Abstract
Background
The study was focused on identifying the pathogenic potential of native entomopathogenic fungi (EPF) viz., Metarhizium anisopliae (Metsch.) and Lecanicillium longisporum (Zimm.) against adult tea mosquito bug (TMB), Helopeltis theivora (Waterhouse) under in vitro conditions.
Results
Four EPF were isolated and the sequence has deposited to NCBI Genbank with accession numbers of MZ930378 (Metarhizium anisopliae isolate UPASI_1), MZ930384 (Lecanicillium longisporum isolate UPASI_2), MZ930388 (Metarhizium anisopliae isolate UPASI_3) and MZ930389 (Metarhizium anisopliae isolate UPASI_4). Isolates were evaluated against adult TMB using dipping and direct spray methods under in vitro conditions. The M. anisopliae isolates achieved 100 and 69–81% of adult mortality in dipping and direct spray method, respectively after the 10th day of application with 1 × 109 spores/ml. Among the tested isolates, a significant (p < 0.001) and highest mortality were observed in M. anisopliae (MZ930388). On the other hand, spraying of L. longisporum with 1 × 109, 1 × 107 and 1 × 105 spores/ml caused the adult mortality of 76, 55 and 46% respectively after the 10th day of application.
Conclusions
The study found that the indigenous EPF, M. anisopliae (MZ930388) was an effective and promising biocontrol agent against adult TMB under in vitro conditions.
Similar content being viewed by others
Background
Tea, Camellia sinensis (L.) Kuntze, is one of the most inexpensive and reasonable beverages in the world. The evergreen, perennial and monoculture crop provides a relatively optimum microclimate and continuous food supply to various insects and mites. Hence, the tea plantations are intensively affected by a wide range of pests. Among the various pests, the tea mosquito bug (TMB), Helopeltis theivora Waterhouse (Hemiptera: Miridae), is a major pest in mid-elevation areas of south Indian tea-growing districts such as Vandiperiyar (Kerala) and Valparai (Tamil Nadu) (Sachin et al., 2008). Infestation accounts for up to 17% of crop loss and sometimes it can able to cause 100% in south India (Srikumar et al., 2017; Suganthi et al., 2018). At present, the population has been started to build up in lower elevation areas of Munnar and Neliyampathi (Kerala). TMB infestation leads to a decline in both the quantity and quality of the tea. Both adults and nymphs suck the plant sap from buds, young leaves, petioles and tender stems of tea plants with the help of proboscis (Santhana Bharathi et al., 2022). The severe infestation leads to curling up, leaf defoliation and might also encourage the growth of secondary fungal pathogen Fusarium sp., which causes dieback disease in tea shoots and results in further loss to the tea plantations (Ekka et al., 2019). The conventional practices have been followed to control TMB as per the instructions by the Tea Board of India through the Plant Protection Code (PPC). The conventional practices lead to various secondary issues such as major pest resurgence, secondary pest outbreak, resistance development, residues in made tea, health hazards to warm-blooded animals, environmental contaminations and increased cost of application (Somnath & Muraleedharan, 2014). Biological control measures are one of the alternate tools to overcome the concern through the integrated pest management (IPM) system (Rachel et al., 2009). Among the various biological controlling agents, entomopathogenic fungi (EPF) are the most effective and promising biocontrol agents over the various insect pests in several agricultural and horticultural crops (Litwin et al., 2020). Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) (Sahayaraj & Borgio Francis, 2010) and Lecanicillium longisporum (Ascomycota: Hypocreomycetidae) (Rachel et al., 2009) are commercially used as a biological control agent against various insect pests. Many previous studies have found that M. anisopliae was a promising biocontrol agent on various hemipteran pests such as Bactericera cockerelli (Hemiptera: Triozidae), Lygus lineolaris (Hemiptera: Miridae), Blissus antillus (Hemiptera: Lygaeidae) and Myzus persicae (Hemiptera: Aphididae) (Lacey et al., 2011; Houping et al., 2002; Samuels et al., 2002; Ullah et al., 2022). Similarly, L. longisporum also reported effectiveness against the following hemipterans viz., Planococcus citri (Hemiptera: Pseudococcidae) and Cinara pini (Hemiptera: Lachnidae) (Ghaffari et al., 2017; Nazemi et al., 2014). Initial EPF infection starts with the adhesion of spores on the insect cuticle and during spore germination, EPF produces lytic enzymes such as proteo-, lipo- and chitinolytic that disintegrate the insect’s body shells to reach the insect body cavity through the penetration process. At this stage, it produces some toxic secondary metabolites which cause paralysis and disrupt the insect’s metabolic process which leads to insect mortality (Ferron, 1978). In this background, the present study was focused on identifying the effective and promising native biocontrol agent, entomopathogenic fungi to control TMB.
Methods
Soil collection and isolation of native entomopathogen
Soil samples (approx. 500 g) were collected randomly at the depth of 15 cm from each field during the South-West monsoon season (June–July 2020) in Aanaimalais, Coimbatore, Tamil Nadu using sterile plastic bags. The EPF was isolated from the soil samples using the insect bait method (Zimmermann, 1986) with a small modification. Corcyra cephalonica larvae were used in the insect bait method instead of Galleria mellonella. C. cephalonica larval mortality was observed after the 5th day of the inoculation and continuously monitored every 24 h until the 15th day. Dead larvae were carefully examined as per the standard protocols. Larval cadavers were initially washed with sterilized distilled water to remove surface soil then the surface was sterilized with 0.1% mercuric chloride followed by three times sterilized distilled water wash. Washed larvae were incubated in a potato dextrose agar (PDA) plate (9.5 cm diameter) at 27 ± 1 °C with a 12L:12D period for 7 days. After 7 days, the selected colonies were subcultured to isolate the native entomopathogens.
Identification of native isolated entomopathogens through 18s rRNA sequencing
The EXpure Microbial DNA isolation kit developed by Bogar Bio Bee stores Pvt Ltd was used for the DNA isolation from the well-grown fungal colonies. The isolated DNA was quantified using Qubit 3.0 Fluorometer (Thermo Fisher Scientific, United States). Added 5 μL of isolated DNA in 25 μL of PCR reaction solution containing, each 1.5 μL of forward and reverse primers, 5 μL of nucleic acid-free water, and 12 μL of Taq Master Mix followed by performed the PCR. The PCR was carried out in the following condition: initial denaturation at 95 °C for 2 min, 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min followed by the final extension at 72 °C for 10 min. The final PCR product was purified using Montage PCR Clean up kit (Millipore) to remove the unincorporated PCR primers and dNTPs from PCR products. The purified PCR product was sequenced using the ITS1 (5′ TCCGTAGGTGAACCTGCGG 3′) and ITS4 (5′ TCCTCCGCTTATTGATATGC 3′) primers. Sequencing reactions were performed using an ABI PRISM® BigDyeTM Terminator Cycle Sequencing Kits with AmpliTaq® DNA polymerase (FS enzyme) (Applied Biosystems). The sequence was subjected to the NCBI blast similarity searching tool for the phylogeny analysis with the closely related sequence. The multiple alignments of sequences were performed by MUSCLE 3.7 (Edgar, 2004) and the phylogeny analysis was carried out in PhyML 3.0 aLRT using HKY85 as a substitution model followed by the phylogenetic tree rendering was completed through Tree Dyn 198.3 (Dereeper et al., 2008).
Preparation of spore suspension
Identified fungal cultures were continuously reared on PDA plates at 27 °C and stored on PDA slants at 4 °C for further studies. The spore suspensions were prepared using 15 days old fungal cultures from the PDA plates. Fungal spore mate was scraped from the PDA plate with the help of a sterile glass slide and the plate was washed thrice using sterile distilled water which contains 0.05% of Tween 80 as a surfactant. Washed fungal spores were kept in a shaker for an hour for a uniform distribution, after that suspension was filtered through sterile double layered muslin cloth to remove the solid debris. Three different spore concentrations of 1 × 105, 1 × 107 and 1 × 109 spores/mL were fixed using a new improved hemocytometer.
Laboratory bioassays
The bioassays were conducted using direct spray and dipping methods to identify the pathogenic potential of isolated native entomopathogens on adult TMB under laboratory conditions. In the direct spray method, the prepared spore suspensions were directly sprayed on the tea shoots (three-leaf and bud) and then transferred to the experimental container (38 cm height × 15 cm width) which contains experimental adults. New shoots were added at the interval of alternate days with initially sprayed tea shoots for 5 days.
In the dipping method, each adult insect was socked in a spore suspension of isolated native entomopathogens for 5 s and then released into the experimental container which contains tea shoots (three-leaf and bud) as a feeding source and tea shoots were changed on alternative days. The mortality was observed on the 5th, 7th and 10th days after the application. All insect cadavers were surface sterilized with 0.1% mercuric chloride and kept on the sterilized moisture tissue paper in a sterilized Petri plate for the conformation of fungal growth over the insect cadaver. Fungal growth was conformed using WESWOX Optic BXL CCTV Microscope. Each treatment contains 20 numbers of the mixed adult population with five replicates and the bioassays were performed at 25 ± 1 °C and 75 ± 5% RH (12D:12L).
Statistical analysis
The data were subjected to one way ANOVA and means were compared by Duncan multiple range test (DMRT). Probit analysis was performed to identify the LC50 and LC90 values of the respective fungal species. All statistical analysis was done using SPSS V16.0 software.
Results
In the present study, a total of 43 soil samples were collected from UPASI experimental farm and Sirukundra Estate, Valparai, Tamil Nadu. Among 43 samples, four soil samples (F. Nos. 65, 39, 24 and 27) were harboured entomopathogenic fungi. The isolated strains from F. No. 65 (10° 15′ 56.0″ N 76° 57′ 59.8″ E), Field No. 39 (10° 18′ 53.9″ N 76° 57′ 26.4″ E), Field No. 24 (10° 17′ 41.6″ N 76° 57′ 27.3″ E) and Field No.27 (10° 17′ 57.6″ N 76° 57′ 51.9″ E) were identified as Metarhizium anisopliae (UPASI-1), Lecanicillium longisporum (UPASI-2), Metarhizium anisopliae (UPASI-3) and Metarhizium anisopliae (UPASI-4) respectively using 18S rRNA sequencing. The sequences were submitted to NCBI GenBank and obtained the accession numbers of MZ930378 (M. anisopliae isolate UPASI_1), MZ930384 (L. longisporum isolate UPASI_2), MZ930388 (M. anisopliae isolate UPASI_3) and MZ930389 (M. anisopliae isolate UPASI_4). The constricted phylogenic trees are given in Figs. 1, 2, 3 and 4.
During the isolation process, M. anisopliae (MZ930388) from F. No. 24 has more virulent than other isolates. It took 3 and 8 days to kill C. cephalonica and spore production respectively. Meanwhile, other isolates such as M. anisopliae (MZ930378), L. longisporum (MZ930384) and M. anisopliae (MZ930389) took on an average of 8 and 13 days to kill the host and spore production, respectively (Figs. 5, 6).
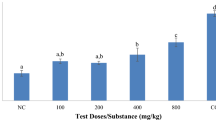
The overall in vitro bioassay showed that the isolated EPF were able to produce 69 to 81% and 76 to 100% of adult mortality in the direct spray method and dipping method, respectively on the 10th day after the application (Fig. 9). The tested treatments such as 1 × 105, 1 × 107 and 1 × 109 spores/mL were produced highly significant (df = 2, f = 1.54, p < 0.0001) adult mortality in both bioassay methods (df = 1, F = 3.64, p < 0.0001). Similarly, significant mortality was observed after the application viz., on the 5th day, on the 7th day and 10th day (df = 2, f = 452.61, p < 0.0001). The mycelium growth and spore production were noted on the 7th and 10th day respectively for M. anisopliae over adult TMB. Well-grown spores were observed on TMB after 15 and 21 days for M. anisopliae and L. longisporum, respectively (Figs. 7, 8).
In the dipping method, all four isolates produced significant mortality (p < 0.05) while spraying with 1 × 107 and 1 × 105 spores/mL. However, UPASI-1, UPASI-3 and UPASI-4 were achieved on par mortality within them at the concentration of 1 × 109 spores/mL on the 10th day of the after application. All isolated M. anisopliae achieved 100% mortality in 1 × 109 spores/mL. However, UPASI-3, UPASI-4 and UPASI-1 isolates were acquired 5, 7 and 10 days respectively to cause 100% mortality. In addition, the other two concentrations such as 1 × 107 and 1 × 105 spores/mL also produced notable mortality with 98 and 90% respectively on the 10th day. Among the all tested isolates, UPASI-3 produced 77 to 100% mortality of TMB on the 5th day. On the other hand, UPASI-2 strain was achieved at about 76% of mortality even on the 10th day. When compared with other strains, UPASI-2 has produced significantly less mortality (p > 0.05) than others in all tested concentrations (Fig. 9).
Mortality percentage (means ± SD) of four isolated entomopathogens on adult TMB at the concentrations of 1 × 105 (A), 1 × 107 (B) & 1 × 109 (C) spores/mL by dipping method and the concentrations of 1 × 105 (D), 1 × 107 (E) and 1 × 109 (F) spores/mL by direct spray method. Treatments followed by the same letter(s) in day(s) after spray (5th, 7th and 10th) is(are) not significant at 5% level by DMRT
In the direct spray method, a maximum of 81% of adult mortality was observed in UPASI-3 isolate with a spore concentration of 1 × 109 spores/ml on the 10 day (Fig. 9). Similarly, it achieves about 76 and 65% of adult mortality while spraying with 1 × 107 and 1 × 105 spores/mL respectively. The other isolates such as UPASI-1, UPASI-2 and UPASI-4 were produced on par adult mortality within them on the 10th day while spraying 1 × 109 spores/mL. UPASI-2 isolate produced very low mortality viz., 12 and 35% in 1 × 105 and 1 × 107 spores/mL respectively. It failed to achieve prominent results in the direct spray method. Probit analysis showed that M. anisopliae (UPASI-3) isolate requires 1 × 105 and 1 × 109 spores/ml to produce 90% of adult mortality in dipping and direct spray method respectively on the 10th day after the application (Table 1).
Discussion
During the study, three M. anisopliae and one L. longisporum were identified from the soil ecosystem of tea plantations. Tropical and subtropical regions of tea plantations provide excellent moisture weather conditions and an undisturbed soil ecosystem which plays a major role in the diversity of the entomopathogenic fungi in the tea soil ecosystem (Debnath, 1996; Meyling & Eilenberg, 2006; Ye et al., 2013). In addition, temperature (Inglis et al., 2001), soil texture and pH values are also playing a vital role in privileging the fungal diversity in the soil ecosystem (Groden & Lockwood, 1991).
The in vitro studies show that a significant pathogenic property (90–100%) was noted with native M. anisopliae in the dipping method. Moreover, the adhesion of fungal spores is highly facilitated in the dipping method than in the direct spray method. This indicates that the adhesion of EPF spores over TMB is very high in the dipping process and adhesion spores will germinate, penetrate to the haemocoel then rapidly cause insect mortality (Ghaffari et al., 2017; Aw & Hue , 2017). Kumhar et al. (2020) reported that the mortality was significantly dependent on spore concentration. A similar observation was made in the present study too. High adult mortality (81%) was observed in high spore concentration (1 × 109 spores/mL).
During the study, the native EPF produced a promising control over TMB. The isolate, M. anisopliae (MZ930388) has achieved a maximum of 81% of adult mortality in the direct spray method. Native soil isolates could be able to adopt the biotic and abiotic factors of the native environment. This might be a reason for the promising mortality of EPF over the various pests. Babu and Kumhar (2014) reported that the native entomopathogen, Beaveria bassiana was highly efficient against TMB and they also reported that a biological organism from the same ecosystem is more effective than commercial formulations. Similarly, Ekka et al. (2019) also found that the native soil isolate of B. bassiana (BPA/B7) from Tinsukia (Assam) has produced 90% of mortality over TMB within 6 days of treatment at the concentration of 10 ml/L under laboratory conditions. Likewise, Kumar and Chaudhary (2021) too found that the native M. anisopliae caused 83.57% of mortality on Helicoverpa armigera under laboratory conditions and it found that highly virulent than other isolated EPFs such as B. bassiana, Lecanicillium lecanii and Nomuraea rileyi. Apart from the adult mortality, Navik et al. (2015) reported that M. anisopliae is also capable to cause 66% of nymphal mortality on Helopeltis antonii Signoret after the 10th day of the application in cashew plantation.
In this study, L. longisporum almost achieves on par mortality of M. anisopliae at higher concentration in the direct spray method and contrast, it achieves low mortality in the dipping method while all M. anisopliae achieves 100% mortality. L. longisporum performed very well over various pests such as Macrosiphum euphorbiae (Alavo et al., 2002), Aphis craccivora (El-Salam & El-Hawary, 2011), Planococcus citri (Ghaffari et al., 2017), Trialeurodes vaporariorum (Fazeli-Dinan et al., 2016). In addition, Nazemi et al. (2014) have found that L. longisporum produced 90% of adult mortality on the aphid, Cinara pini (Hemiptera: Lachnidae) while spraying the concentration of 108 spores/ml after the 7th day of the application under laboratory conditions. Besides the M. anisopliae and L. longisporum, many fungi viz., Fusarium sp., Aspergillus flavus, Aspergillus niger, Cladosporium sp., Curvularia sp., Acremonium, Trichoderma (Bordoloi et al., 2012), Beauveria bassiana (Deka et al., 2021; Kishor et al., 2020) were reported as capable of causing mortality on adult TMB.
Conclusions
From the above study, M. anisopliae isolates produced 81–100% of adult mortality under in vitro conditions. However, field evaluations of these indigenously isolated promising fungal strains against these target insect pests and their natural enemies constitute the future perspectives of this work.
Availability of data and materials
All generated data for the current study are presented in this research article and the corresponding author has no objection to the availability of data.
Abbreviations
- UPASI:
-
United Planters’ Association of Southern India
- TRF:
-
Tea Research Foundation
- PPC:
-
Plant Protection Code
- EPF:
-
Entomopathogenic Fungi
- NCBI:
-
National Center for Biotechnology Information
- F. Nos.:
-
Field Numbers
- F. No.:
-
Field Number
- TMB:
-
Tea Mosquito Bug
References
Alavo, T., Sermann, H., & Bochow, H. (2002). Virulence of strains of the entomopathogenic fungus Verticillium lecanii to aphids: Strain improvement. Archives of Phytopathology and Plant Protection, 34, 379–398.
Aw, K. M. S., & Hue, S. M. (2017). Mode of infection of Metarhizium spp. fungus and their potential as biological control agents. Journal of Fungi, 3, 30. https://doi.org/10.3390/jof3020030
Babu, A., & Kumhar, K. C. (2014). Pathogenicity and compatibility of native isolate of Beauveria bassiana (BKN-2 0): Biological agent for control of tea mosquito, Helopeltis Theivora. Tocklai News, 21, 3.
Bordoloi, M., Madhab, M., Dutta, P., Borah, T., Nair, S. C., Phukan, I., Debnath, S., & Borthakur, B. K. (2012). Potential of entomopathogenic fungi for the management of Helopeltis theivora (Waterhouse). Two Bud, 59(1), 24–26.
Debnath, S. (1996). Fungi on tea pests. Two and a Bud, 43(1), 46.
Deka, B., Babu, A., Peter, A. J., Pandey, A. K., Kumhar, K. C., Sarkar, S., Rajbongshi, K., Dey, P., Amalraj, E. L. D., & Talluri, V. R. (2021). Beauveria bassiana: As a potential microbial biocontrol agent for tea mosquito bug, Helopeltis theivora Waterhouse (Hemiptera: Miridae) in Dooars and Darjeeling, India. Egyptian Journal of Biological Pest Control, 31, 134. https://doi.org/10.1186/s41938-021-00478-3
Dereeper, A., Guignon, V., Blanc, G., Audic, S., Buffet, S., Chevenet, F., Dufayard, J. F., Guindon, S., Lefort, V., Lescot, M., Claverie, J. M., & Gascuel, O. (2008). Phylogeny.fr: Robust phylogeneticanalysis for the non-specialist. Nucleic Acids Research, 1, 36.
Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5), 1792–1797.
Ekka, P., Babu, A., & Saikia, L. R. (2019). Potential of new strain of Beauveria bassiana isolated from Tinsukia (Assam) against tea mosquito bug Helopeltis theivora Waterhouse (Heteroptera: Miridae). Jbiopest, 12(1), 104–108.
El-Salam, A., & El-Hawary, F. M. A. (2011). Lethal and pathogenic effects of Beauveria bassiana and Lecanicillium lecanii on the adult and nymph of Aphis craccivora Koch. Archives of Phytopathology and Plant Protection, 44, 57–66.
Fazeli-Dinan, M., Talaei-Hassanloui, R., & Goettel, M. (2016). Virulence of the entomopathogenic fungus Lecanicillium longisporum against the greenhouse whitefly, Trialeurodes vaporariorum and its parasitoid Encarsia formosa. Int. J. Pest Manage, 62, 251–260.
Ferron, P. (1978). Biological control of insect pests by entomopathogenous fungi. Annual Review of Entomology, 23, 409–442.
Ghaffari, S., Karimi, J., Kamali, S., & Mehdikhani, E. (2017). Biocontrol of Planococcus citri (Hemiptera: Pseudococcidae) by Lecanicillium longisporum and Lecanicillium lecanii under laboratory and greenhouse conditions. Journal of Asia-Pacific Entomology, 20, 605–612. https://doi.org/10.1016/j.aspen.2017.03.019
Groden, E., & Lockwood, J. L. (1991). Effects of soil fungistasis on Beauveria bassiana and its relationship to disease incidence in the colorado potato beetle, Leptinotarsa decemlineata, in Michigan and Rhode Island soils. Journal of Invertebrate Pathology, 57, 7–16.
Houping, L., Margaret, S., Parker, B. L., & Michael, B. (2002). Pathogenicity of Beauveria bassiana, Metarhizium anisopliae (Deuteromycotina: Hyphomycetes), and other Entomopathogenic Fungi Against Lygus lineolaris (Hemiptera: Miridae). Journal of Economic Entomology, 95(4), 675–681. https://doi.org/10.1603/0022-0493-95.4.675.
Inglis, G. D., Goettel, M. S., Butt, T. M., & Strasser, H. (2001). Use of hyphomycetous fungi for managing insect pests. In T. M. Butt, C. Jackson, & N. Magan (Eds.), Fungi as biocontrol agents: Progress, problems and potential. CABI Publishing. https://doi.org/10.1079/9780851993560.0023
Kumar, M., & Chaudhary, V. (2021). Isolation and characterization of native entomopathogenic fungi from Uttar Pradesh. Indian Journal of Entomology, 83(3), 468–470.
Kumhar, K. C., Babu, A., Arulmarianathan, J. P., Deka, B., Bordoloi, M., Rajbongshi, H., & Dey, P. (2020). Role of beneficial fungi in managing diseases and insect pests of tea plantation. Egyptian Journal of Biological Pest Control, 30(78), 1–9. https://doi.org/10.1186/s41938-020-00270-9
Lacey, L. A., & X-Liu T, Buchman J L, Munyaneza J E, Goolsby J A, Horton D R,. (2011). Entomopathogenic fungi (Hypocreales) for control of potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae) in an area endemic for zebra chip disease of potato. Biological Control, 56, 271–278. https://doi.org/10.1016/j.biocontrol.2010.11.012
Litwin, A., Nowak, M., & Różalska, S. (2020). Entomopathogenic fungi: Unconventional applications. Reviews in Environmental Science & Biotechnology, 19, 23–42.
Meyling, N. V., & Eilenberg, J. (2006). Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agriculture, Ecosystems & Environment, 113, 336–341.
Navik, O. S., Godase, S. K., Turkhade, P. D., & Narangalkar, A. L. (2015). Evaluations of entomopathogenic fungi and botanicals against tea mosquito bug Helopeltis antonii Signoret. Current Advances in Agricultural Sciences, 7(2), 203–204.
Nazemi, A. H., Moravvej, G., Karimi, J., & Talaei-Hassanlouei, R. (2014). Pathogenicity of Lecanicillium longisporum (Ascomycota: Hypocreomycetidae) on the aphid Cinara pini (Hemiptera: Lachnidae) in laboratory conditions. Journal of Crop Protection, 3(2), 159–171.
Rachel, E. D., Cuthbertson, A. G. S., Mathers, J. J., & Walters, K. F. A. (2009). Dissemination of the entomopathogenic fungi, Lecanicillium longisporum and L. muscarium, by the predatory bug, Orius laevigatus, to provide concurrent control of Myzus persicae, Frankliniella occidentalis and Bemisia tabaci. Biological Control, 50, 172–178.
Sachin, P. J., Selvasundaram, R., Babu, A., & Muraleedharan, N. (2008). Behavioral and electroantennographic responses of the tea mosquito, Helopeltis theivora, to female sex pheromones. Environmental Entomology, 37(6), 1416–1421.
Sahayaraj, K., & Borgio Francis, J. F. (2010). Virulence of entomopathogenic fungus Metarhizium anisopliae (Metsch.) Sorokin on seven insect pests. Indian Journal of Agricultural Research, 44(3), 195–200.
Samuels, R. I., Coracini, D. L. A., Martins dos Santos, C. A., & Gava, C. A. T. (2002). Infection of Blissus antillus (Hemiptera: Lygaeidae) eggs by the Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana. Biological Control, 23, 269–273. https://doi.org/10.1006/bcon.2001.1009
Santhana Bharathi, N., Mahendran, P., Antony, A., & Rabeesh, T. P. (2022). Behavioural response and mass trapping of males of tea mosquito bug Helopeltis theivora Waterhouse. Indian Journal of Entomology. https://doi.org/10.5958/IJE.2022.165
Somnath, R., & Muraleedharan, N. (2014). Microbial management of arthropod pests of tea: Current state and prospects. Applied Microbiology Biotechnology, 98, 5375–5386.
Srikumar, K. K., Smitha, S., Kumar, B. S., & Radhakrishnan, B. (2017). Electro antennographic response of Helopeltis theivora to synthetic pesticides used in tea plantations. Acta Biologica Hungarica, 68(4), 358–367. https://doi.org/10.1556/018.68.2017.4.2
Suganthi, M., Arvinth, S., & Senthilkumar, P. (2018). Defensive responses in Tea (Camellia sinensis) against Tea mosquito bug infestation. Research Journal of Biotechnology, 13(6), 7–11.
Ullah, S., Raza, A. B. M., Alkafafy, M., Sayed, S., Hamid, M. I., Majeed, Z. M., Muhammad, A. R., Gaber, N. M., & Asim, M. (2022). Isolation, identification and virulence of indigenous entomopathogenic fungal strains against the peach-potato aphid, Myzus persicae Sulzer (Hemiptera: Aphididae), and the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Egyptian Journal of Biological Pest Control, 32(2), 1–11. https://doi.org/10.1186/s41938-021-00500-8
Ye, G. Y., Xiao, Q., Chen, M., Chen, X., Yua, Z., Stanley, D., & Hu, C. (2013). Tea: Biological control of insect and mite pests in China. Biological Control, 68, 73–91.
Zimmermann, G. (1986). The Galleria baits method for detection of entomopathogenic fungi in soil. Journal of Applied Entomology, 102, 213–215.
Acknowledgements
The authors are thankful to The Director I/C, UPASI TRF Tea Research Institute for his support and encouragement during the study period. The authors are also thankful to NTRF for their financial support.
Funding
The study was performed as a part of the research project under the funding of the National Tea Research Foundation (NTRF), C/o Tea Board, Kolkata 700001, India (Grant No. 208/2019).
Author information
Authors and Affiliations
Contributions
PM: designed the experiment; NSB: conducted all the experiments and drafted the manuscript; PM, SA and TPR: reviewed the manuscript; KJ and NSB: executed statistical analysis; SA and KJ: performed molecular identification of the isolates. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they do not have a competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bharathi, N.S., Mahendran, P., Sujatha, K. et al. Pathogenic potential of Metarhizium anisopliae and Lecanicillium longisporum on tea mosquito bug, Helopeltis theivora Waterhouse (Hemiptera: Miridae). JoBAZ 83, 33 (2022). https://doi.org/10.1186/s41936-022-00297-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-022-00297-4