diptera: Culicidae): implications for malaria control. Parasit Vectors. 2013;6:320.
Mwangangi JM, Muturi EJ, Shililu J, Muriu SM, Jacob B, Kabiru EW, et al. Contribution of different aquatic habitats to adult Anopheles arabiensis and Culex quinquefasciatus (Diptera: Culicidae) production in a rice agroecosystem in Mwea, Kenya. J Vector Ecol. 2008;33:129–38.
Mala AO, Irungu LW. Factors influencing differential larval habitat productivity of Anopheles gambiae complex mosquitoes in a western Kenyan village. J Vector Borne Dis. 2011;48:52–7.
Floore TG. Mosquito larval control practices: past and present. J Am Mosq Control Assoc. 2006;22:527–33.
Mwangangi JM, Mbogo CM, Muturi EJ, Nzovu JG, Githure JI, et al. Spatial distribution and habitat characterisation of Anopheles larvae along the Kenyan coast. J Vector Borne Dis. 2007;44:44–51.
Kweka EJ, Zhou G, Lee M-C, Gilbreath TM, Mosha F, Munga S, et al. Evaluation of two methods of estimating larval habitat productivity in western Kenya highlands. Parasit Vectors. 2011;4:110.
Tabbabi A, Bousse`s P, Rhim A, Brengues C, Daaboub J, Ben-Alaya-Bouafif N, et al. Larval habitats characterization and species composition of Anopheles mosquitoes in Tunisia, with particular attention to Anopheles maculipennis complex. Am J Trop Med Hyg. 2015;92:653–9.
Muturi EJ, Mwangangi J, Shililu J, Jacob BG, Mbogo C, Githure J, et al. Environmental factors associated with the distribution of Anopheles arabiensis and Culex quinquefasciatus in a rice agro-ecosystem in Mwea, Kenya. J Vector Ecol. 2008;33:56–63.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region). Johannesburg: Johannesburg publications of the South African Institute for Medical Research; 1987.
Mwangangi JM, Shililu J, Muturi EJ, Muriu S, Jacob B, Kabiru EW, et al. Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar J. 2010;9:228.
Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G. Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. Am J Trop Med Hyg. 1999;61:1010–6.
Kenea O, Balkew M, Gebre-Michael T. Environmental factors associated with larval habitats of anopheline mosquitoes (Diptera: Culicidae) in irrigation and major drainage areas in the middle course of the Rift Valley, central Ethiopia. J Vector Borne Dis. 2011;48:85–92.
Sogoba N, Doumbia S, Vounatsou P, Baber I, Keita M, Maiga M, et al. Monitoring of larval habitats and mosquito densities in the Sudan savanna of Mali: implications for malaria vector control. Am J Trop Med Hyg. 2007;77:82–8.
Konradsen F, Matsuno Y, Amerasinghe FP, Amerasinghe PH, van der Hoek W. Anopheles culicifacies breeding in Sri Lanka and options for control through water management. Acta Trop. 1998;71:131–8.
Imbahale SS, Paaijmans KP, Mukabana WR, van Lammeren R, Githeko AK, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 2011;10:81.
Mutero CM, Blank H, Konradsen F, van der Hoek W. Water management for controlling the breeding of Anopheles mosquitoes in rice irrigation schemes in Kenya. Acta Trop. 2000;76:253–63.
Animut A, Gebre-Michael T, Balkew M, Lindtjørn B. Abundance and dynamics of anopheline larvae in a highland malarious area of south-central Ethiopia. Parasit Vectors. 2012;5:117.
Dida GO, Gelder FB, Anyona DN, Abuom PO, Onyuka JO, Matano AS, et al. Presence and distribution of mosquito larvae predators and factors influencing their abundance along the Mara River, Kenya and Tanzania. SpringerPlus. 2015;4:1–14.
Majambere S, Fillinger U, Sayer DR, Green C, Lindsay SW. Spatial distribution of mosquito larvae and the potential for targeted larval control in The Gambia. Am J Trop Med Hyg. 2008;79:19–27.
Hardy AJ, Gamarra JGP, Cross DE, Macklin MG, Smith MW, Kihonda J, et al. Habitat hydrology and geomorphology control the distribution of malaria vector larvae in rural Africa. PLoS ONE. 2013;8:e81931.
Edillo FE, Tour´e YT, Lanzaro GC, Dolo G, Taylor CE. Survivorship and distribution of immature Anopheles gambiae s.l. (Diptera: Culicidae) in Banambani village, Mali. J Med Entomol. 2004;41:333–9.
Gimnig JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval anopheline (Diptera: Culicidae) habitats in western Kenya. J Med Entomol. 2001;38:282–8.
Koennraadt CJM, Takken W. Cannibalism and predation among larvae of the Anopheles gambiae complex. Med Vet Entomol. 2003;17:61–6.
Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack OJ, Githeko AK, et al. Association between land cover and habitat productivity of malaria vectors in Western Kenyan highlands. Am J Trop Med Hyg. 2006;74:69–75.
Fillinger U, Sombroek H, Majambere S, van Loon E, Takken W, Lindsay SW. Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malar J. 2009;8:62.
Afrane YA, Lawson BW, Brenya R, Kruppa T, Yan G. The ecology of mosquitoes in an irrigated vegetable farm in Kumasi, Ghana: abundance, productivity and survivorship. Parasit Vectors. 2012;5:233.
Mutuku FM, Bayoh MN, Gimnig JE, Vulule JM, Kamau L, Walker ED, et al. Pupal habitat productivity of Anopheles gambiae complex mosquitoes in a rural village in western Kenya. Am J Trop Med Hyg. 2006;74:54–61.
Fillinger U, Sonye G, Killeen GF, Knols BGJ, Becker N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop Med Int Health. 2004;9:1274–89.
Gone T, Balkew M, Gebre-Michael T. Comparative entomological study on ecology and behaviour of Anopheles mosquitoes in highland and lowland localities of Derashe District, southern Ethiopia. Parasit Vectors. 2014;7:483.
Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, et al. Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar J. 2005;4:4.
Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R. Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. Am J Trop Med Hyg. 2007;76:103–10.
Tuno N, Okeka W, Minakawa N, Takagi M, Yan G. Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) larvae in western Kenya highland forest. J Med Entomol. 2005;42:270–7.
Bayoh MN, Lindsay SW. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae). Bull Entomol Res. 2003;93:375–81.
Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mir S, Mulla MS, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303.
Gu W, Utzinger J, Novak RJ. Habitat-based larval interventions: a new perspective for malaria control. Am J Trop Med Hyg. 2008;78:2–6.
Acknowledgements
We would like to thank local chiefs, communities of Ghibe farm enterprise and local communities for their assistance during larval sampling. We are grateful to Bereket Taphano for his assistance in larval collection. A special thanks to Dr Essayas Aklilu for his support in larval identification. We are grateful to Bedasa Tesema for his assistance in data analysis. We would like to thank Dr Seth R. Irish for his support in editing the manuscript. We thank the Ethiopian National Meteorological Agency for providing weather data of the study area.
Funding
This study was financially supported by Addis Ababa University. The funding body was not involved in the design of the study and data collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
DG, MB and HT conceived the idea of the research, developed the experimental design and protocols. DG performed field work, conducted laboratory activities, analysed the data and drafted and produced the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval to conduct the study was obtained from the ethical committee of College of Natural Sciences Institutional Ethics Review Board, Addis Ababa University. Consent was also obtained from Abeshge district (woreda) administration and the manager of each study site after the purpose of the investigation was explained to them.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Getachew, D., Balkew, M. & Tekie, H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar J 19, 65 (2020). https://doi.org/10.1186/s12936-020-3145-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-3145-8