Abstract
Background
Creeping fat is a pathological feature of small bowel Crohn’s disease (CD), with literature suggesting that bowel resection with extended mesenteric resection is related to less postoperative recurrences. Conventional imaging is unable to accurately quantify the disease involvement (i.e., fibrosis) of creeping fat. Quantification of disease involvement could be useful in decision-making for additional extended mesenteric resection. We investigated the feasibility of magnetic resonance elastography (MRE) of the mesentery and if MRE is capable to detect fibrotic disease involvement of mesentery in active CD.
Methods
Multifrequency MRE yielded spatial stiffness (shear wave speed, SWS, |G*|) and fluidity maps (φ). Viscoelastic properties of seven CD patients’ mesentery were compared to age- and sex-matched healthy volunteers (HV) (Mann–Whitney U-test). Within CD patients, the affected and “presumably” unaffected mesentery were compared (Wilcoxon-signed rank test). Repeatability was tested in 15 HVs (Bland–Altman analysis, coefficient of variation [CoV]). Spearman rank correlations were used to investigate the relation between microscopically scored amount of mesenteric fibrosis and viscoelastic parameters.
Results
SWS, |G*|, and φ of affected mesentery in CD were higher compared to HV (p = 0.017, p = 0.001, p = 0.017). Strong correlations were found between percentage of area of mesenteric fibrosis and SWS and |G*| (p < 0.010). No differences were found within CD between affected and presumably unaffected mesentery. Repeatability of SWS showed 95% limits of agreement of (-0.09, 0.13 m/s) and within-subject CoV of 5.3%.
Conclusion
MRE may have the potential to measure fibrotic disease involvement of the mesentery in CD, possibly guiding clinical decision-making with respect to extended mesenteric resection.
Trial registration
Dutch trial register, NL9105, registered 7 December 2020.
Relevance statement
MRE may have the potential to measure the amount of mesenteric fibrosis of the affected mesenteric fat in active Crohn’s disease, giving more insight into disease progression and could potentially play a role in clinical decision-making for extended mesenteric resection.
Key points
• MRE of the mesentery in patients with active CD is feasible.
• Fluidity and stiffness of the mesentery increase in active CD, while stiffness correlates with the histopathological amount of mesenteric fibrosis.
• MRE provides biomarkers to quantify mesenteric disease activity in active CD.
Graphical Abstract

Similar content being viewed by others
Background
Creeping fat is a pathological feature of small bowel Crohn’s disease (CD) at cross-sectional imaging and histopathology. It constitutes thickened, hypertrophic mesenteric adipose tissue (hereafter: mesentery) that wraps around the affected bowel wall. The exact mechanism of creeping fat is still debated and has been hypothesized as follows: (1) a passive and protective response to a dysfunctional gut barrier [1] and (2) an active part in the disease course with adipocytes secreting cytokines [2].
The presence of creeping fat at imaging is not an innocuous finding as it is associated with fibrosis and stricture formation of the affected bowel [3,4,5,6]. Currently, new surgical techniques are being studied to reduce postoperative recurrences. These techniques involve the mesentery either directly or indirectly (such as bowel resection with extended mesenteric resection or mesenteric exclusion by means of an anti-mesenteric Kono-S handsewn side-to-side anastomosis), and both techniques show less postoperative recurrences [7,8,9,10]. Current surgical guidelines [11] advise performing a mesenteric-sparing resection due to the benign character of CD. Extended mesenteric resection is not without risks and could result in a more extensive bowel resection as the latter vascularization is derived from the mesentery. Hence, critical thinking is imperative when extended mesenteric resection is considered. Characterizing the degree of mesenteric disease involvement (i.e., fibrosis) could facilitate this decision-making.
Several studies have tried to quantify creeping fat either directly [12, 13] or indirectly [14,15,16,17]. A higher mesenteric fat index, an example of an indirect quantification, was shown to be correlated with an increased risk of early postoperative endoscopic recurrence of CD [15,16,17]. Recently, Li et al. [18] developed a novel mesenteric creeping fat index (MCFI) to quantify the amount of creeping fat. This was based on the extent of fat around the bowel circumference encompassed by vessels in the fat on computed tomography and showed an excellent correlation with the extent of fatty wrapping in resection specimens. Although promising, these methods do not provide information about the disease involvement (i.e., fibrosis) of the mesentery.
Literature shows that creeping fat is histologically characterized by an increase in connective tissue (i.e., fibrosis) and adipocyte hyperplasia [7]. Since an increase in connective tissue would lead to an increase in tissue stiffness, more advanced imaging techniques like elastography could play a role in identifying the amount of fibrosis. Lo Re et al. [19] supported this in a pilot study with ultrasound elastography in which fat around the affected bowel wall was stiffer compared to the fat around unaffected bowel wall in CD patients. Although promising, ultrasound elastography has some technical limitations such as a low penetration depth, dependence on transducer placement, and mapping is only performed in two dimensions [20].
On the contrary, magnetic resonance elastography (MRE) is a noninvasive technique that is able to quantify viscoelastic properties of soft tissue. This technique uses external mechanical vibrations [21] and measures the shear wave propagation throughout the entire abdomen. Using multiple transducers, at multiple frequencies, a three-dimensional overview of the local soft tissue stiffness in the abdomen is obtained. The research conducted with MRE to date has predominantly centered on diseases affecting the liver, such as liver fibrosis, cirrhosis, and hepatitis, as well as some other abdominal organs (e.g., pancreas), certain brain, and musculoskeletal disorders [22]. MRE has been found to be particularly useful in assessing the severity of liver fibrosis, a condition in which there is excessive scarring of liver tissue that can lead to liver damage and dysfunction, as it can accurately measure the stiffness of liver tissue, which is a key indicator of fibrosis progression [23].
The aim of this study was to study the feasibility of mesenteric MRE and explore if this technique is able to detect mesenteric disease involvement (i.e., fibrosis) in small bowel mesentery of active CD patients.
Methods
Study design
This prospective exploratory study was performed at a tertiary referral center (Amsterdam University Medical Center, location Academic Medical Center). Patients were recruited simultaneously with an overarching study (Dutch Trial Register NL9105; https://trialsearch.who.int/Trial2.aspx?TrialID=NL9105) from July 2021 until March 2022 and provided written informed consent. The study was conducted in agreement with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Amsterdam University Medical Center, location Academic Medical Center). Healthy volunteers signed informed consent and were recruited for protocol optimization with approval of the local Medical Ethics Committee by means of a waiver.
Patients and healthy controls
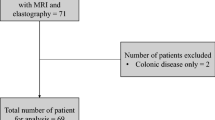
Patients were eligible when they had endoscopic or histological-confirmed CD. Furthermore, patients were eligible when there was active CD of the small bowel and were scheduled for small bowel segment resection or had one or more small bowel stricture(s) (confirmed on endoscopy or cross-sectional imaging) that were scheduled for treatment with anti-inflammatory therapy. Patients were excluded if they had an isolated colonic stricture, were under 18 years of age, were unable to give informed consent, or if they had general MR imaging contraindications. Patient data consisted of sex, age, body mass index, Harvey-Bradshaw index, smoking status, disease duration in years, disease location and behavior according to the Montreal classification, type of previous surgery, and treatment given.
Fifteen healthy volunteers aged 18 years and older were recruited for repeatability analysis, which included seven age-sex-matched healthy controls (± 5 years). Healthy volunteers were excluded if they had a disease history of bowel disease (such as inflammatory bowel disease, celiac disease, diverticulitis, and a history of large or small bowel cancer) or had previous small or large bowel surgery. From the healthy volunteers, we collected disease history, sex, age, body mass index (kg/m2), and smoking status.
Magnetic resonance protocol
All scanning was performed on a 3 T scanner (Ingenia, Philips, Best, the Netherlands). All subjects fasted 4 h before scanning. Patients drank 1,600 mL of 1.9%-mannitol solution (Baxter B. V., Utrecht, the Netherlands) as an intraluminal contrast agent within 1 h before scanning as part of their routine MR enterography examination. Healthy volunteers drank 500 mL of water 15 min before scanning (mannitol was not allowed in healthy volunteers based on the protocol optimization waiver). MRE scans were performed using four pneumatic drivers placed at the height of the ileocecal junction (McBurney’s point was used as a derivate for the ileocecal junction), two anterior and two posterior. The four drivers were held in place with an elastic band (Fig. 1). A multifrequency spin-echo echo-planar imaging with spectral presaturation with inversion recovery MRE sequence was used (Table 1). For this sequence, free breathing was used with motion encoding gradients (MEGs) in three orthogonal directions to capture the full three-dimensional displacement field. Eight wave-phase offsets were measured, and mechanical frequencies ranged from 30 to 60 Hz with a 10-Hz increment. Furthermore, the magnetic resonance protocol for all participants consisted of T2-weighted images in coronal and axial orientation for anatomical reference. Repeatability was tested by performing two consecutive MRE acquisitions without repositioning, to test the within-session repeatability and ensure the most consistency in the location of the mesentery between both scans.
Research setup for magnetic resonance elastography of the small bowel mesentery. Four transducers are placed at the height of the ileocecal junction, two anterior and two posterior, and held in place by an elastic strap. The transducers are connected to a pressure box that controls the airflow to create a vibration
Post-processing
Post-processing of the MRE data was performed using the multifrequency dual elasto-visco (MDEV) and k-MDEV reconstruction algorithms [24, 25]. A Gaussian smoothing filter was applied before unwrapping the phase data for denoising. Unwrapping was done using a gradient method and a Laplacian filter for the MDEV and k-MDEV, respectively. A high-pass filter was used to filter the longitudinal waves; for the MDEV, an additional low-pass Butterworth filter was used. Taking the mean for all frequencies resulted in the mean shear stiffness (mean |G*|) in kilopascal (kPa) and phase angle (φ) in radians (rad) for the MDEV and the shear wave speed (SWS) in meters/seconds (m/s) for the k-MDEV. Furthermore, the octahedral shear strain signal-to-noise ratio (OSS-SNR) was calculated as a measure for MRE quality [21].
Viscoelastic properties
Stiffness in tissue was determined by the SWS or |G*|, with higher SWS and |G*| values reflecting a higher tissue stiffness. Phase angle (φ) is a measure of tissue fluidity, φ > π⁄4 indicates predominantly fluid properties, while φ < π⁄4 indicates predominantly solid behavior [21]. An increase in the amount of water present in tissue increases fluidity and can be described as a viscous fluid [26].
Segmentation
Segmentation was done manually by drawing a region of interest (ROI) on the MRE magnitude data with guidance of axial and coronal T2-weighted images. All segmentations were performed in ITK snap (version 3.4.0, Nov 30, 2015). Each ROI was placed in the mesentery close to the targeted small bowel loop, using the feeding mesenteric vessels to identify the proper mesentery. In CD patients, ROIs were placed in the mesentery of the most severely affected small bowel loop, defined as the part with the thickest bowel wall (> 3 mm) and smallest luminal diameter (≥ 50% luminal reduction compared to an unaffected bowel loop). In CD patients a ROI was also placed in the mesentery of healthy upstream small bowel at least 25 cm proximal to the diseased small bowel. Segmentations were supervised by an abdominal radiologist with 28 years of experience in gastrointestinal MR imaging (J.S.).
Histopathology
In the fresh resection specimen, the small bowel segment with the thickest bowel wall and the most luminal narrowing was identified by a clinician (K.B.) and marked with a pin. Thereafter, the fresh resection specimen was kept for at least 24 h in formalin. After fixation, the indicated bowel segments were sectioned, photographed, and processed using hematoxylin and eosin staining.
The amount of fatty wrapping around the gut was macroscopically scored by means of the mesenteric creeping fat index (MCFI) based on photos made after formalin fixation. MCFI is based on the amount of fatty tissue surrounding the bowel wall divided into eight clockwise segments (see Fig. 2a). The scoring was performed independently by two study coordinators (A.S. and K.B.). Afterwards, discrepancies were discussed, and the definitive MCFI scores per segment were determined, supervised by a pathologist specialized in gastrointestinal diseases with 31 years of experience (A.N.B.).
Examples are of a 21-year-old-male Crohn’s disease patient. a Macroscopic image of the resected small bowel section. Scoring is based on eight segments; four segments (1–4) are involved as is demonstrated. b Histopathological analysis (Elastica van Gieson stain) of a segment of the affected mesentery, the black arrows indicate fibrotic tissue. c Magnitude magnetic resonance elastography image with the affected mesentery in pink with the corresponding shear wave speed (SWS) map visible on the corresponding elastogram in the lower right corner
In order to quantitatively measure mesenteric fibrosis, the pathologist determined one section per patient which contained the most affected mesentery and delineated the affected area of interest. After digitalization of the sections, the area of interest was scored for fibrotic severity. This was done using an in-house built semiautomatic MATLAB software (v2021a, the MathWorks, Natick, MA, USA) that identified fibrosis within this area. Fibrosis was identified by a watershed segmentation technique, which was checked manually. The fibrotic severity was determined by the percentage of fibrosis with respect to the previously total delineated affected area, which yielded the microscopically scored amount of mesenteric fibrosis, denoted as the mesenteric fibrotic score in % (Fig. 2b).
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median with interquartile range (IQR) when skewed. Because of the small groups, we tested for normality with the Shapiro–Wilk test. Categorical variables were presented in proportions and percentages.
Average MRE parameters were determined by taking the mean with standard deviation over the ROI. Differences in viscoelastic properties for mesentery between active CD patients and healthy volunteers were analyzed by means of an unpaired t-test or Mann–Whitney U-test in case of skewed data. For differences in viscoelastic properties within CD patients, a paired t-test or Wilcoxon-signed rank test was used. Correlation analysis was done using the Pearson correlation coefficient or Spearman’s rho in case of non-normality.
Repeatability of MRE findings was analyzed by means of a Bland–Altman analysis resulting in the limits of agreement (LoA). Within-subject coefficients of variation (CoV) were determined for all MRE. Values of p lower than 0.05 were considered significant in all other assessments. No power calculation was performed because of the exploratory nature of this study.
Results
Study population
Our case–control population (Table 2) consisted of seven patients (median age 39 years; 5 males) with active stricturing small bowel CD and seven healthy volunteers (median age 34 years; 5 males), without a difference in age (p = 0.902). All patients had either stricturing or penetrating disease according to the Montreal classification [27]. On conventional magnetic resonance imaging, all patients had a small bowel stricture (bowel wall thickness > 3 mm and luminal narrowing ≥ 50% compared to normal bowel loop). The patients had a relatively long disease duration of 7 years (IQR 2–12). Six of the seven patients underwent ileocecal resection (n = 5) or ileocecal re-resection (n = 1) within median of 39 days (IQR 23–61) after MRE acquisition. One patient started with anti-inflammatory treatment. The repeatability study population consisted of 15 healthy volunteers, aged 31 ± 7 years (mean ± standard deviation), and 6 males. All scans were of sufficient quality for analysis.
Viscoelastic parameters of the mesentery
All ROIs had a minimal size of 31.25 mm2. SWS, |G*|, and φ of affected mesentery in CD patients were higher compared to the mesentery of healthy volunteers (p = 0.017, p = 0.001, and p = 0.017, respectively) (Fig. 3). SWS, |G*|, and φ between affected mesentery and “presumably” unaffected mesentery (at least 25 cm proximal to the diseased small bowel) within CD patients showed no significant differences (p = 0.318, p = 0.620, and p = 0.209, respectively). A representative patient is shown in Fig. 4. The median SWS of affected mesentery in CD patients was 0.76 m/s (IQR 0.68–0.79), while the median mesentery of healthy volunteers was 0.64 m/s (IQR 0.53–0.74). All viscoelastic parameters are reported in the supplementary material (Table S1). Mean OSS-SNR values for the healthy mesentery were 3.6 ± 1.8 dB, while the affected mesentery in CD patients had a OSS-SNR of 5.5 ± 1.4 dB (p = 0.123).
Region of interest of affected bowel mesentery in a representative patient with Crohn’s disease on T2-weighted images, on top in the inset is the corresponding magnitude magnetic resonance elastography image with the shear wave speed (SWS) and phase angle (φ) map. Segmented affected mesentery is highlighted in pink, and the red arrow shows the affected bowel wall
Histopathology of the mesentery
Histopathological analysis was done in six patients who underwent ileocecal (re-)resection. The mean macroscopic MCFI score based on six resection specimens was 4 ± 1. No correlation was found between macroscopic MCFI score and viscoelastic parameters: (r(4) = 0.062, p = 0.908 for SWS and |G*|; r(4) = -0.555, p = 0.252 for φ (Fig. 5a–c). A strong positive correlation between mesenteric fibrotic score for both SWS (r(4) = 1.000, p < 0.010) and |G*| (r(4) = 1.000, p < 0.010) was found, but not for φ (r(4) = 0.429, p = 0.397). Macro- and microscopic scores as a function of viscoelastic parameters are shown in Fig. 5d–f. The mesenteric fibrotic score for all patients was 3.9 ± 2.2% (mean ± standard deviation). Figure 2 depicts an example of an elastogram of a 21-year-old male with active CD with histopathological analysis after ileocecal resection.
Macroscopic scoring. a Shear wave speed (SWS), (b) shear stiffness (|G*|), and (c) phase angle (φ) as a function of mesenteric creeping fat index (MCFI). Microscopic scoring: (d) SWS, (e) |G*|, and (f) (φ) as a function of mesenteric fibrosis score (percentage of area of fibrosis (%) within a region of interest). Spearman’s rho and corresponding p-values are given
Repeatability
In the repeatability population (n = 15), mean SWS, |G*|, and φ were 0.64 ± 0.11 m/s, 0.63 ± 0.10 kPa, and 0.46 ± 0.12 rad (mean ± standard deviation), respectively. LoA were [-0.09, 0.13 m/s], [-0.09, 0.12 kPa], and [-0.12, 0.13 rad], respectively. Within-subject CoV were 8.7%, 8.4%, and 14.0%, respectively. Bland–Altman analysis of the repeatability in healthy volunteers is shown in Fig. 6.
Discussion
We demonstrated that MRE of the small bowel mesentery can show differences in viscoelastic properties between active CD patients and age- and sex-matched healthy volunteers. The increase in SWS and |G*| in the mesentery of CD patients indicates an increased stiffness, while the increased φ indicates increased fluidity. To our knowledge, this is the first study to look into viscoelastic properties of small bowel mesentery with MRE in patients with active CD and corresponding histopathology.
Increased stiffness suggests an increase in connective tissue present in the mesentery in active CD patients. This is also supported by recent literature which demonstrated that 88% of CD patients with stricturing or penetrating CD (according to the Montreal classification) had penetrating fibrosis at histopathology defined as fibrosis that was continuous through all intestinal layers with extension into the mesenteric fat [28, 29].
The amount of mesenteric fibrosis at histopathology (mesenteric fibrotic score) showed a strong positive linear relation with stiffness (SWS) and shear stiffness (|G*|) measured by means of MRE within active CD patients. This suggests that measuring the SWS and |G*| with MRE could be used to determine the amount of mesenteric fibrosis in patients with active CD. This also suggests that MRE could potentially be used as a biomarker, which gives insight into the degree of mesenteric disease involvement (i.e., fibrosis).
For both the CD patients and healthy volunteers, φ was below π/4, meaning the mesentery presents as an elastic-solid. The increased fluidity can indicate the presence of disorganized proteins, vascularity, or increased amount of adipocytes, which increases pressure and mechanical friction [29,30,31]. However, this has not been explored yet; further research in fluidity is necessary to corroborate this.
Previous literature states that 50% is the minimal amount of fatty wrapping for the definition of creeping fat [7]. The MCFI showed an average value of 4, which implies at least 50% of the bowel surface of the resected specimens was covered with fatty tissue and therefore creeping fat. Nonetheless, the MCFI showed no correlation with any mesenteric viscoelastic parameter and suggests that mesenteric viscoelastic properties have no interdependence with creeping fat. However, the cohort is too small to corroborate hard conclusions based on this finding.
In regards to the comparison within CD patients between affected and presumably unaffected mesentery, no significant differences were found for each of the viscoelastic parameters. One can speculate based on these findings that mesenteric involvement in active CD may be more extensive than seen at conventional imaging. This is supported by Büning et al. [32] who showed that patients with stricturing CD had a higher visceral adipose tissue/total fat mass ratio and proinflammatory cytokines (interleukin-6) compared to patients with nonstricturing CD. Likewise, the study showed that there were more patients with higher visceral adipose tissue/total fat mass ratios in short-term remission than in long-term remission.
Our study displays a reliable repeatability of MRE of the mesentery in healthy volunteers. The LoA and CoV are in line with literature that has used similar methods in other abdominal applications [33,34,35]. Furthermore, the measured difference between CD patients and healthy volunteers surpasses the within-subject CoV, meaning that the measured differences are greater than the acquisition variability. However, it is important to note that no repositioning took place between MRE scans. This was to ensure the most optimal overlap in the ROI between both scans of the same part of the mesentery. This approach was considered more crucial in a feasibility study with histopathology correlation, although it may have affected the repeatability outcome by potentially inflating it. Several limitations exist in our study.
First, we performed an exploratory study with a small patient cohort. This precludes firm conclusions, although the findings suggest a role of MRE in determining the amount of mesenteric disease involvement (i.e., fibrosis) in active CD patients. Moreover, the spin-echo echo-planar imaging sequence requires fat suppression to reduce EPI artifacts due to high fat signal. Smaller fat signals in proximity to the main water peak are not suppressible with spectral presaturation with inversion recovery yet cause a minimal water-fat shift. The remaining signal is lower, but is not zero as a result of incomplete fat suppression. Nonetheless, the OSS-SNR in subjects was of sufficient amount to determine the viscoelastic properties and notably larger in affected mesentery in CD patients. This could be due to infiltration of other tissues (i.e., fibrosis) in the mesenteric fat. Therefore, differences in viscoelastic properties between active CD patients and healthy volunteers are caused by differences in underlying tissue structures.
Furthermore, our patient population all had long-standing Crohn’s disease, and they underwent surgical resection as this study was designed to include histopathological verification. Therefore, this patient cohort does not reflect the entire CD patient population. It would be interesting to investigate mesenteric viscoelastic properties in patients with a shorter disease duration. If the mesenteric viscoelastic properties between patients with long-standing disease differ between patients with a shorter disease duration, this could possibly play a role in clinical decision-making with respect to either surgical or medical treatment.
To conclude, our findings suggest that MRE may have the potential to measure the amount of mesenteric fibrosis of the affected mesenteric fat in active CD. Therefore, it could give more insight into disease progression and the role of the mesentery in the disease course. Future research should look into the value of MR elastography concerning viscoelastic properties of small bowel mesentery in a more heterogeneous group of patients with CD, with advanced and less advanced disease progression. Moreover, the quantitative nature of MRE allows for the exploration of patient’s stratification options, based on the amount of mesenteric fibrosis. Defining cutoff values for mesenteric fibrosis detection could help surgeons in their decision-making whether to perform an extended mesenteric resection or conventional bowel surgery. This could contribute to a proper identification of the added value MRE could play in managing patients with active CD.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- |G*|:
-
Shear stiffness
- 3D:
-
Three dimensional
- CD:
-
Crohn’s disease
- CoV:
-
Coefficient of variation
- IQR:
-
Interquartile range
- LoA:
-
Limits of agreement
- MCFI:
-
Mesenteric creeping fat index
- MDEV:
-
Multifrequency dual elasto-visco
- MEGs:
-
Motion encoding gradients
- MRE:
-
Magnetic resonance elastography
- OSS-SNR:
-
Octahedral shear strain signal-to-noise ratio
- ROI:
-
Region of interest
- SWS:
-
Shear wave speed
References
Ha CWY, Martin A, Sepich-Poore GD et al (2020) Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 183:666−683 e617 doi:https://doi.org/10.1016/j.cell.2020.09.009
Goncalves P, Magro F, Martel F (2015) Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm Bowel Dis 21:453–467. https://doi.org/10.1097/MIB.0000000000000209
Borley NR, Mortensen NJ, Jewell DP, Warren BF (2000) The relationship between inflammatory and serosal connective tissue changes in ileal Crohn’s disease: evidence for a possible causative link. J Pathol 190:196–202. https://doi.org/10.1002/(SICI)1096-9896(200002)190:2%3c196::AID-PATH513%3e3.0.CO;2-5
Mao R, Kurada S, Gordon IO et al (2019) The mesenteric fat and intestinal muscle interface: creeping fat influencing stricture formation in Crohn’s disease. Inflamm Bowel Dis 25:421–426. https://doi.org/10.1093/ibd/izy331
Sheehan AL, Warren BF, Gear MW, Shepherd NA (1992) Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg 79:955–958. https://doi.org/10.1002/bjs.1800790934
Meng J, Mao Y, Zhou J et al (2022) Mesenteric abnormalities play an important role in grading intestinal fibrosis in patients with Crohn’s disease: a computed tomography and clinical marker-based nomogram. Therap Adv Gastroenterol 15:17562848221122504. https://doi.org/10.1177/17562848221122504
Coffey CJ, Kiernan MG, Sahebally SM et al (2018) Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis 12:1139–1150. https://doi.org/10.1093/ecco-jcc/jjx187
Zhu Y, Qian W, Huang L et al (2021) Role of extended mesenteric excision in postoperative recurrence of Crohn’s colitis: a single-center study. Clin Transl Gastroenterol 12:e00407. https://doi.org/10.14309/ctg.0000000000000407
van der Does de Willebois EML, group Ss (2022) Mesenteric sparing versus extensive mesenterectomy in primary ileocolic resection for ileocaecal Crohn’s disease (spicy): study protocol for randomized controlled trial. BJS Open 6 doi:https://doi.org/10.1093/bjsopen/zrab136
Luglio G, Rispo A, Imperatore N et al (2020) Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: the supreme-cd study - a randomized clinical trial. Ann Surg 272:210–217. https://doi.org/10.1097/SLA.0000000000003821
Gionchetti P, Dignass A, Danese S et al (2017) 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis 11:135–149. https://doi.org/10.1093/ecco-jcc/jjw169
Rimola J, Alfaro I, Fernandez-Clotet A et al (2018) Persistent damage on magnetic resonance enterography in patients with Crohn’s disease in endoscopic remission. Aliment Pharmacol Ther 48:1232–1241. https://doi.org/10.1111/apt.15013
Li XH, Feng ST, Cao QH et al (2021) Degree of creeping fat assessed by ct enterography is associated with intestinal fibrotic stricture in patients with Crohn’s disease: a potentially novel mesenteric creeping fat index. J Crohns Colitis. https://doi.org/10.1093/ecco-jcc/jjab005doi:10.1093/ecco-jcc/jjab005
Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V (2011) Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin Gastroenterol Hepatol 9:684−687 e681 doi:https://doi.org/10.1016/j.cgh.2011.05.005
Li Y, Zhu W, Gong J et al (2015) Visceral fat area is associated with a high risk for early postoperative recurrence in crohn’s disease. Colorectal Dis 17:225–234. https://doi.org/10.1111/codi.12798
Connelly TM, Juza RM, Sangster W, Sehgal R, Tappouni RF, Messaris E (2014) Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn’s disease patients. Dig Surg 31:219–224. https://doi.org/10.1159/000365359
Gu P, Chhabra A, Chittajallu P et al (2022) Visceral adipose tissue volumetrics inform odds of treatment response and risk of subsequent surgery in IBD patients starting antitumor necrosis factor therapy. Inflamm Bowel Dis 28:657–666. https://doi.org/10.1093/ibd/izab167
Li XH, Feng ST, Cao QH et al (2021) Degree of creeping fat assessed by computed tomography enterography is associated with intestinal fibrotic stricture in patients with Crohn’s disease: a potentially novel mesenteric creeping fat index. J Crohns Colitis 15:1161–1173. https://doi.org/10.1093/ecco-jcc/jjab005
Lo Re G, Picone D, Vernuccio F et al (2017) Comparison of us strain elastography and entero-mri to typify the mesenteric and bowel wall changes during Crohn’s disease: a pilot study. Biomed Res Int 2017:1–6. https://doi.org/10.1155/2017/4257987
Low G, Kruse SA, Lomas DJ. (2016) General review of magnetic resonance elastography. World J Radiol. 28;8(1):59−72. https://doi.org/10.4329/wjr.v8.i1.59.
Manduca A, Bayly PJ, Ehman RL et al (2020) MR elastography: principles, guidelines, and terminology. Magn Reson Med 85:2377–2390. https://doi.org/10.1002/mrm.28627
Glaser KJ, Manduca A, Ehman RL (2012) Review of MR elastography applications and recent developments. J Magn Reson Imaging 36:757–774. https://doi.org/10.1002/jmri.23597
Tang A, Cloutier G, Szeverenyi NM, Sirlin CB (2015) Ultrasound elastography and mr elastography for assessing liver fibrosis: part 2, diagnostic performance, confounders, and future directions. AJR Am J Roentgenol 205:33–40. https://doi.org/10.2214/AJR.15.14553
Papazoglou S, Xu C, Hamhaber U et al (2009) Scatter-based magnetic resonance elastography. Phys Med Biol 54:2229–2241. https://doi.org/10.1088/0031-9155/54/7/025
Tzschätzsch H, Guo J, Dittmann F et al (2016) Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal 30:1–10. https://doi.org/10.1016/j.media.2016.01.001
Reiter R, Shahryari M, Tzschätzsch H et al (2021) Influence of fibrosis progression on the viscous properties of in vivo liver tissue elucidated by shear wave dispersion in multifrequency MR elastography. J Mechan Behav Biomed Mater 121:104645. https://doi.org/10.1016/j.jmbbm.2021.104645
Satsangi J, Silverberg MS, Vermeire S, Colombel J-F (2006) The montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55:749–753. https://doi.org/10.1136/gut.2005.082909
Xiong S, Whitehurst CE, Li L et al (2022) Reverse translation approach generates a signature of penetrating fibrosis in Crohn’s disease that is associated with anti-TNF response. Gut 71:1289–1301. https://doi.org/10.1136/gutjnl-2020-323405
Geboes K (2003) Histopathology of Crohn ’ s disease and ulcerative colitis. Crohn 18:726–733. https://doi.org/10.1038/ncpgasthep0528
Shahryari M, Tzschatzsch H, Guo J et al (2019) Tomoelastography distinguishes noninvasively between benign and malignant liver lesions. Can Res 79:5704–5710. https://doi.org/10.1158/0008-5472.CAN-19-2150
Streitberger KJ, Lilaj L, Schrank F et al (2020) How tissue fluidity influences brain tumor progression. Proc Natl Acad Sci U S A 117:128–134. https://doi.org/10.1073/pnas.1913511116
Buning C, von Kraft C, Hermsdorf M et al (2015) Visceral adipose tissue in patients with Crohn’s disease correlates with disease activity, inflammatory markers, and outcome. Inflamm Bowel Dis 21:2590–2597. https://doi.org/10.1097/MIB.0000000000000527
An H, Shi Y, Guo Q, Liu Y (2016) Test–retest reliability of 3D EPI MR elastography of the pancreas. Clin Radiol 71:1068.e1067–1068.e1012. https://doi.org/10.1016/j.crad.2016.03.014
Pagé G, Tardieu M, Besret L et al (2019) Assessing tumor mechanics by mr elastography at different strain levels. J Magn Reson Imaging 50:1982–1989. https://doi.org/10.1002/jmri.26787
Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL (2015) Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J Magn Reson Imaging 41:369–375. https://doi.org/10.1002/jmri.24572
Acknowledgements
Gerrit Hooijer for support of the logistics of the resection specimens and scanning the pathology images and Eelco Schol for processing the resection specimens according to protocol and a lot of patience and attention for segmenting the sections and making photographs. Furthermore, we would like to thank all the patients and the healthy volunteers who agreed on scanning their mesenteries.
Funding
For this project, no funding was involved.
Author information
Authors and Affiliations
Contributions
Study design, AS, NW, KB, ES, JR, and JS; data collection, AS, KB, and NW; data analysis, AS and KB; data interpretation, AS, KB, and JS; drafting manuscript, AS, KB, and JS; radiological guidance, JS; pathological guidance, ANB; surgical interventions, CB and JvB; revising manuscript, AS, KB, NW, JR, ES, ANB, KG, CB, JvB, AN, and JS; and approval of manuscript, KB, AS, NW, ES, JR, KG, CB, JvB, ANB, JS and AN.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in agreement with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the (Amsterdam UMC, location AMC). Healthy volunteers signed informed consent and were recruited for protocol optimization with approval of the local Medical Ethics Committee by means of a waiver. All included patients and healthy volunteers provided written informed consent.
Consent for publication
Not applicable.
Competing interests
Jaap Stoker is a member of the European Radiology Experimental Editorial Board. He has not taken part in the review or selection process of this article. The remaining authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Comparison of viscoelastic properties of affected small bowel mesentery in Crohn’s Disease patients and mesentery in healthy volunteers, and between affected and ‘presumably’ unaffected mesentery within Crohn’s Disease patients (*p<0.05, ***p ≤0.001).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Schelt, AS., Beek, K.J., Wassenaar, N.P.M. et al. Viscoelastic properties of small bowel mesentery at MR elastography in Crohn’s disease: a prospective cross-sectional exploratory study. Eur Radiol Exp 7, 53 (2023). https://doi.org/10.1186/s41747-023-00366-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41747-023-00366-5