Abstract
Background
Deuterium metabolic imaging (DMI) has emerged as a promising non-invasive technique for studying metabolism in vivo. This review aims to summarize the current developments and discuss the futures in DMI technique in vivo.
Methods
A systematic literature review was conducted based on the PRISMA 2020 statement by two authors. Specific technical details and potential applications of DMI in vivo were summarized, including strategies of deuterated metabolites detection, deuterium-labeled tracers and corresponding metabolic pathways in vivo, potential clinical applications, routes of tracer administration, quantitative evaluations of metabolisms, and spatial resolution.
Results
Of the 2,248 articles initially retrieved, 34 were finally included, highlighting 2 strategies for detecting deuterated metabolites: direct and indirect DMI. Various deuterated tracers (e.g., [6,6′-2H2]glucose, [2,2,2′-2H3]acetate) were utilized in DMI to detect and quantify different metabolic pathways such as glycolysis, tricarboxylic acid cycle, and fatty acid oxidation. The quantifications (e.g., lactate level, lactate/glutamine and glutamate ratio) hold promise for diagnosing malignancies and assessing early anti-tumor treatment responses. Tracers can be administered orally, intravenously, or intraperitoneally, either through bolus administration or continuous infusion. For metabolic quantification, both serial time point methods (including kinetic analysis and calculation of area under the curves) and single time point quantifications are viable. However, insufficient spatial resolution remains a major challenge in DMI (e.g., 3.3-mL spatial resolution with 10-min acquisition at 3 T).
Conclusions
Enhancing spatial resolution can facilitate the clinical translation of DMI. Furthermore, optimizing tracer synthesis, administration protocols, and quantification methodologies will further enhance their clinical applicability.
Relevance statement
Deuterium metabolic imaging, a promising non-invasive technique, is systematically discussed in this review for its current progression, limitations, and future directions in studying in vivo energetic metabolism, displaying a relevant clinical potential.
Key points
• Deuterium metabolic imaging (DMI) shows promise for studying in vivo energetic metabolism.
• This review explores DMI’s current state, limits, and future research directions comprehensively.
• The clinical translation of DMI is mainly impeded by limitations in spatial resolution.
Graphical Abstract

Similar content being viewed by others
Background
Metabolic imaging is a class of non-invasive techniques that enable in vivo visualization and quantification of metabolic processes such as glucose uptake, glycolysis, and tricarboxylic acid (TCA) cycle [1, 12, 14, 15, 23, 25, 54, 59, 68, 75]. It can detect malignant tumors with high sensitivity and specificity by imaging their increased metabolic rates for glucose, amino acids, or lipids; additionally, it offers valuable insights into risk stratification and treatment response evaluation of malignant tumors [1, 12, 14, 15, 23, 25, 54, 59, 68, 75]. So far, many metabolic imaging techniques have emerged, including fluorine-18-fluorodeoxyglucose positron emission tomography, 1H-magnetic resonance spectroscopic imaging (1H-MRSI), chemical shift saturation transfer, hyperpolarized-13C-magnetic resonance imaging (hyperpolarized-13C-MRI), and magnetic resonance (MR)-based deuterium metabolic imaging (DMI) [9, 14, 16, 25, 30, 36, 54, 58, 75].
Fluorine-18-fluorodeoxyglucose positron emission tomography is widely used in oncology for tumor staging, prognostic prediction, and treatment response evaluation [1, 5, 9, 33, 78]. However, it is limited by radiation exposure (about 25 mSv for each examination) and incapability in monitoring downstream metabolism except for glucose intake [6, 33]. On the other hand, MRI and MRS, such as 1H-MRS/MRSI and chemical shift saturation transfer, can monitor metabolism in vivo without exposing patients to ionizing radiation [30, 36, 54]. However, these techniques can only be applied to observe the steady-state metabolism with complicated peak overlaps between different molecules (e.g., water, lipids) [54, 70]. To address these issues, a hyperpolarized MRI technique has been developed in the past decade [14, 23, 25, 59, 68, 75]. By utilizing the dynamic nuclear polarization technique, the MR signal of certain nuclei, such as 13C, can be enhanced for more than 10,000 folds [26, 51]. Hyperpolarized MRI enables the visualization of metabolic pathways in real-time following the administration of exogenous hyperpolarized-13C-labeled tracers (e.g., hyperpolarized-13C-pyruvate, hyperpolarized-13C-fumarate) [23, 26, 59, 75]. However, the clinical translation of hyperpolarized MRI is slow due to the short-term enhanced signal (1–2 min) and the high cost of the equipment.
DMI is a relatively new MR-based imaging technique that utilizes exogenous non-radioactive and biocompatible deuterium-labeled metabolic tracers such as [6,6′-2H2]glucose and [2H3]acetate to visualize different metabolic pathways in vivo [15, 16, 58]. Compared to the aforementioned metabolic imaging techniques, DMI offers several distinct advantages including non-ionizing radiation, stable isotope labeling, biochemical safety, and relatively simple techniques [15, 16, 44, 58]. Compared with hyperpolarized-13C-MRI, DMI can be applied to observe a longer-term metabolic process (more than hours) [16]. Although deuterium has a lower gyromagnetic ratio than the 1H proton (6.536 MHz/T versus 42.577 MHz/T), it has a rapid longitudinal relaxation that enhances its detection [16, 81]. Unlike 1H-MRSI, DMI does not require additional water suppression due to the low abundance of natural deuterated water (0.0115%, 10.12 mM), leading to a low specific absorption rate of radiofrequency power [15, 16, 44]. Moreover, because of the reduced coupling effects of deuterium, the spectrum in DMI is much simpler to interpret than that of 1H that has complex peak-splitting patterns [15, 16]. The combination of the low natural abundance of deuterium and the selective deuterium-labeling of metabolites by the tracer contribute to the further simplification of the spectrum in DMI. So far, many outstanding reviews or comments have been published on the applications of DMI [12, 16, 53, 58, 67, 76, 79, 82]. To avoid repetition, this systematic review aims to summarize the current development of the DMI technique in vivo, outline present limitations, and discuss the potential research and development directions for the future.
Methods
Strategy of literature review
A systematic literature review was performed to summarize the deuterium metabolic MRI or spectroscopy in vivo based on the PRISMA 2020 statement [55]. The PubMed database was used for the MeSH term search for previously published studies on deuterium metabolic MR imaging or spectroscopy from 1 January 2003 to 13 May 2023 (encompass earlier works that laid the foundation for subsequent advancements). The search strategy is described in Additional file 1: Table S1. A reference check was also performed. The inclusion criteria include the following: (1) original articles published with specific technique descriptions of MR imaging or spectroscopy involving deuterium detection to explore the metabolic processes in vivo and (2) studies were performed in living humans or animals, excluding studies performed on cell lines or isolated samples from humans or animals. Studies were selected for inclusion by two authors, who have 15 years of experience in diagnostic radiology and 15 years of experience in MR experiments. Decisions were made by consensus among all authors.
Data extraction and summarization
After conducting the literature review, a comprehensive extraction of specific technical details (including study subjects, examined body parts, target diseases/specific physiological conditions, deuterated tracers, administration dose/route, and MR equipment/scanning parameters) was performed from the relevant articles. Additionally, potential applications, such as examination purposes and metabolic biomarkers, were collected. Two authors (FP and PS) performed the data extraction from all included studies. Subsequently, several key topics were summarized, including strategies of deuterated metabolites detection, deuterium-labeled tracers and corresponding metabolic pathways in vivo, potential clinical applications, routes of tracer administration, quantitative evaluations of metabolisms, and spatial resolution.
Results
The initial literature search resulted in a total of 2,248 articles. Following the screening process, 2,217 articles were excluded based on the inclusion criteria. Additionally, 3 articles were identified through citation searching. Finally, 34 published articles were involved (Table 1). The details of the database retrieval are shown in Fig. 1.
Strategies of deuterated metabolites detection: direct and indirect DMI
From the included articles (Table 1), detecting deuterium-labeled metabolites by DMI typically involves 2 strategies: direct (30 studies, including 25 animal studies and 6 human studies, with 1 study conducted in both animals and humans) and indirect (4 studies, including 3 human studies and 1 animal study) DMI. In the former strategy, 2H-MRS/MRSI sequences were applied to selectively excite the deuterium nuclei and specifically capture the deuterium-labeled metabolite signals over time (Figs. 2 and 3) [15, 62, 65, 73, 81]. In the latter strategy, the deuterium-labeled metabolites were not excited but detected indirectly through the corresponding 1H-MR signal decrease, due to the chemical exchange between hydrogen and deuterium atoms following the administration of deuterium-labeled tracers (Fig. 4) [3, 10, 52, 60].
A representative 2H-MRS obtained from the liquid cultured medium for a tumor cell line (MC38 murine colon adenocarcinoma) after being exposed to [6,6′-2H2]glucose at different time points (a). Over time, the 2H-labeled glucose level gradually decreased but the 2H-labeled lactate gradually increased indicating a typical Warburg effect of the malignant cell line (b). Figures were provided by author QB. All experiment was performed in Bruker 500-MHz MR scanner
A representative deuterium metabolic imaging visualizing the Warburg effect in an MC38 tumor inoculated C57BL/6 male mouse after a bolus intravenous injection of [6-6′-2H2]glucose (1.0 g/kg mouse weight). Over time, the 2H-labeled glucose level gradually decreased, but the 2H-labeled lactate gradually increased in the tumor region, indicating a typical Warburg effect of the malignancy. Figures were provided by author QB. All experiment was performed in Bruker 9.4-T MR scanner
An exemplary 1H-MR difference spectrum from a voxel (3 mm × 3 mm × 3 mm) of MC38 tumor in the C57BL/6 male mouse thigh with an illustration of the dynamic change of lactate signals after an intravenous infusion of [6,6′-2H2]glucose (1.0 g/kg mouse weight) (a). Over time, the signal difference of lactate gradually increased with a gradual decrease of the lactate signal intensity (SI) ratio after/before tracer injection (b). Figures were provided by author QB. All experiment was performed in 9.4-T MR scanner
Deuterium-labeled tracers and corresponding metabolic pathways in vivo
Deuterated tracers used in DMI are always the metabolites that are part of the cellular regular metabolism pathways [34]. They contain deuterium atoms and can be metabolized like their non-labeled counterparts. When administered, these tracers can be incorporated into the corresponding metabolic pathways (e.g., glycolysis, TCA cycle, fatty acid oxidation) and metabolized into downstream deuterated compounds which can be detected and quantified by MRS/MRSI (Fig. 5) [34]. Although a high-dose deuterium intake is harmful, the tracer doses widely employed in DMI, especially within the range of 0.6–0.8 g/kg [6,6′-2H2]glucose, have been extensively demonstrated to be non-toxic in vivo through previous studies [3, 10, 15, 18, 32, 43, 52, 62, 65].
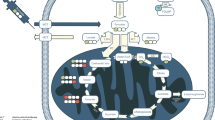
An overview of cellular metabolic key pathways detected by deuterium metabolic imaging: glycolysis, TCA cycle, fatty acid oxidation, deoxyribonucleic acid synthesis, and Kennedy pathway. ATP Adenosine triphosphate, CO2 Carbon dioxide, GLUT Glucose transporter, Glx Glutamine and glutamate, HDO Semiheavy water, TCA Tricarboxylic acid
Most tracers were produced from the deuteration of protonated metabolites that are associated with energy metabolism or cellular proliferation, including glucose, pyruvate, acetate, fumarate, and choline [2, 3, 10, 15, 17, 18, 24, 28, 29, 31, 32, 37, 41, 43, 44, 46,47,48, 50, 52, 56, 60,61,62, 65, 66, 69, 72,73,74, 81, 83]. For instance, [6,6′-2H2]glucose, as a mostly used metabolic tracer, can be used to detect downstream deuterated products, including glutamate, glutamine, lactate, and semiheavy water (Fig. 5) [2, 3, 10, 15, 17, 18, 24, 28, 29, 31, 32, 37, 41, 43, 44, 46,47,48, 50, 52, 56, 60,61,62, 65, 66, 69, 72,73,74, 81, 83]. Among these downstream metabolites, glutamate and glutamine (Glx) can be regarded as products from TCA cycle and lactate from glycolysis [2, 3, 10, 15, 17, 18, 24, 28, 29, 31, 32, 37, 41, 43, 44, 46,47,48, 50, 52, 56, 60,61,62, 65, 66, 69, 72,73,74, 81, 83]. By measuring the incorporation of deuterium into these products, the rates of glycolysis and TCA cycle can be estimated [2, 3, 10, 15, 17, 18, 24, 28, 29, 31, 32, 37, 41, 43, 44, 46,47,48, 50, 52, 56, 60,61,62, 65, 66, 69, 72,73,74, 81, 83]. However, the low deuterium enrichment of [6,6′-2H2]glucose restricts its signal intensity [15]. Therefore, scientists are interested in developing novel glucose tracers with more deuterium atoms within the molecules, such as [2,3,4,6,6′-2H5]-d-glucose and [2H7]glucose [15, 45, 46, 83]. These tracers may have some advantages over [6,6′-2H2]glucose, such as higher signal intensities and enhanced tolerability to label loss [15, 45, 46, 83].
As examples for other tracers, fatty acid oxidation involves the breakdown of fatty acids to generate acetyl-coenzyme A, which can enter the TCA cycle for further metabolism; so, by administering a deuterium-labeled fatty acid [2,2,2′-2H3]acetate, the fatty acid oxidation can be traced by the products of Glx (Fig. 5) [15, 60, 74]. Similarly, [2,3-2H2]fumarate, a precursor of malate, and [U-2H]pyruvate, a precursor of lactate, can be specifically used to measure the rates of TCA cycle and glycolysis, respectively (Fig. 5) [2, 28, 29].
In addition, some essential materials for cell proliferation can also be deuterated as tracers in DMI, such as [2H9]choline and heavy water (Fig. 5) [8, 31, 72]. Choline is a precursor for phosphatidylcholine, a major component of cell membranes produced in the Kennedy pathway. By using [2H9]choline as a tracer, DMI can image and quantify the total pool of choline in vivo, which can reflect the cellular proliferation activity [31, 72]. Exogenous heavy water administrated into the body water pool can also be used as a tracer, which can be incorporated into various macromolecules such as lipids, proteins, and deoxyribonucleic acid [7, 8, 20, 42]. However, most synthesized molecules such as lipids and proteins will gradually lose deuterium labels over time owing to the energy metabolism except de novo deoxyribonucleic acid; thus, measuring long-term deuterium enrichments can help to quantify the de novo deoxyribonucleic acid which can serve as a proxy indicator for cell proliferation [7, 8, 20].
Moreover, another tracer type is the non-metabolizable analog of metabolic precursors. For example, deuterated 3-O-methylglucose is a glucose analog that is taken up by cells but not metabolized further, thus specifically reflecting the glucose uptake [27].
Potential clinical applications
As a novel and still evolving technique, most in vivo DMI studies have been performed in animal brains and gliomas because of relatively static organs, and the results from these studies have shown great potential for a range of clinical applications (Table 2) [2, 15, 27, 31, 37, 41, 47, 56, 60, 66, 69, 72, 81, 83]. The most common application of DMI is to diagnose malignant tumors by detecting abnormal metabolic changes in the material requirement, glycolysis, and TCA cycle [2, 15, 27, 37, 41, 47, 56, 60, 66, 69, 72, 81, 83]. Because most malignancies have increased demands for proliferation, an elevated consumption or uptake of different materials (including glucose and choline) can be detected by DMI following administrating tracers [71]. Moreover, compared to normal tissue, malignant tumors present a so-called Warburg effect, presenting significantly increased glycolysis (elevated lactate) and reduced TCA cycle activity (decreased Glx) [35, 71]. This metabolism alteration can be specifically detected after applying various deuterated tracers (including [6,6′-2H2]glucose, [U-2H]pyruvate, and [2,3-2H2]fumarate) in DMI, which demonstrates higher lactate levels, lower Glx synthesis/flux rates, higher lactate/Glx ratios, or lower fumarate/malate conversion rate [2, 15, 41, 60, 66, 69, 83]. Specifically, the biomarker “lactate/Glx ratio,” as a rational indicator of the Warburg effect, is considered sensitive to detect solid malignancies located in organs with high-glucose intakes and consumptions, such as the brains and kidneys [11, 15, 37, 41, 57, 83]. In these organs, where positron emission tomography is limited due to the possible similarity in glucose uptake between tumors and background tissues, the lactate/Glx ratio serves as a valuable alternative for assessing metabolic abnormalities associated with malignancies.
DMI has also presented great potential in estimating residual tumors and early response after various anticancer treatments (e.g., chemotherapy, targeted therapy, radiotherapy) (Table 2) [2, 15, 24, 28, 37, 69]. From previous studies, DMI presented a higher sensitivity in identifying tumoral necrosis and residue than conventional imaging techniques that mainly rely on morphological changes [2, 15, 24, 28, 37, 69]. A necrotized tumor after treatments first demonstrated a significantly declined Warburg effect, accompanied by a recovered TCA cycle activity and suppressed glycolysis, before the reduction of glucose intake and following morphological necrosis [15, 24, 37, 69]. Thus, DMI can also be applied in the early treatment-response evaluation with a potentially higher sensitivity than conventional methods; for example, previous studies revealed that early tumoral necrosis presented a significantly increased fumarate/malate conversion rate (recovered TCA cycle activity) after administrating [2,3-2H2]fumarate in DMI [28, 75].
Moreover, some animal studies found that DMI can also reflect various metabolic abnormalities under other diseases or physiological conditions, such as chronic graft-versus-host disease after hematopoietic stem cell transplantation, preeclampsia, ischemic stroke, cold acclimation, deep anesthesia, and analgesia condition (Table 2) [8, 44, 48, 50, 61]. Although these experiments were performed on animals, they can also be extended to human beings [8, 44, 48, 50, 61].
Routes of tracer administration: oral, intravenous bolus/infusion, and intraperitoneal
In DMI, deuterated tracers can be administered in several ways (Table 3): oral intake, intravenous (IV) bolus injection, IV infusion, intraperitoneal (IP) bolus injection, IP infusion, applying a bolus variable infusion protocol, and IV bolus injection followed by oral intake.
Oral administration is the only attempted administration route in humans so far, but it may result in delayed or variable tracer absorption, depending on a range of varying factors (e.g., food intake, gastric pH), leading to unstable kinetics [63]. In contrast, IV administration allows for more precise control of the dose and timing of the tracer than oral intake, ensuring uniform distributions of the tracer throughout the body and benefiting a more precise kinetic quantification [63]. Nevertheless, a rapid IV bolus administration might affect the physiology of the body (e.g., changes in blood pressure, heart rate) and alter the intracellular metabolic states in some cases by competing receptors, transporters, and enzymes [31, 72, 77]. Instead, an IV infusion protocol reduces these adverse effects and is particularly useful when studying steady-state metabolism [15, 17]. Still, this infusion protocol has some issues that need to be considered, one of which is the relatively longer time required to reach a real steady-state concentration of the tracer [31, 49, 60]. To achieve a steady state faster, a bolus variable infusion protocol was developed involving a bolus injection of the tracer, followed by a slow but constant infusion [31, 49, 60]. This method may improve the stability and linearity of metabolic quantification during a more than 1-h imaging period [31, 49, 60]. Yet, the bolus variable infusion protocol has challenges that should be considered, such as optimizing variable infusion parameters (e.g., dose, duration, infusion rate). IP administration is another convenient way when performing animal studies [15, 17, 74, 81]. The technique of IP injection is easier than that of IV administration. It allows for the direct delivery of the tracer to the blood circulation through peritoneal absorption. However, this route is probably useless in humans because of the potential risk of bowel injury. Like IV administration, IP administration can also be implemented via a bolus injection or continuous infusion [15, 17, 74, 81].
Quantitative evaluations of metabolic processes
Before the metabolic quantification, various corrections and normalization procedures should be implemented to obtain accurate molar concentrations of the tracer and metabolites in DMI:
-
1)
Correction for signal variations due to incomplete longitudinal relaxation and RF transmit B1+ magnetic field inhomogeneities (the actual RF flip angles) based on measured T1 relaxation times for various metabolites in vivo (e.g., water 320 ms, glucose 64 ms, lactate 297 ms, Glx 146 ms, at 11.7 T) and quantitative B1+ maps [15, 29, 37, 50]
-
2)
Normalization of metabolite signals by referring to the naturally abundant semiheavy water signal in the body (about 10.12 mM, acquired before administering tracer as an internal reference), which can automatically correct the inhomogeneity of the receive sensitivity (B1−) of the deuterium RF coil [2, 24, 50, 61, 62, 65]
-
3)
Corrections for deuterium-label loss for each metabolite (e.g., 8.1% label loss for 2H-lactate) [15, 50]
In metabolic quantification using DMI, kinetic analysis is most commonly applied. It involves monitoring the blood concentrations of the tracer, the MR signals of the tracers, and the downstream metabolites in each voxel over time, to calculate the flux rate of each target metabolic process in response to different diseases or interventions [37, 44, 47, 48, 66, 83]. So far, IV bolus injection is mostly used in kinetic analysis because it offers a fixed initial concentration of the tracer benefiting the assessment of the flux rate for each metabolite [37, 44, 66]. On the other hand, as discussed above, a bolus injection might introduce neuroendocrine activation and hemodynamic changes, leading to inevitable changes in an individual’s physiological state and metabolic rates [31, 72, 77]. As an alteration, the infusion kinetic analysis reduces the adverse effects caused by tracer administration. It hypothesizes the input rate equals the elimination rate [3, 8, 27, 60, 74]. This method allows for the calculation of stable metabolic rates (e.g., the slope or rate constant) using a relatively simple linear or exponential curve fitting [3, 8, 27, 60, 74].
Besides, there are two other accessible quantification methods: (1) Calculation of the area under the curves of target metabolites over time [24, 28, 29, 61, 69, 73]. A previous study for hyperpolarized-MRI demonstrated the significantly improved reliability of area under the curves-based analysis compared to multiple kinetic analysis, which may shed light as well on the data analysis for DMI [13]. (2) Single time point quantifications [15, 18, 31, 32, 41, 43, 50, 65, 72, 81]. Instead of focusing on dynamic metabolic processes, this method only measured the relative abundance of deuterium-labeled metabolites at a particular time point (commonly after 1 h) [15, 18, 31, 32, 41, 43, 50, 65, 72, 81]. Although it does not provide dynamic information about specific metabolisms, it has been proven as a feasible way to diagnose malignant tumors and evaluate early anti-tumor treatment responses in humans [15]. Compared to kinetic analysis, these two quantification methods have three advantages: simplicity, robustness, and practicability.
Spatial resolution
The clinical transformation of DMI faces one major challenge: an insufficient spatial resolution with a low signal-to-noise ratio (SNR) [18] (Table 4). Despite the administration of exogenous deuterium-labeled tracers, the concentration of deuterium is still low leading to weak signals. This contrasts with hyperpolarized-13C-MRI which provides a higher spatial resolution (0.05 mL versus 0.21 mL) with a shorter acquisition time (6 s versus 60 s) at 4.7 T than DMI [73]. In a study exploring DMI at 3 T, the spatial resolution reached 3.3 mL with an acquisition time of 10 min [32]. If fixing the acquisition time, spatial resolution and SNR can be improved directly by increasing the B0 intensity [18]. A previous study has shown the SNR increased from 169.2 ± 13.3 at 4 T to 423.1 ± 25.7 at 7 T [18]. In animal MR scanners above 9 T, the spatial resolution can reach 0.1 mL with an acquisition time of 5–10 min (Table 4). However, high-field magnet cost and safety issues (e.g., higher radiofrequency energy deposition, dizziness, nausea) limit the clinical translation of DMI [39]. Presently, only 7-T MRI equipment is validated for DMI in clinics, offering a spatial resolution of approximately 1 mL; conversely, this comes with the trade-off of an acquisition time of approximately 30 min (Table 4 and Additional file 1: Table S2) [18]. Although increasing acquisition time is the easiest way to increase spatial resolution by accumulating sufficient signals (Table 4), it introduces the risk of motion artifacts and patient discomfort during the examination. Interpolation and zero-filling are commonly used to enhance spatial resolution, but these enhancements are fake [44, 47].
Another way to address the spatial resolution or SNR issue in DMI is to improve imaging sequences and coils. The implementation of an Ernst-angle approach, considering an assumed or measured in vivo T1, has been shown to enhance signals effectively; for instance, employing a flip angle of 68° for breast cancer in rats with an in vivo measured T1 of 250 ms has demonstrated promising results (Table 4) [3, 8, 27, 62]. Another study proposed a multi-echo balanced steady-state free precession approach to enhance SNR (Table 4) [56]. Compared to conventional chemical shift imaging (repetition time/flip-angle = 95ms/90°), the multi-echo balanced steady-state free precession imaging (echo time 2.2 ms, repetition time 12 ms, flip angle 60°) demonstrated a predicted SNR increase of three to five times with matched spatial resolution and scan time, while maintaining good agreement in the time courses of all metabolites [56]. In a previous study, a dual-tuned array coil was introduced, featuring ten transmitting/receiving channels for 1H and eight transmitting/two receiving channels for 2H, paired with an Ernst-angle three-dimensional chemical shift imaging sequence [62]. This configuration provided a nominal spatial resolution of 0.003 mL with an acquisition time of 10 min and achieved a successful DMI implementation in humans at 7 T (Table 4) [62].
Discussion
In this work, we provide an extensive overview of the current development of the DMI technique. By analyzing 34 published articles, we introduced and summarized specific technical details and potential applications of DMI in vivo. In the following part, we will discuss present limitations, potential research, and development directions of DMI for the future.
So far, direct DMI is still the mainstream technique. While the SNR is constrained by the low gyromagnetic ratio of the deuterium isotope, this limitation is offset by the relatively short T1 of the deuterium-labeled tracer [16, 81]. This characteristic facilitates rapid signal acquisition and allows for a large number of excitations without a significant signal saturation [16, 81]. Compared with direct DMI, indirect DMI has a reported five times higher SNR and can even be performed at commercial 3-T MRI scanners [52, 60]. This eliminates the need for extra spectrometers, specialized deuterium coils, and dedicated MRI sequences. However, from a previous study, the correlation between indirect and direct metabolite quantification was not high (r = 0.62) [60]. It indicates the 1H-MR signal reduction might be affected by several factors, such as tracer administration methods, stress reaction after tracer administration, participant movement (e.g., participants moved in and out of the scanner to drink the tracer), and so on [31, 72, 77]. After all, the basic hypothesis of indirect DMI is the difference in the absolute metabolite concentrations over time at each pixel. If the absolute metabolite concentrations were affected by tracer administration, the signal reduction would not indicate the real deuterium-labeled metabolite concentrations. Thus, we propose exploring methods to maintain intra-environmental stability of metabolism to address these concerns.
In some cases, the deuteration of certain molecules may affect their metabolism, such as cytochrome P450-mediated oxidative metabolism [4, 64]. Especially, it is essential to address safety considerations in the development of analog tracers [27]. After all, intracellular accumulation of the analog might inhibit glycolysis and bring potential toxicity [38, 80]. However, the deuterated tracers reported in Table 5 rarely interrupt the natural metabolism at the indicated doses and can be safely used in vivo [2, 3, 10, 15, 17, 18, 24, 28, 29, 31, 32, 37, 41, 43, 44, 46,47,48, 50, 52, 56, 60,61,62, 65, 66, 69, 72,73,74, 81, 83]. With the help of these different tracers, we can understand the metabolic status of various tissues and organs in living bodies and find the metabolic alterations in various diseases, such as cancer, preeclampsia, and neurological disorders [15, 34, 48, 50]. New development of tracers is still on the way; it is worthwhile to develop tracers on more specific metabolic pathways while prioritizing safety and minimizing label loss.
Overall, DMI holds potential for various medical applications in the future, including cancer diagnosis, early response evaluation after anti-tumor treatment, nutritional studies, and metabolic disease research. As another deduction that merits further exploration, DMI might also help identify the “pseudoprogression” after immune checkpoint inhibitor therapies, characterized by an initial increase followed by a subsequent decrease in the size of existing tumors after treatment [22, 40]. In addition, due to the non-radioactive feature, DMI presents a potential utilization in human fetuses and pregnant women, although definitive evidence is yet to be established; however, the feasibility of DMI has been demonstrated in pregnant mice [48].
There are different administration routes in DMI, but the comparisons between varied routes in vivo are still limited. After all, the different routes of administration may affect the plasma glucose level and the deuterium enrichment of downstream metabolites, which can influence the sensitivity and accuracy of DMI. One previous study explored the discrepancies between IV and IP infusions of [6,6′-2H2]glucose and found that IV infusions caused the glucose signal to rapidly reach a plateau in the liver, while IP infusions showed a continuous rise in the glucose signal, far surpassing the water signal [17]. Another study comparing IV bolus injection and infusion in mice found that while infusion led to a smoother glucose response without an initial spike, minimal differences were observed in downstream lactate metabolite quantification compared to bolus injection [48]. So far, while the emphasis on oral administration for humans has stemmed from its perceived safety, simplicity, and well-tolerance, alternative administration routes have not been extensively explored rather than being deemed inherently infeasible. Therefore, for clinical translation, it is essential to comprehensively compare different tracer administration routes in further investigations.
Although kinetic analysis is the most commonly applied quantification in DMI, it still has several challenges. First, it requires multiple time point measurements, which can be time-consuming and resource-intensive. Second, it requires complex mathematical modeling to calculate kinetic parameters, requiring a sufficiently short acquisition time of DMI. Third, even the infusion kinetic analysis may lead to inaccurate estimation of metabolic rates because the tracer is always compartmentalized within specific tissues or metabolic pathways resulting in a very complex kinetic system [3, 8, 27, 60, 74]. To tackle these issues, quantification methods, including calculation of the area under the curves of target metabolites over time and single time-point quantifications, can be considered [15, 18, 24, 28, 29, 31, 32, 41, 43, 50, 61, 65, 69, 72, 73, 81]. However, these two methods are limited because they may not capture the dynamic information as effectively as kinetic modeling. Overall, future research is likely to focus on addressing the challenges associated with kinetic analysis while exploring alternative quantification methods to enable more accurate and efficient assessment of metabolic processes in clinical scenarios.
To date, the clinical transformation of DMI heavily hinges on addressing the limitation of spatial resolution and SNR. Enhancing deuterium enrichment of tracers (e.g., [2,3,4,6,6′-2H5]glucose and [2H7]glucose) and applying the indirect DMI strategy are both potentially applicable to enhance SNR for DMI, as introduced in previous sections (Table 4 and Additional file 1: Table S2), but these advancements have yet to fully overcome existing challenges [3, 46, 52, 60, 83]. Recently, deep learning techniques have offered a promising avenue for DMI denoising, resulting in improved SNR. In a pioneering study, researchers proposed a novel machine learning-based approach that synergistically integrates physics-based subspace modeling and data-driven deep learning to achieve effective denoising, enabling high-resolution DMI [41]. In addition, a less explored technique in DMI is compressed sensing, which may hold the potential to reduce the acquisition time while preserving data quality by leveraging the inherent sparsity of metabolic signals in a relatively wide spectrum, meriting further investigations [19, 21].
In conclusion, DMI holds promise for improving clinical diagnostics and treatment protocols by offering new insights into metabolic disorders and diseases. Despite significant advancements, limitations in spatial resolution still hinder the clinical translation of DMI techniques. Additionally, to unlock the full clinical potential of DMI, it is essential to optimize tracer synthesis, administration protocols, and quantitative analysis methodologies.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- DMI:
-
Deuterium metabolic imaging
- Glx:
-
Glutamine and glutamate
- IP:
-
Intraperitoneal
- IV:
-
Intravenous
- MR:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- MRSI:
-
Magnetic resonance spectroscopic imaging
- SNR:
-
Signal-to-noise ratio
- TCA:
-
Tricarboxylic acid
References
Barrington SF, Mikhaeel NG, Kostakoglu L et al. (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048-3058. https://doi.org/10.1200/JCO.2013.53.5229
Batsios G, Taglang C, Tran M et al (2022) Deuterium metabolic imaging reports on TERT expression and early response to therapy in cancer. Clin Cancer Res 28:3526–3536. https://doi.org/10.1158/1078-0432.CCR-21-4418
Bednarik P, Goranovic D, Svatkova A et al (2023) 1H magnetic resonance spectroscopic imaging of deuterated glucose and of neurotransmitter metabolism at 7 T in the human brain. Nat Biomed Eng 7:1001–1013. https://doi.org/10.1038/s41551-023-01035-z
Belete TM (2022) Recent updates on the development of deuterium-containing drugs for the treatment of cancer. Drug Des Devel Ther 16:3465–3472. https://doi.org/10.2147/DDDT.S379496
Belhocine T, Spaepen K, Dusart M et al (2006) 18FDG PET in oncology: the best and the worst (review). Int J Oncol 28:1249–1261. https://doi.org/10.3892/ijo.28.5.1249
Brix G, Lechel U, Glatting G et al. (2005) Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med 46:608-613. https://jnm.snmjournals.org/content/jnumed/46/4/608.full.pdf
Busch R, Neese RA, Awada M et al (2007) Measurement of cell proliferation by heavy water labeling. Nat Protoc 2:3045–3057. https://doi.org/10.1038/nprot.2007.420
Buxbaum NP, Farthing DE, Maglakelidze N et al. (2017) In vivo kinetics and nonradioactive imaging of rapidly proliferating cells in graft-versus-host disease. JCI Insight 2. https://doi.org/10.1172/jci.insight.92851
Castell F, Cook GJ (2008) Quantitative techniques in 18FDG PET scanning in oncology. Br J Cancer 98:1597–1601. https://doi.org/10.1038/sj.bjc.6604330
Cember ATJ, Wilson NE, Rich LJ et al (2022) Integrating 1H MRS and deuterium labeled glucose for mapping the dynamics of neural metabolism in humans. Neuroimage 251:118977. https://doi.org/10.1016/j.neuroimage.2022.118977
Chen Z, Han F, Du Y et al (2023) Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther 8:70. https://doi.org/10.1038/s41392-023-01332-8
Corbin ZA (2019) New metabolic imaging tools in neuro-oncology. Curr Opin Neurol 32:872–877. https://doi.org/10.1097/WCO.0000000000000758
Daniels CJ, Mclean MA, Schulte RF et al (2016) A comparison of quantitative methods for clinical imaging with hyperpolarized 13C-pyruvate. NMR Biomed 29:387–399. https://doi.org/10.1002/nbm.3468
Day SE, Kettunen MI, Gallagher FA et al (2007) Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med 13:1382–1387. https://doi.org/10.1038/nm1650
De Feyter HM, Behar KL, Corbin ZA et al (2018) Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv 4:eaat7314. https://doi.org/10.1126/sciadv.aat7314
De Feyter HM, De Graaf RA (2021) Deuterium metabolic imaging - back to the future. J Magn Reson 326:106932. https://doi.org/10.1016/j.jmr.2021.106932
De Feyter HM, Thomas MA, Behar KL et al (2021) NMR visibility of deuterium-labeled liver glycogen in vivo. Magn Reson Med 86:62–68. https://doi.org/10.1002/mrm.28717
De Graaf RA, Hendriks AD, Klomp DWJ et al (2020) On the magnetic field dependence of deuterium metabolic imaging. NMR Biomed 33:e4235. https://doi.org/10.1002/nbm.4235
Desai A, Middlebrooks EH (2023) Cerebral aneurysm imaging at 7 T with use of compressed sensing. Radiology 306:e221277. https://doi.org/10.1148/radiol.221277
Farthing DE, Buxbaum NP, Bare CV et al (2013) Sensitive GC-MS/MS method to measure deuterium labeled deoxyadenosine in DNA from limited mouse cell populations. Anal Chem 85:4613–4620. https://doi.org/10.1021/ac400309d
Fu Q, Lei ZQ, Li JY et al (2023) Subtractionless compressed-sensing-accelerated whole-body MR angiography using two-point Dixon fat suppression with single-pass half-reduced contrast dose: feasibility study and initial experience. J Cardiovasc Magn Reson 25:41. https://doi.org/10.1186/s12968-023-00953-w
Fujimoto D, Yoshioka H, Kataoka Y et al (2019) Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol 14:468–474. https://doi.org/10.1016/j.jtho.2018.10.167
Gallagher FA, Kettunen MI, Day SE et al (2008) Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453:940–943. https://doi.org/10.1038/nature07017
Ge X, Song KH, Engelbach JA et al (2022) Distinguishing tumor admixed in a radiation necrosis (RN) background: 1H and 2H MR with a novel mouse brain-tumor/RN model. Front Oncol 12:885480. https://doi.org/10.3389/fonc.2022.885480
Golman K, Zandt RI, Lerche M et al (2006) Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res 66:10855–10860. https://doi.org/10.1158/0008-5472.CAN-06-2564
Griffin RG, Swager TM, Temkin RJ (2019) High frequency dynamic nuclear polarization: new directions for the 21st century. J Magn Reson 306:128–133. https://doi.org/10.1016/j.jmr.2019.07.019
Hartmann B, Muller M, Seyler L et al (2021) Feasibility of deuterium magnetic resonance spectroscopy of 3-O-methylglucose at 7 Tesla. PLoS One 16:e0252935. https://doi.org/10.1371/journal.pone.0252935
Hesse F, Somai V, Kreis F et al. (2021) Monitoring tumor cell death in murine tumor models using deuterium magnetic resonance spectroscopy and spectroscopic imaging. Proc Natl Acad Sci U S A 118. https://doi.org/10.1073/pnas.2014631118
Hesse F, Wright AJ, Bulat F et al (2022) Deuterium MRSI of tumor cell death in vivo following oral delivery of 2H-labeled fumarate. Magn Reson Med 88:2014–2020. https://doi.org/10.1002/mrm.29379
Horska A, Barker PB (2010) Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin N Am 20:293–310. https://doi.org/10.1016/j.nic.2010.04.003
Ip KL, Thomas MA, Behar KL et al (2023) Mapping of exogenous choline uptake and metabolism in rat glioblastoma using deuterium metabolic imaging (DMI). Front Cell Neurosci 17:1130816. https://doi.org/10.3389/fncel.2023.1130816
Kaggie JD, Khan AS, Matys T et al (2022) Deuterium metabolic imaging and hyperpolarized 13C-MRI of the normal human brain at clinical field strength reveals differential cerebral metabolism. Neuroimage 257:119284. https://doi.org/10.1016/j.neuroimage.2022.119284
Kapoor V, Mccook BM, Torok FS (2004) An introduction to PET-CT imaging. Radiographics 24:523–543. https://doi.org/10.1148/rg.242025724
Kim IY, Park S, Kim Y et al (2022) Tracing metabolic flux in vivo: basic model structures of tracer methodology. Exp Mol Med 54:1311–1322. https://doi.org/10.1038/s12276-022-00814-z
Kim SH, Baek KH (2021) Regulation of cancer metabolism by deubiquitinating enzymes: the Warburg effect. Int J Mol Sci 22. https://doi.org/10.3390/ijms22126173
Kogan F, Hariharan H, Reddy R (2013) Chemical exchange saturation transfer (CEST) imaging: description of technique and potential clinical applications. Curr Radiol Rep 1:102–114. https://doi.org/10.1007/s40134-013-0010-3
Kreis F, Wright AJ, Hesse F et al (2020) Measuring tumor glycolytic flux in vivo by using fast deuterium MRI. Radiology 294:289–296. https://doi.org/10.1148/radiol.2019191242
Kurtoglu M, Maher JC, Lampidis TJ (2007) Differential toxic mechanisms of 2-deoxy-D-glucose versus 2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells. Antioxid Redox Signal 9:1383–1390. https://doi.org/10.1089/ars.2007.1714
Ladd ME, Bachert P, Meyerspeer M et al (2018) Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc 109:1–50. https://doi.org/10.1016/j.pnmrs.2018.06.001
Lee JH, Long GV, Menzies AM et al (2018) Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol 4:717–721. https://doi.org/10.1001/jamaoncol.2017.5332
Li Y, Zhao Y, Guo R et al (2021) Machine learning-enabled high-resolution dynamic deuterium MR spectroscopic imaging. IEEE Trans Med Imaging 40:3879–3890. https://doi.org/10.1109/TMI.2021.3101149
Liu X, Shi L, Shi L et al (2022) Towards mapping mouse metabolic tissue atlas by mid-infrared imaging with heavy water labeling. Adv Sci (Weinh) 9:e2105437. https://doi.org/10.1002/advs.202105437
Liu Y, De Feyter HM, Fulbright RK et al (2022) Interleaved fluid-attenuated inversion recovery (FLAIR) MRI and deuterium metabolic imaging (DMI) on human brain in vivo. Magn Reson Med 88:28–37. https://doi.org/10.1002/mrm.29196
Lu M, Zhu XH, Zhang Y et al (2017) Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J Cereb Blood Flow Metab 37:3518–3530. https://doi.org/10.1177/0271678X17706444
Mahar R, Donabedian PL, Merritt ME (2020) HDO production from [2H7]glucose quantitatively identifies Warburg metabolism. Sci Rep 10:8885. https://doi.org/10.1038/s41598-020-65839-8
Mahar R, Zeng H, Giacalone A et al (2021) Deuterated water imaging of the rat brain following metabolism of [2H7]glucose. Magn Reson Med 85:3049–3059. https://doi.org/10.1002/mrm.28700
Markovic S, Roussel T, Agemy L et al (2021) Deuterium MRSI characterizations of glucose metabolism in orthotopic pancreatic cancer mouse models. NMR Biomed 34:e4569. https://doi.org/10.1002/nbm.4569
Markovic S, Roussel T, Neeman M et al. (2021) Deuterium magnetic resonance imaging and the discrimination of fetoplacental metabolism in normal and L-NAME-induced preeclamptic mice. Metabolites 11. https://doi.org/10.3390/metabo11060376
Mauler J, Heinzel A, Matusch A et al (2020) Bolus infusion scheme for the adjustment of steady state [11C]Flumazenil levels in the grey matter and in the blood plasma for neuroreceptor imaging. Neuroimage 221:117160. https://doi.org/10.1016/j.neuroimage.2020.117160
Meerwaldt AE, Straathof M, Oosterveld W et al (2023) In vivo imaging of cerebral glucose metabolism informs on subacute to chronic post-stroke tissue status - a pilot study combining PET and deuterium metabolic imaging. J Cereb Blood Flow Metab 43:778–790. https://doi.org/10.1177/0271678X221148970
Ni QZ, Daviso E, Can TV et al (2013) High frequency dynamic nuclear polarization. Acc Chem Res 46:1933–1941. https://doi.org/10.1021/ar300348n
Niess F, Hingerl L, Strasser B et al (2023) Noninvasive 3-dimensional 1H-magnetic resonance spectroscopic imaging of human brain glucose and neurotransmitter metabolism using deuterium labeling at 3T: feasibility and interscanner reproducibility. Invest Radiol 58:431–437. https://doi.org/10.1097/RLI.0000000000000953
Ouwerkerk R (2020) Deuterium MR spectroscopy: a new way to image glycolytic flux rates. Radiology 294:297–298. https://doi.org/10.1148/radiol.2019192024
Oz G, Alger JR, Barker PB et al (2014) Clinical proton MR spectroscopy in central nervous system disorders. Radiology 270:658–679. https://doi.org/10.1148/radiol.13130531
Page MJ, Mckenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Peters DC, Markovic S, Bao Q et al (2021) Improving deuterium metabolic imaging (DMI) signal-to-noise ratio by spectroscopic multi-echo bSSFP: a pancreatic cancer investigation. Magn Reson Med 86:2604–2617. https://doi.org/10.1002/mrm.28906
Petrova V, Annicchiarico-Petruzzelli M, Melino G et al (2018) The hypoxic tumour microenvironment. Oncogenesis 7:10. https://doi.org/10.1038/s41389-017-0011-9
Polvoy I, Qin H, Flavell RR et al. (2021) Deuterium metabolic imaging-rediscovery of a spectroscopic tool. Metabolites. 11. https://doi.org/10.3390/metabo11090570
Ravoori MK, Singh SP, Lee J et al (2017) In vivo assessment of ovarian tumor response to tyrosine kinase inhibitor pazopanib by using hyperpolarized 13C-pyruvate MR spectroscopy and 18F-FDG PET/CT imaging in a mouse model. Radiology 285:830–838. https://doi.org/10.1148/radiol.2017161772
Rich LJ, Bagga P, Wilson NE et al (2020) 1H magnetic resonance spectroscopy of 2H-to-1H exchange quantifies the dynamics of cellular metabolism in vivo. Nat Biomed Eng 4:335–342. https://doi.org/10.1038/s41551-019-0499-8
Riis-Vestergaard MJ, Laustsen C, Mariager CO et al (2020) Glucose metabolism in brown adipose tissue determined by deuterium metabolic imaging in rats. Int J Obes (Lond) 44:1417–1427. https://doi.org/10.1038/s41366-020-0533-7
Ruhm L, Avdievich N, Ziegs T et al (2021) Deuterium metabolic imaging in the human brain at 94 Tesla with high spatial and temporal resolution. Neuroimage 244:118639. https://doi.org/10.1016/j.neuroimage.2021.118639
Salvi De Souza G, Mantovani DBA, Mossel P et al (2023) Oral administration of PET tracers: current status. J Control Release 357:591–605. https://doi.org/10.1016/j.jconrel.2023.04.008
Schofield J, Derdau V, Atzrodt J et al (2015) Effect of deuteration on metabolism and clearance of Nerispirdine (HP184) and AVE5638. Bioorg Med Chem 23:3831–3842. https://doi.org/10.1016/j.bmc.2015.03.065
Seres Roig E, De Feyter HM, Nixon TW et al (2023) Deuterium metabolic imaging of the human brain in vivo at 7 T. Magn Reson Med 89:29–39. https://doi.org/10.1002/mrm.29439
Simoes RV, Henriques RN, Cardoso BM et al (2022) Glucose fluxes in glycolytic and oxidative pathways detected in vivo by deuterium magnetic resonance spectroscopy reflect proliferation in mouse glioblastoma. Neuroimage Clin 33:102932. https://doi.org/10.1016/j.nicl.2021.102932
Straathof M, Meerwaldt AE, De Feyter HM et al (2021) Deuterium metabolic imaging of the healthy and diseased brain. Neuroscience 474:94–99. https://doi.org/10.1016/j.neuroscience.2021.01.023
Sun P, Wu Z, Lin L et al (2023) MR-Nucleomics: the study of pathological cellular processes with multinuclear magnetic resonance spectroscopy and imaging in vivo. NMR Biomed 36:e4845. https://doi.org/10.1002/nbm.4845
Taglang C, Batsios G, Mukherjee J et al (2022) Deuterium magnetic resonance spectroscopy enables noninvasive metabolic imaging of tumor burden and response to therapy in low-grade gliomas. Neuro Oncol 24:1101–1112. https://doi.org/10.1093/neuonc/noac022
Tkac I, Deelchand D, Dreher W et al (2021) Water and lipid suppression techniques for advanced 1H MRS and MRSI of the human brain: experts’ consensus recommendations. NMR Biomed 34:e4459. https://doi.org/10.1002/nbm.4459
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. https://doi.org/10.1126/science.1160809
Veltien A, Van Asten J, Ravichandran N et al. (2021) Simultaneous recording of the uptake and conversion of glucose and choline in tumors by deuterium metabolic imaging. Cancers (Basel) 13. https://doi.org/10.3390/cancers13164034
Von Morze C, Engelbach JA, Blazey T et al (2021) Comparison of hyperpolarized 13C and non-hyperpolarized deuterium MRI approaches for imaging cerebral glucose metabolism at 4.7 T. Magn Reson Med 85:1795–1804. https://doi.org/10.1002/mrm.28612
Wang T, Zhu XH, Li H et al (2021) Noninvasive assessment of myocardial energy metabolism and dynamics using in vivo deuterium MRS imaging. Magn Reson Med 86:2899–2909. https://doi.org/10.1002/mrm.28914
Wang ZJ, Ohliger MA, Larson PEZ et al (2019) Hyperpolarized 13C MRI: state of the art and future directions. Radiology 291:273–284. https://doi.org/10.1148/radiol.2019182391
Wilkinson DJ, Brook MS, Smith K et al (2017) Stable isotope tracers and exercise physiology: past, present and future. J Physiol 595:2873–2882. https://doi.org/10.1113/JP272277
Woitek R, Gallagher FA (2021) The use of hyperpolarised 13C-MRI in clinical body imaging to probe cancer metabolism. Br J Cancer 124:1187–1198. https://doi.org/10.1038/s41416-020-01224-6
Xia Q, Liu J, Wu C et al (2015) Prognostic significance of 18FDG PET/CT in colorectal cancer patients with liver metastases: a meta-analysis. Cancer Imaging 15:19. https://doi.org/10.1186/s40644-015-0055-z
Zhang G, Keshari KR (2021) Deuterium metabolic imaging of pancreatic cancer. NMR Biomed 34:e4603. https://doi.org/10.1002/nbm.4603
Zhang SQ, Yung KK, Chung SK et al (2018) Aldo-keto reductases-mediated cytotoxicity of 2-deoxyglucose: a novel anticancer mechanism. Cancer Sci 109:1970–1980. https://doi.org/10.1111/cas.13604
Zhang Y, Gao Y, Fang K et al (2022) Proton/deuterium magnetic resonance imaging of rodents at 9.4T using birdcage coils. Bioelectromagnetics 43:40–46. https://doi.org/10.1002/bem.22382
Zhu XH, Lu M, Chen W (2018) Quantitative imaging of brain energy metabolisms and neuroenergetics using in vivo X-nuclear 2H, 17O and 31P MRS at ultra-high field. J Magn Reson 292:155–170. https://doi.org/10.1016/j.jmr.2018.05.005
Zou C, Ruan Y, Li H et al (2023) A new deuterium-labeled compound [2,3,4,6,6’-(2) H(5)]-D-glucose for deuterium magnetic resonance metabolic imaging. NMR Biomed 36:e4890. https://doi.org/10.1002/nbm.4890
Acknowledgements
The authors would like to express sincere gratitude to Prof. Chaoyang Liu from the Laboratory of State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, for his invaluable expertise and assistance in the paper writing. The authors declared LLMs were not used.
Funding
This study was supported by a research grant awarded by the National Key Research and Development Projects from the Ministry of Science and Technology of the People’s Republic of China (grant number: 2023YFE0113300). The funders had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: F.P., Q.B., and L.Y. Methodology: F.P., Q.B., and L.Y. Software: F.P., Q.B., and L.Y. Validation: X.L., J.W., Y.G., X.Z., Q.B., and L.Y. Formal analysis: F.P., X.Z., J.W., Q.B., and L.Y. Investigation: F.P., P.S., Q.B., and L.Y. Resources: F.P., Q.B., and L.Y. Data curation: F.P., P.S., Q.B., and L.Y. Writing—original draft preparation: F.P. Writing—review and editing: all authors. Visualization: X.L., J.W., Y.G., and Q.B. Supervision: F.P., Q.B., and L.Y. Project administration: F.P., Q.B., and L.Y. Funding acquisition: none. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors declare all figures depicting animal experiments in this review were approved by the institutional review board of the Laboratory of State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences (No. APM23022T).
Consent for publication
Not applicable.
Competing interests
P.S., X.Z., and J.W. are employees of Philips Healthcare. All the remaining authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Search strategy. Table S2. Comparisons of spatial-temporal resolution among different studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, F., Liu, X., Wan, J. et al. Advances and prospects in deuterium metabolic imaging (DMI): a systematic review of in vivo studies. Eur Radiol Exp 8, 65 (2024). https://doi.org/10.1186/s41747-024-00464-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41747-024-00464-y