Abstract
Background
Poor sleep may negatively affect parents’ health-related quality of life (HRQoL). This longitudinal study aimed to describe and compare sleep, insomnia and HRQoL in mothers and fathers of preterm and full-born infants, and to assess possible associations between sleep, insomnia, and HRQoL from birth up to 12 months in the total sample.
Methods
A longitudinal study of parents of preterm (n = 25 couples) and full-born (n = 76 couples) infants was conducted. To assess sleep, parents wore wrist actigraphs and filled out sleep diaries for 2 consecutive weeks before responding to a digital questionnaire regarding insomnia symptoms and HRQoL. Actigraphy and sleep diary data were collected at the infant age of 2 months, while questionnaire data on insomnia and HRQoL were collected at the infant ages of 2, 6, and 12 months. Statistical analyses included linear regression and linear mixed models for repeated measures.
Results
There were no statistically significant differences in total sleep time (actigraphy and sleep diary) between the parent groups (preterm and full-born) at 2 months postpartum. Sleep efficiency was significantly higher for the full-born group. All mothers reported significantly shorter total sleep time and lower sleep efficiency compared to fathers (all p < 0.01). In the whole sample, insomnia incidence at 2 months postpartum was high (> 43.5%), and for mothers, it remained high at 6 and 12 months (> 50%). No significant HRQoL differences were identified between the parent groups over time. Fathers in both groups reported significantly higher physical HRQoL levels compared to mothers (p = 0.04). There were no significant associations between total sleep time or sleep efficiency and HRQoL at 2 months postpartum. Insomnia symptoms were associated with reduced mental and physical HRQoL at all measurement points.
Conclusions
Sleep efficiency (actigraphy and sleep diary) was significantly higher for the full-born group compared to the preterm group. Mothers (both groups) experienced significantly shorter total sleep time and lower sleep efficiency compared to fathers. The incidences of insomnia symptoms were high at 2 months postpartum for the whole sample and remained high at follow-up for mothers. Fathers (both groups) reported higher physical HRQoL compared to mothers. Insomnia symptoms had a significantly negative impact on parents’ long-term HRQoL.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Sleep is important for the normal function and recovery of all body systems (Carscadon and Dement 2017). Sleep affects physical, cognitive, and mental health; the immune system; and social functions (Redeker 2011). Normal, healthy sleep can be defined by sufficient duration, quality, appropriate timing and regularity, and lack of sleep disturbances and disorders (Medic et al. 2017). Deficits in sleep quantity and quality that impact the continuity of sleep are often referred to as sleep disruption (Medic et al. 2017). Total sleep time (TST) refers to the amount of sleep time (minutes) from sleep onset to sleep offset (Shrivastava et al. 2014). A low total sleep time at night may indicate that individuals slept insufficiently (Shrivastava et al. 2014). Sleep efficiency (SE) refers to the percentage of total time in bed, actually spent asleep (Shrivastava et al. 2014). To evaluate whether an individual's sleep is normal or not, SE is a valuable indicator, values above 85% are indicative of normal sleep (Bjorvatn 2012). SE is often used as a primary outcome measure in insomnia research (Reed and Sacco 2016). Insomnia is a highly prevalent sleep disorder among adults (Sivertsen et al. 2021). The diagnostic criteria for insomnia diagnosis are defined as ‘difficulty initiating or maintaining sleep for at least 3 months, in addition to impaired daytime functioning caused by the sleep disturbance’ (Bjorvatn et al. 2018). Sleep disruption and insomnia has the potential to impact health (Banks et al. 2017) and HRQOL (Luyster 2013) negatively. This longitudinal study focuses on postpartum parents’ TST, SE and insomnia.
The ‘postpartum period’ refers to the immediate period from childbirth up to 6 months after (Romano et al. 2010). During this period, parents often experience reduced opportunities for sleep, along with increased wakefulness at night, daytime sleepiness, fatigue, and reduced neurobehavioral performance (Insana et al. 2014). Maternal sleep is often the most affected (Insana et al. 2013), but parental sleep often gradually improves from 10–12 weeks postpartum (Dørheim et al. 2009). Between 6 and 12 months postpartum, many infants develop a more stabilized sleep pattern and sleep through the night (Bruni et al. 2014). However, some parents develop more significant sleep problems than others and are more vulnerable to changes following childbirth (Sivertsen and Dorheim 2015). Postpartum sleep disturbances have been associated with a wide array of negative health consequences, including postpartum depression (Busse et al. 2013; Vigod et al. 2010).
Although sleep deprived, most parents experience the birth of an infant as a happy life event (Lee and Hsu 2012). In contrast, parents have described a preterm birth as a crisis and a traumatic life experience (Lee and Hsu 2012). Preterm births are defined as deliveries before 37 completed weeks of gestation (World health organization.Preterm birth, from 2018). The early birth and admission of a preterm infant to the neonatal intensive care unit (NICU) has been associated with considerable stress for caregivers, particularly if infants are in the lower birthweight categories and have a higher risk of mortality and morbidity (very low birthweight ≥ 1,500 g) (Singer et al. 1999). Preterm infants can be hospitalized for weeks or even months and discharged from the hospital with complex health needs (McAndrew et al. 2019). For parents, the consequences of a preterm birth can be far-reaching and influence several life aspects (Moura et al. 2017). Two reviews reported that the sleep quality and quantity of mothers of preterm infants are poor in the first few postpartum months (Haddad et al. 2019; Marthinsen et al. 2018). High levels of perceived stress negatively affect these mothers’ sleep outcomes (Busse et al. 2013; Lee and Hsu 2012; Haddad et al. 2019). Stress can lead to poor sleep and, in turn, increase the risk of fatigue, anxiety, and depression, as well as reduce health-related quality of life (HRQoL), early after childbirth (Lee and Hsu 2012; Lee and Kimble 2009). Previous research has mainly focused on sleep among mothers of preterm infants early in the postpartum period, while there has been little focus on fathers’ sleep (Haddad et al. 2019; Marthinsen et al. 2018). There is also a lack of comparative studies to determine whether sleep differs between parents of preterm and full-born infants over time (Marthinsen et al. 2018).
Pregnancy and postpartum have been identified as vulnerable periods for developing sleep problems such as insomnia, particularly for women (Sivertsen and Dorheim 2015; Sivertsen et al. 2017). Previous publications have reported that insomnia prevalence for postpartum mothers is very high (60%) immediately after childbirth and remains high (41%) after 2 years (Sivertsen and Dorheim 2015; Sivertsen et al. 2017). Different factors, such as genetic, environmental, social, medical, and mental conditions (including stressful life events), can contribute to the onset of insomnia (Morin et al. 2003; Zambotti et al. 2018; Sivertsen et al. 2014). Although parents of preterm infants may be at risk of developing insomnia due to the stressful birth event, few studies have examined the prevalence of insomnia for these parents. In a Swedish study, Blomqvist et al. (Blomqvist et al. 2017) identified a high insomnia prevalence in parents of preterm infants early after childbirth, particularly mothers. Insomnia has been associated with significant physical and mental health impairments, including poor HRQoL, in other populations (Baglioni et al. 2011; Hertenstein et al. 2019).
HRQoL is an important outcome and a prominent indicator of how various conditions affect individuals’ lives (Pagels et al. 2020). HRQoL is a multidimensional concept covering physical, psychological, social, and spiritual aspects of life (Post 2014). Previous cohort studies have compared the quality of life (QoL) of parents of preterm and full-term infants. Some studies have reported no differences in QoL (Donohue et al. 2008; Eiser et al. 2005), while others have indicated lower QoL for parents of preterm infants (Witt et al. 2012; Klassen et al. 2004; Hill and Aldag 2007). None of these studies, however, have included an assessment of sleep or insomnia. To our knowledge, no longitudinal study has explored sleep and its associations with health and HRQoL over time in this vulnerable parent population (Haddad et al. 2019; Marthinsen et al. 2018). Previous literature has shown that parents of preterm infant’s experience practical and mental challenges after discharge from hospital (Aagaard et al. 2023). Considering that a preterm delivery might be a stressful life event (Busse et al. 2013; Lee and Hsu 2012; DaF et al. 2017) with the potential to adversely affect parental sleep over time, we found it important to describe and compare sleep, and examine if these parents were more prone to sleep disturbances compared to parents of full- born infants over time. Based on the literature, our presumptions were that parents of premature infants sleep poorly, particularly in the early postpartum period, and will be more susceptible to sleep disturbances like insomnia compared to parents of full-born infants (comparison group) (Haddad et al. 2019; Blomqvist et al. 2017; McMillen et al. 1993) from birth up to 12 months postpartum. We presume that mothers will sleep poorer than fathers, regardless of whether the infant is premature or not (Blomqvist et al. 2017; Lee et al. 2007). Lastly, we anticipate that poor sleep can reduce HRQoL in parents postpartum (Lee and Hsu 2012).
This longitudinal study aimed to describe and compare sleep, insomnia and HRQoL in mothers and fathers of preterm and full-born infants and to assess possible associations between sleep, insomnia, and HRQoL from birth up to 12 months in the total sample.
Methods
Study design, setting, and population
A comparative longitudinal study was conducted between June 2019 and March 2020. Parents were recruited as couples into two separate groups: one group included parents with preterm infants (born before the 37th week of pregnancy), recruited from NICUs and maternity wards, whereas the other group (comparison group) included parents with full-born infants (born after the 37th week of pregnancy), recruited from two maternity wards.
The inclusion criteria for both parent groups were that parents needed to be over 16 years of age, be living together, and have a sufficient command of a Nordic language (written and oral). Both mother–father parents and same-sex parents (referred to as birth-giving and non-birth-giving mothers) were included in the study.
Parents were excluded if they had a serious drug addiction (recorded in the patient journal; cf. International Classification of Diseases (ICD-10) or Diagnostic and Statistical Manual for Mental Disorders [4th ed.]), the newborn had serious deformities/or a life-threatening condition that could affect survival, the mother had given birth to multiple infants, or the mother had a condition/diagnosis which made participation in the project ethically challenging (serious, life-affecting health issues). Parents with shift work were excluded, as working at night impacts sleep. Those with sleep diagnoses (according to International Classification of Sleep disorders ICSD-3) were also excluded (American Academy of Sleep medicine: International Classification of Sleep Disorders- Third Edition 2014). See Fig. 1a and b for a flow chart of the participants.
Parents with preterm infants were recruited from four different NICUs and one maternity ward in southeastern Norway. Three of the NICUs were at Level 3c, while one was at Level 3b. In Norway, Level 3c units have the highest medical competence to treat extremely preterm infants, starting from gestational age (GA) 23. Level 3b units have the second-highest competence and treat preterm infants from GA 26 (Helsedirektoratet.Nyfødtavdelinger, kompetanse og kvalitet, from 2017). Parents with full-born infants were recruited from two maternity wards in the same geographic area (southeastern Norway) which treated healthy mothers with uncomplicated births.
Sample size
The study sample size was estimated using data from two previous studies reporting on differences in total sleep time (TST) for mothers of preterm (Lee and Hsu 2012) and full-born infants (Montgomery-Downs et al. 2010). Previous research has shown that mothers of preterm infants sleep, on average, for 6.3 (SD 2) hours per night (Lee and Hsu 2012), compared to 7.0 (SD 1) hours for a group of mothers of full-born infants (Montgomery-Downs et al. 2010). Thus, we assumed a one-hour difference in the TST between the groups. To account for multiple testing, we estimated that it would be sufficient to include ≥ 75 couples with a preterm infant and ≥ 75 couples with a full-born infant.
Recruitment
A collaborating nurse within each hospital ward was responsible for the recruitment of parents of preterm and parents of full-born (comparison group) infants. The nurses identified eligible parent couples and received informed consent from parents who wanted to participate.
The original plan was to recruit the targeted number of parent couples to the preterm and full-born groups between June and December 2019. For the preterm group, the recruitment period was more extensive due to a low recruitment rate. The total recruitment period for the preterm group was 33 weeks, until March 2020 when coronavirus disease 2019 (COVID-19) restrictions ended all recruitment efforts. By then, only 25 parent couples had been recruited to the preterm group.
For the full-born group, 78 parent couples were recruited within 15 weeks from the two maternity wards. Two couples withdrew their consent before the baseline measurement. Therefore, a total of 76 parent couples participated in the full-born group.
Data collection
This sub-study was part of a prospective, comparative, longitudinal study that compared sleep, psychosocial variables and HRQoL between parents of premature and full-born infants over time during the postpartum period. Descriptive data for some of the longitudinal outcomes are published tabularly in a feasibility study (Marthinsen et al. 2022). A tabular overview of the sample (parents and infants), descriptive overview over results for subjective and objective sleep (baseline), and descriptive results for psychosocial variables (depression, fatigue, stress, insomnia, social support, self- efficacy and HRQoL) at 2,6 and 12 months can be viewed in the feasibility study (Marthinsen et al. 2022).
All parent couples were included before 5 weeks postpartum so that actigraphs and sleep diaries could be sent to parents’ home addresses before the first data collection. Subjective and objective sleep data were collected around the infant age of 2 months (6–8 weeks postpartum). Participants received a postal mail with two preprogrammed actigraphs – Respironics Actiwatch 2 (Philips Healthcare, Philips.com; https://www.philips.no/healthcare) – and two preplotted (dates only) sleep diaries to be completed over the same period as the actigraphs. After 2 weeks of sleep recordings, parents responded to a digital questionnaire regarding insomnia and HRQoL. The actigraphs and sleep diaries were returned to the first author in a prepaid envelope. The data collection occurred before and during the first wave of the COVID-19 pandemic. The pandemic introduced great uncertainties regarding how the virus was spread; and it was decided to not distribute actigraphs and sleep diaries to parents after March 2020. Sleep data from actigraphs and sleep diaries were therefore collected at 2 months only.
Parents responded to a digital questionnaire assessing insomnia and HRQoL at three measurement points (2, 6, and 12 months). The questionnaire included items on parental sociodemographic variables such as age, educational level, income, employment status, ethnicity, weight, height, and parity. The questionnaire also included items on the infants’ data, including GA at birth, birthweight, and infant length. All sociodemographic variables have been associated with sleep in previous research (Haddad et al. 2019).
Measures
Subjective sleep (sleep diary)
Subjective sleep data was collected using sleep diaries. At baseline, both parents independently completed a sleep diary for 2 weeks. A sleep diary similar to Carney et al.’s (Carney et al. 2012) recommendation was used. The diary consisted of the parents’ estimates of own daytime and nighttime sleep patterns, including perceived sleep quality. The following measures were reported in the sleep diary: number of daytime naps, daytime nap duration, daytime function (1, very good; 5, very poor), sleep onset latency (SOL – the number of minutes to fall asleep), wake after sleep onset (WASO – the number of minutes awake between sleep onset and sleep offset), number of nighttime awakenings, early morning awakening, total wake time (SOL + WASO + early morning awakening), TST (number of minutes asleep in bed after ‘lights off’, considered nighttime sleep), time in bed (TIB = the duration spent in bed), sleep efficiency (SE = % TST as a percentage of TIB), and sleep quality rating (1, very restless; 5, very poor) (Fekedulegn et al. 2020; Fung et al. 2013).
Objective sleep (wrist actigraphy)
Objective sleep-wake data was assessed at baseline using wrist actigraphs – Actiwatch 2 (Philips Healthcare, Philips.com; https://www.philips.no/healthcare). Actigraphs are noninvasive motion sensors used to assess sleep–wake patterns in adults (Martin and Hakim 2011; Marino et al. 2013). Actigraphy has been tested and found reliable compared to polysomnography, especially for TST estimates (Weiss et al. 2010). The sensitivity of the Actiwatch was set to medium. Data was collected in 30-s epochs (activity counts) and transferred to a computer for analysis. Parents were asked to press an event marker to indicate when they went to bed to sleep for the night and when they got out of bed in the morning. Parents wore the Actiwatch on their nondominant wrist. The following measures were derived from the actigraphs: SOL, TIB, SE, WASO, total wake time, and TST.
Insomnia
The Bergen Insomnia Scale (BIS) was used to assess insomnia symptoms at all three measurement points (2, 6, and 12 months) (Pallesen et al. 2008). The BIS was originally developed to correspond with the diagnostic criteria for insomnia presented in the Diagnostic and Statistical Manual for Mental Disorders (4th ed., Text Revision) (American Psychiatric Association 2000). Each BIS item is scored along a scale from 0 to 7, indicating the number of days per week for which a specific insomnia symptom was experienced (0–7 days). The first four items measure sleep impairment, assessing sleep onset (sleep latency > 30 min), WASO (> 30 min), early morning awakening (> 30 min), and nonrestorative sleep, and the last two items measure daytime impairment and dissatisfaction with sleep (Pallesen et al. 2008). The BIS provides a total score and may be used as a dichotomous score for the presence or absence of chronic insomnia (Sivertsen and Dorheim 2015). Chronic insomnia disorder was defined as scoring 3 days per week or more on at least one of the first three items, as well as 3 days per week or more on at least one of the latter two items (Pallesen et al. 2008). The BIS originally defined insomnia as symptoms experienced during the past month but was modified to a wider time frame of the last 3 months in the updated Diagnostic and Statistical Manual for Mental Disorders (5th ed.)/International Classification of Sleep Disorders-3 diagnostic criteria (American Psychiatric Association 2013). The BIS has acceptable test–retest reliability and good validity in relation to other self-report measures and polysomnography (Pallesen et al. 2008).
HRQoL
HRQoL was assessed using the RAND Medical Outcomes Study 36-Item Short Form Survey (RAND-36) (Hays and Morales 2001). The RAND-36 is a self-reported generic HRQoL questionnaire that includes eight domains: general health, bodily pain, physical function, role limitation (physical), mental health, vitality, social function, and role limitations (emotional). The domains can be combined into physical and mental scales reflecting both physical (physical component summary [PCS]) and mental (mental component summary [MCS]) well-being (Hays and Morales 2001; Ware and Sherbourne 1992). The RAND-36 is scored using values from 0 to 100, with 100 representing optimal health (Kvien et al. 1998). The Norwegian version of the RAND-36 has been reported as a valid and reliable instrument for assessing HRQoL (Garratt and Stavem 2017).
Attrition
Attrition refers to the failure of participants to complete their measurements after being enrolled in a study (DeMauro et al. 2019). Not all participants responded to the questionnaire. Consequently, the number of participants at a given assessment point did not always correspond to the number of returned questionnaires. Therefore, we decided to compute attrition based on the number of participants (mothers and fathers) and not couples. Attrition rates were calculated as participants (mothers or fathers) who completed the questionnaire at 6 and 12 months divided by the number of participants (mothers and fathers) at baseline, 2 months (Fig. 1a and b).
Statistical analysis
IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA) was used for the statistical analyses. Descriptive statistics were used to describe the study sample. Categorical data are presented as frequencies and percentages, and continuous data are presented as median, minimum, and maximum values.
The mean values of sleep obtained from 7 days of actigraphy and sleep diary data were analysed. To analyse objective sleep (actigraphy), activity counts and light off/on were used to determine sleep (rest) and wake intervals in actograms (Marino et al. 2013; Ancoli-Israel et al. 2015; Chow et al. 2016). We used sleep diaries to control sleep onset and sleep offset time, and control artefacts and uncertainties in actigraphy data (Mazza et al. 2020). In the case of missing data, the 7 days with the least combined missing data from both measures (actigraphy and sleep diary) were used. Daytime nap time was not collected from actigraphs since many participants had forgotten to use the Actiwatch’s button to register daytime sleep. Therefore, daytime sleep was registered in sleep diaries only.
In the regression analysis, sleep length -TST, sleep quality/continuity- SE, and insomnia were the selected sleep variables to be examined. To assess possible differences in TST and SE between the parent groups (preterm compared to full-born) and genders (mothers compared to fathers) we fitted four linear regression models with TST and SE as the dependent variables and parent (mother or father) and group (preterm or full-born) as the independent variables. To investigate if there was an interaction between the group and parent variables, in addition to the additive effect (e.g. if being a mother of a preterm infant presented an additional burden), we fitted an interaction term (group * parent). This interaction was not significant for any of the models, so we further adopted models fitted with group and parent variables only.
To assess whether there was a difference in TST and SE between parents of preterm and full-born infants, we fitted four different regression models assessing four dependent variables: the sleep diary variables were a) TST and b) SE, while the actigraphy variables were c) TST and d) SE. The independent (indicator) variables were.
-
Group (preterm or full-born)
-
Parent (mother or father)
-
Interaction (parent and group)
Longitudinal data analyses were performed using linear mixed models for repeated measures to include multiple repeated measurements of each patient’s outcome over time. Possible associations between sleep (TST, SE), insomnia, and HRQoL were assessed with multiple linear regression.
To ease interpretation of the linear regression results, we also computed effect sizes (ESs) (Sawilowsky 2009). The ES is interpreted as follows: d < 0.1, very small effect; d < 0.2, small effect; d < 0.5, moderate effect; and d < 0.8, large effect (Sawilowsky 2009).
Results
Attrition and missing data
The longitudinal sample included 25 parent couples with preterm infants and 76 couples with full-born infants. At 6 months, 21 (preterm) and 61 (full-born) couples participated in the study. At 12 months, 17 (preterm) and 60 (full-born) couples participated in the final measurement (Fig. 1a and b).
For the preterm group, 25 couples (25 mothers and 25 fathers) were recruited for the study. Nine mothers and 6 fathers responded to the questionnaire at 6 months, producing attrition rates of 64% (mothers) and 76% (fathers). At 12 months, 10 mothers and 8 fathers responded, producing attrition rates of 60% (mothers) and 68% (fathers; Fig. 1a).
For the full-born group, 76 parent couples (76 mothers, 73 fathers, and 3 non-birth-giving mothers) were recruited for the study. Thirty-nine mothers and 31 fathers responded to the questionnaire at 6 months, producing attrition rates of 49% and 59%, respectively. At 12 months, 30 mothers and 31 fathers responded; attrition rates were 61% and 59%, respectively (Fig. 1b).
Characteristics of the sample
The median maternal age was 30.5 years (preterm group) and 31.5 years (full-born group) (Table 1). For fathers, the median age was 32.0 years (preterm) and 33.0 years (full-born). The parent groups were quite similar according to age, body mass index, education, and income level. Most participants were first-time parents (primiparas) and had Norwegian ethnicity and an income level ≥ NOK 500,000 (about €50,000) per year (Marthinsen et al. 2022). As many as 15 (60%) of the infants in the preterm group were in the ‘moderate/late’ category, with GA between 32 and 36 weeks. Only 2 (8%) were in the extremely preterm (GA < 28) and very preterm (GA 28–31) categories (Marthinsen et al. 2022).
Subjective and objective sleep in the parent sample
There was a general tendency towards mothers (preterm and full-born) reporting lower SE (sleep diary) compared to fathers (Table 1). Fathers’ SE was above the cutoff for normal values, which is considered ≥ 85% (Desjardins et al. 2019). Mothers (preterm group) reported the highest WASO and seemed to compensate for this by sleeping during daytime (Marthinsen et al. 2022). Mothers with preterm infants reported the highest daytime sleep length at baseline, with median nap duration on 24 min per week. Parents’ daytime function and self-rated sleep quality were quite similar and comparable for all responders (Marthinsen et al. 2022).
Group differences in subjective and objective sleep at 2 months postpartum
Total sleep time (TST)
Regarding TST (baseline), there were no statistically significant differences between the parent groups for either the sleep diary or actigraphy (data not shown).
Sleep efficiency (SE)
For SE, there were statistically significant differences between the parent groups. Parents in the full-born group reported higher SE values compared to those in the preterm group when assessed with actigraphy (B = 2.20, 95% CI [0.28 to 4.13], p < 0.025) and the sleep diary (B = 3.68, 95% CI [0.67 to 6.69], p = 0.017).
Gender differences in subjective and objective sleep at 2 months postpartum
Total sleep time (TST)
Comparing TST differences regarding gender, mothers (preterm and full-born) reported, on average, 40 min shorter TST compared to fathers in their sleep diaries (B = 39.15; 95% CI [22.62 to 55.67], p < 0.001). No similar differences were assessed by actigraphy.
Sleep efficiency (SE)
When genders were compared for SE differences, mothers (preterm and full-born) reported significantly lower SE levels (4% lower) compared to fathers on actigraphy (B = 4.26, 95% CI [2.58 to 5.94], p < 0.001). When SE was assessed with the sleep diary, mothers (preterm and full-born) reported, on average, about 12% lower SE compared to fathers (B = 11.93, 95% CI [9.29 to 14.58], p < 0.01). Gender differences were more pronounced when SE was assessed with a sleep diary than when using actigraphy data.
Insomnia
The prevalence of insomnia symptoms was high for both parents in both groups at baseline (Table 2), but parents in the preterm group demonstrated higher baseline incidences: 62.5% CI [35.4 to 84.8] for mothers and 71.4% CI [41.9 to 91.6] for fathers. The incidences for the full-born group were 53.4% CI [39.9 to 66.6] for mothers and 43.5% CI [28.9 to 58.9] for fathers. Over time, the insomnia incidence remained high (> 50%) for mothers (preterm and full-born) at both 6 and 12 months postpartum. However, the tendency between the parent groups was reversed, as parents in the full-born group reported a higher incidence of insomnia. There was also a tendency towards mothers (preterm and full-born) reporting a higher incidence of insomnia compared to fathers at the 6- and 12-month measurements.
Health related quality of life (HRQoL)
HRQoL was assessed at 2, 6, and 12 months postpartum (Table 2). Considering the entire follow-up, there was no statistically significant differences between parents of preterm and full-born infants regarding PCS (B = 1.42; 95% CI [-1.11 to 3.94], p = 0.273) or MCS (B = -0.10, 95% CI [-3.83 to 3.6], p = 0.962).
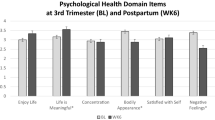
However, fathers (preterm and full-born) reported significantly higher PCS levels compared to mothers (preterm and full-born; B = 2.24; 95% CI [0.11 to 4.37], p = 0.04). The difference between mothers and fathers was present at baseline and did not change over time. Further, the PCS levels remained unchanged over the entire observation period. For the MCS, our data did not reveal any differences between mothers and fathers (B = 0.76; 95% CI [-2.41 to 3.93], p = 0.640). Notably, MCS levels remained unchanged from baseline (2 months) to 6 months but decreased significantly for both groups at 12 months (B = -2.16; 95% CI [-4.31 to -0.02], p = 0.048).
Associations between sleep, insomnia, and HRQoL
Associations between parents’ TST, SE, and insomnia and their HRQoL were analysed using multiple linear regression. For SE and TST (sleep diary and actigraphy), there were no significant associations between the sleep variables at baseline and the PCS or MCS (Supplementary tables 1–4). Using mixed models for repeated measures adjusted for gender, insomnia was strongly associated with lower HRQoL (Table 3). The effect was more pronounced for the MCS (B = -6.93 95% CI [-10.31 to -3.55] p < 0.001) compared to the PCS (B = -3.64 95% CI [-6.04 to -1.25], p = 0.003). For mental well-being (MCS), the reduction for those with insomnia was twice as large as that estimated for physical well-being (PCS). However, due to high data variations, the ESs were small (0.24 for the mental component and 0.18 for the physical component). Time, gender, and group did not affect the highly significant association between insomnia and HRQoL at any measurement point. Table 3 lists the changes over time in the PCS and MCS.
Discussion
To the best of our knowledge, this is the first study to examine parental sleep, insomnia symptoms, and HRQoL in mothers and fathers of preterm and full-born infants over time during the first postpartum year. Our key findings were that SE was lower for parents in the preterm group compared to those in the full-born group at 2 months postpartum. Moreover, our data revealed significant differences in sleep between the genders in the total sample. Mothers (preterm and full-born) reported significantly shorter TST and lower SE than fathers. Gender differences were most pronounced when sleep was assessed using subjective sleep diaries.
Additionally, we identified a very high prevalence of insomnia symptoms in both parent groups at 2 months postpartum. Although the prevalence decreased, the insomnia incidence remained stable and high for mothers (preterm and full-born) at the 6- and 12-month measurements. We also identified a statistically highly significant association between insomnia symptoms (at 2 months postpartum) and parents’ mental and physical well-being. The association was most pronounced for mental well-being.
In line with our presumptions, our results confirmed that parents of preterm infants are significantly more susceptible to experiencing poor sleep, with lower SE, than parents of full-born infants. The explanation for these results might be that parents of preterm infants’ transition to parenthood is far more stressful, with a higher risk of infant hospitalization and concerns for the infant’s medical condition and long-term health outcomes (Schappin et al. 2013). Stress has been highlighted as a major factor causing sleep problems for these parents (Lee and Hsu 2012; Haddad et al. 2019; Costa et al. 2021). Parents have described the NICU environment as unfamiliar and frightening (Heidari et al. 2013). They are often constantly present (Edell-Gustafsson et al. 2015; Lee et al. 2013) and underprioritize their own sleep (DaF et al. 2017; García et al. 2020). The NICU environment is primarily dedicated for the provision of advanced medical care and not for parental sleep (Edell-Gustafsson et al. 2015; Stremler et al. 2014). High levels of parental stress, together with concerns for the infant, and hospitalization in the NICU (Edell-Gustafsson et al. 2015; Lee et al. 2013) might explain the sleep differences in our parent samples.
We also hypothesized there could be gender differences in sleep between mothers and fathers. Our baseline results confirmed this hypothesis: the mothers in our sample (preterm and full-born) experienced shorter TST and lower SE compared to fathers. The literature supports our results (Montgomery-Downs et al. 2013; Richter et al. 2019; Kenny et al. 2020). Postpartum mothers often struggle to achieve restorative sleep during the first postpartum months (Montgomery-Downs et al. 2013). Despite maternal sleep pattern changes being a ‘normal phenomenon’ (McGuire 2013), it is important to note that some mothers develop more serious sleep problems of a more chronic nature (Polo-Kantola 2022). Mothers with significant sleep problems may have a higher risk of developing mental health issues – such as postpartum depression (Dørheim et al. 2009), which has been associated with negative outcomes for the whole family (Letourneau et al. 2012).
Our results indicated that gender differences regarding sleep were most pronounced when sleep was assessed using subjective sleep diaries. This is in line with previous research, which has reported discrepancies between subjective and objective sleep measurements (Trimmel et al. 2021; Rezaie et al. 2018). Subjective and objective sleep measurements are often used in combination to provide more in-depth sleep assessments (Ibáñez et al. 2018; Natale et al. 2009). Although subjective and objective sleep measurements (such as sleep diary and actigraphy, respectively) have different strengths and limitations, both measurements have been found reliable to give a valid indication of TST and SE.
Our results particularly raise concerns for mothers giving birth to preterm infants. These mothers reported the lowest SE among all participants. This is in line with previous studies that have raised concerns about these mothers’ sleep and mental health outcomes in the early postpartum period (Lee and Hsu 2012; Lee and Kimble 2009; Lee et al. 2012; Schaffer 2012; Shelton et al. 2014). Mothers of preterm infants have a higher risk of postpartum depression compared to those of full-born infants (Lefkowitz et al. 2010; Beck 2003). Sleep disturbances and stress are often closely linked (Lee and Hsu 2012). The high levels of maternal stress described in the literature (Lee and Hsu 2012; Haddad et al. 2019; Lee and Kimble 2009; DaF et al. 2017) may partly explain these mothers’ poor sleep outcomes, but further research is required. Efforts to reduce stress may be one way to support sleep for future mothers with preterm infants (Lee and Hsu 2012).
There was an overall pattern indicating a high prevalence of insomnia symptoms for both parent groups at 2 months postpartum. For mothers, the prevalence remained high at 6 and 12 months postpartum. Our results are in line with the literature confirming that postpartum mothers generally have a high prevalence of insomnia, which could be chronic (Sivertsen and Dorheim 2015; Sivertsen et al. 2017; Paavonen et al. 2017). The high prevalence of maternal insomnia symptoms is of concern since insomnia is associated with mental health issues (Emamian et al. 2019; Harvey et al. 2011; Ross et al. 2005; Cox and Olatunji 2020).
For parental HRQoL, we did not identify any differences between the groups or genders, which was surprising. We hypothesized that for parents with preterm infants, poor sleep could have a negative impact on parents’ HRQoL, as described in the literature (Luyster 2013). We did not find any significant associations between the baseline variables for TST or SE and parental HRQoL. However, there was a highly significant association between insomnia symptoms and reduced parental HRQoL over time, particularly for the subscale mental well-being. This is of concern since the prevalence of insomnia symptoms was high in our sample.
Insomnia is a rising public health problem with both individual and economic consequences (Sivertsen et al. 2014; Sivertsen et al. 2011; Daley et al. 2009). Our results indicate the need for insomnia prevention and treatment in postpartum parents, with a particular focus on mothers. The use of screening tools to evaluate sleep, insomnia symptoms, and depression should be implemented as standard care to identify vulnerable parents (Baglioni et al. 2022). Different interventions should also be tested to treat insomnia among new parents. Nonpharmacological treatments, such as cognitive behavioural therapy, have been tested to be effective (Tang 2009).
We suggest a stronger focus on sleep promotion, with guidance from early in the postpartum period. Evaluations of sleep disturbances and depression, with advice for sleep optimization, should be included as standard postpartum care for all parents within neonatal caregiving (Stremler et al. 2017). Nurses/midwives in hospital wards are in a unique position to provide guidance and recommendations to new parents so they can maintain a consistent sleep pattern (Redeker 2002). Such guidance could be implemented as standard information for parents (Schaffer et al. 2013). More studies on factors that affect postpartum parents’ sleep are needed so that sleep-promoting efforts can be developed.
Strengths and limitations
This is, to our knowledge, the first comparative longitudinal study to evaluate sleep, insomnia, and HRQoL in parents of preterm and full-born (comparison group) infants over time. The strengths of this study are the longitudinal study design, with collection of sleep and HRQoL data for both mothers and fathers in both groups over time.
The recruitment of parents of preterm infants represented a challenge in this study. We aimed to recruit a homogenous sample, which was reflected in rather strict inclusion criteria. The volume of twins/multiple infants (48.2%) and non-Nordic speaking individuals (20%) was surprisingly high for parents of preterm infants, and the inclusion criteria contributed to many exclusions (Fig. 1a and b). Around two-thirds of the preterm infant group refused to participate, compared to one-third of the full-born group (Fig. 1a and b). Due to the low number of participants, the statistical power was limited, and many of our estimates had low precision (increased risk of type 2 error). Retainment of parents over time was another challenge. The failure of parents to complete their participation at 6 and 12 months was high for both parent groups. This reduced the power for statistical analyses in this longitudinal study, compared to what was originally planned.
Other strengths of this study are the long-term sleep and HRQoL assessment, as well as the subjective and objective sleep measurements. The measurements give a broad picture of sleep pattern and relevant sleep variables, as high night-to-night variability is common for individuals suffering from insomnia (Perlis et al. 2014) and postpartum parents in general (Montgomery-Downs et al. 2013; Insana and Montgomery-Downs 2013). It was unforeseen that COVID-19 restrictions would interfere with recruitment and the long-term use of sleep measures; thus, we only could present actigraphy and sleep diary data at baseline.
For self- report measures, recall bias has been described as a problem (Ibáñez et al. 2018). Recall bias might have been present in this study; however, it might have been reduced since the sleep diary was filled out every morning and was not so dependent on memory. For BIS, the issue of recall bias might potentially have been more pronounced since this questionnaire assesses sleep as far back as 3 months.
We defined usable data for the actigraphs and sleep diaries as ≥ 1 day with ≥ 24 h of daily wear time; data that did not fulfil these criteria had to be discarded. This represents a limitation for our results since studies suggest that actigraphs should be used for at least 5–7 nights to accurately measure TST and SE (Aili et al. 2017).
Overall, the difficult recruitment of parents with preterm infants, high attrition rates, and the COVID-19 outbreak hampered recruitment and data collection in this longitudinal study. However, the statistically significant association between insomnia symptoms and HRQoL was highly noteworthy, and we considered it clinically relevant.
Conclusions
This study introduces knowledge about sleep and HRQoL in parents of newborn babies. Insomnia was identified as a frequently occurring sleep disorder among the studied parents. The prevalence was high in both groups 2 months postpartum and remained high at 6 and 12 months postpartum for the mothers. An important finding was that insomnia was the only sleep variable that had a significant negative impact on parents’ HRQoL. Mental HRQoL was particularly negatively affected.
Another important result was that parents of preterm children had poorer sleep quality at 2 months postpartum, assessed with subjective and objective measures, compared to parents of full-born infants. Mothers in both groups had significantly lower SE and shorter TST compared to the fathers for the same period. When HRQoL was compared between parent groups, no statistically significant differences were identified. Fathers in both groups had higher physical HRQoL compared to the mothers at all assessment points.
This study is relevant for health professionals working with new parents. Our results indicate that postpartum parents have impaired sleep after childbirth, and this requires increased attention from health professionals. Sleep promotion can be important steps to promote good HRQoL for parents. We suggest a stronger focus on sleep support and the prevention of sleep disorders, especially for new mothers. A particular concern should be paid to developing sleep support for vulnerable groups, such as mothers of preterm infants. Within clinical caregiving, sleep screening should be introduced as a standard routine so that parents with sleep problems can be identified and receive sleep-promoting help. Insomnia prevention is important to promote HRQoL among future postpartum parents.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- BIS:
-
Bergen Insomnia Scale
- COVID-19:
-
Coronavirus disease 2019
- ES:
-
Effect size
- GA:
-
Gestational age
- HRQoL:
-
Health-related quality of life
- MCS:
-
Mental component summary
- NICU:
-
Neonatal intensive care unit
- PCS:
-
Physical component summary
- QoL:
-
Quality of life
- RAND-36:
-
RAND Medical Outcomes Study 36-Item Short Form Survey
- SE:
-
Sleep efficiency
- SOL:
-
Sleep onset latency
- TIB:
-
Time in bed
- TST:
-
Total sleep time
- WASO:
-
Wake after sleep onset
References
Aagaard H, Hall EC, Audulv Å, Ludvigsen MS, Westergren T, Fegran L. Parents’ experiences of transitioning to home with a very-low-birthweight infant: A meta-ethnography. J Neonatal Nurs. 2023;29:444–52. https://doi.org/10.1016/j.jnn.2022.11.012.
Aili K, Astrom-Paulsson S, Stoetzer U, Svartengren M, Hillert L. Reliability of Actigraphy and Subjective Sleep Measurements in Adults: The Design of Sleep Assessments. J Clin Sleep Med. 2017;13:39–47. https://doi.org/10.5664/jcsm.6384.
American Academy of Sleep Medicine. International Classification of Sleep Disorders, third edition (ICSD-3). American Academy of Sleep Medicine. 2014. ISBN 978-0991543410. Retrieved 2024-05-16.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013.
Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor DJ. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav Sleep Med. 2015;13:S4–38. https://doi.org/10.1080/15402002.2015.1046356.
Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. https://doi.org/10.1016/j.jad.2011.01.011.
Baglioni C, Tang NKY, Johann AF, Altena E, Bramante A, Riemann D, Palagini L. Insomnia and poor sleep quality during peripartum: a family issue with potential long term consequences on mental health. J Matern Fetal Neonatal Med. 2022;35:4534–42. https://doi.org/10.1080/14767058.2020.1854718.
Banks DJ, Basnes M, Dinges DF. Sleep deprivation. In: Kryger MR, T. C Dement W, editors. Principles and practice of sleep medicine. 6th ed. Philadelphia: Elsevier; 2017.
Beck CT. Recognizing and screening for postpartum depression in mothers of NICU infants. Adv Neonatal Care. 2003;3:37–46. https://doi.org/10.1053/adnc.2003.50013.
Bjorvatn B. Sleep disorders: modern evaluation and treatment. 1st ed. Bergen: Fagbokforlaget; 2012.
Bjorvatn B, Waage S, Pallesen S. The association between insomnia and bedroom habits and bedroom characteristics: an exploratory cross-sectional study of a representative sample of adults. Sleep Health: Journal of the National Sleep Foundation. 2018;4:188–93. https://doi.org/10.1016/j.sleh.2017.12.002.
Blomqvist YT, Nyqvist KH, Rubertsson C, Funkquist EL. Parents need support to find ways to optimise their own sleep without seeing their preterm infant’s sleeping patterns as a problem. Acta Paediatr. 2017;106:223–8. https://doi.org/10.1111/apa.13660.
Bruni O, Baumgartner E, Sette S, Ancona M, Caso G, Di Cosimo ME, Mannini A, Ometto M, Pasquini A, Ulliana A, Ferri R. Longitudinal study of sleep behavior in normal infants during the first year of life. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine. 2014;10:1119–27. https://doi.org/10.5664/jcsm.4114.
Busse M, Stromgren K, Thorngate L, Thomas KA. Parents’ responses to stress in the neonatal intensive care unit. Crit Care Nurse. 2013;33:52–60. https://doi.org/10.4037/ccn2013715.
Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. https://doi.org/10.5665/sleep.1642.
Carscadon M, Dement W. Normal human sleep: an overview. In: Dement Kryger R, editor. Principles and practice og sleep medicine. 6th ed. Philadelphia: Elsevier; 2017. p. 15–23.
Chow CM, Wong SN, Shin M, Maddox RG, Feilds K-L, Paxton K, Hawke C, Hazell P, Steinbeck K. Defining the rest interval associated with the main sleep period in actigraph scoring. Nature and Science of Sleep. 2016;8:321–8. https://doi.org/10.2147/NSS.S114969.
Cox RC, Olatunji BO. Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Med Rev. 2020;51: 101282. https://doi.org/10.1016/j.smrv.2020.101282.
Da Costa D, Lai JK, Zelkowitz P. A prospective study on the course of sleep disturbances in first-time fathers during the transition to parenthood. Infant Ment Health J. 2021;42:222–32. https://doi.org/10.1002/imhj.21911.
Al Maghaireh DAF, Abdullah KL, Chong MC, Chua YP, Al Kawafha MM. Stress, Anxiety, depression and sleep disturbance among Jordanian mothers and fathers of infants admitted to neonatal intensive care unit: a preliminary study. J Pediatr Nurs. 2017;36:132–40. https://doi.org/10.1016/j.pedn.2017.06.007.
Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64.
de Zambotti M, Goldstone A, Colrain IM, Baker FC. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12–24. https://doi.org/10.1016/j.smrv.2017.06.009.
DeMauro SB, Bellamy SL, Fernando M, Hoffmann J, Gratton T, Schmidt B. Patient, Family, and center-based factors associated with attrition in neonatal clinical research: a prospective study. Neonatology. 2019;115:328–34. https://doi.org/10.1159/000494105.
Desjardins S, Lapierre S, Hudon C, Desgagne A. Factors involved in sleep efficiency: a population-based study of community-dwelling elderly persons. Sleep. 2019;42:1–9. https://doi.org/10.1093/sleep/zsz038.
Donohue PK, Maurin E, Kimzey L, Allen MC, Strobino D. Quality of life of caregivers of very low-birthweight infants. Birth. 2008;35:212–9. https://doi.org/10.1111/j.1523-536X.2008.00242.x.
Dørheim S, Bondevik G, Eberhard-Gran M, Bjorvatn B. Sleep and depression in postpartum women: a population-based study. Sleep. 2009;32:847–55.
Edell-Gustafsson U, Angelhoff C, Johnsson E, Karlsson J, Morelius E. Hindering and buffering factors for parental sleep in neonatal care. A Phenomenogr Study J Clin Nurs. 2015;24:717–27. https://doi.org/10.1111/jocn.12654.
Eiser C, Eiser JR, Mayhew AG, Gibson AT. Parenting the premature infant: balancing vulnerability and quality of life. J Child Psychol Psychiatry. 2005;46:1169–77. https://doi.org/10.1111/j.1469-7610.2005.00415.x.
Emamian F, Khazaie H, Okun ML, Tahmasian M, Sepehry AA. Link between insomnia and perinatal depressive symptoms: A meta-analysis. J Sleep Res. 2019;28: e12858. https://doi.org/10.1111/jsr.12858.
Fekedulegn D, Andrew ME, Shi M, Violanti JM, Knox S, Innes KE. Actigraphy-based assessment of sleep parameters. Ann Work Expos Health. 2020;64:350–67. https://doi.org/10.1093/annweh/wxaa007.
Fung MM, Peters K, Ancoli-Israel S, Redline S, Stone KL, Barrett-Connor E, Osteoporotic Fractures in Men Research G. Total sleep time and other sleep characteristics measured by actigraphy do not predict incident hypertension in a cohort of community-dwelling older men. J Clin Sleep Med. 2013;9:585–91. https://doi.org/10.5664/jcsm.2756.
García M, Manrique G, Fernández SN, Puerta Y, Paredes P, Corchado AM, García-Moreno AB, Jiménez B, Mencía S. Sleep characteristics of the parents of children admitted to a pediatric intensive care unit: risk factors and repercussion on their daily life activities. Sleep Medicine: X. 2020;2: 100020. https://doi.org/10.1016/j.sleepx.2020.100020.
Garratt AM, Stavem K. Measurement properties and normative data for the Norwegian SF-36: results from a general population survey. Health Qual Life Outcomes. 2017;15:51. https://doi.org/10.1186/s12955-017-0625-9.
Haddad S, Dennis CL, Shah PS, Stremler R. Sleep in parents of preterm infants: A systematic review. Midwifery. 2019;73:35–48. https://doi.org/10.1016/j.midw.2019.01.009.
Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev. 2011;31:225–35. https://doi.org/10.1016/j.cpr.2010.04.003.
Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–7. https://doi.org/10.3109/07853890109002089.
Heidari H, Hasanpour M, Fooladi M. The experiences of parents with infants in Neonatal Intensive Care Unit. Iran J Nurs Midwifery Res. 2013;18:208–13.
Helsedirektoratet.Nyfødtavdelinger, kompetanse og kvalitet, from https://www.helsedirektoratet.no/retningslinjer/nyfodtintensivavdelinger-kompetanse-og-kvalitet/bemanning-og-kompetanse-ved-avdelinger-for-syke-nyfodte#kompetanse-i-kategori-3c-avdelinger-behandler-alle-grupper-premature. 2017.
Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Jansson-Fröjmark M, Palagini L, Rücker G, Riemann D, Baglioni C. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105. https://doi.org/10.1016/j.smrv.2018.10.006.
Hill PD, Aldag JC. Maternal perceived quality of life following childbirth. J Obstet Gynecol Neonatal Nurs. 2007;36:328–34. https://doi.org/10.1111/j.1552-6909.2007.00164.x.
Ibáñez V, Silva J, Cauli O. A survey on sleep assessment methods. PeerJ. 2018;6:e4849–e4849. https://doi.org/10.7717/peerj.4849.
Insana SP, Montgomery-Downs HE. Sleep and sleepiness among first-time postpartum parents: a field- and laboratory-based multimethod assessment. Dev Psychobiol. 2013;55:361–72. https://doi.org/10.1002/dev.21040.
Insana SP, Stremler R, Montgomery-Downs H. Postpartum sleep in new mothers and fathers. Open Sleep J. 2013;6:87–97. https://doi.org/10.2174/1874620901306010087.
Insana SP, Garfield CF, Montgomery-Downs HE. A mixed-method examination of maternal and paternal nocturnal caregiving. J Pediatr Health Care. 2014;28:313–21. https://doi.org/10.1016/j.pedhc.2013.07.016.
Kenny S, Burdayron R, Lannes ÉEM, Dubois-Comtois K, Béliveau M-J, Pennestri M-H. Mothers’ and fathers’ sleep: Is there a difference between first-time and experienced parents of 6-month-olds? J Sleep Res. 2020;30:e13238. https://doi.org/10.1111/jsr.13238.
Klassen AF, Lee SK, Raina P, Chan HW, Matthew D, Brabyn D. Health status and health-related quality of life in a population-based sample of neonatal intensive care unit graduates. Pediatrics. 2004;113:594–600. https://doi.org/10.1542/peds.113.3.594.
Kvien TK, Kaasa S, Smedstad LM. Performance of the Norwegian SF-36 Health Survey in patients with rheumatoid arthritis. II. A comparison of the SF-36 with disease-specific measures. J Clin Epidemiol. 1998;51:1077–86.
Lee SY, Hsu HC. Stress and health-related well-being among mothers with a low birth weight infant: the role of sleep. Soc Sci Med. 2012;74:958–65. https://doi.org/10.1016/j.socscimed.2011.12.030.
Lee S-Y, Lee KA, Rankin SH, Weiss SJ, Alkon A. Sleep disturbance, fatigue, and stress among chinese-american parents with ICU hospitalized infants. Issues Ment Health Nurs. 2007;28:593–605. https://doi.org/10.1080/01612840701354505.
Lee SY, Grantham CH, Shelton S, Meaney-Delman D. Does activity matter: an exploratory study among mothers with preterm infants? Arch Womens Ment Health. 2012;15:185–92. https://doi.org/10.1007/s00737-012-0275-1.
Lee SY, Aycock DM, Moloney MF. Bright light therapy to promote sleep in mothers of low-birth-weight infants: a pilot study. Biol Res Nurs. 2013;15:398–406. https://doi.org/10.1177/1099800412445612.
Lee SY, Kimble LP. Impaired sleep and well-being in mothers with low-birth-weight infants. J Obstet Gynecol Neonatal Nurs. 2009;38:676–85. https://doi.org/10.1111/j.1552-6909.2009.01064.x.
Lefkowitz D, Baxt C, Evans J. Prevalence and Correlates of Posttraumatic Stress and Postpartum Depression in Parents of Infants in the Neonatal Intensive Care Unit (NICU). J Clin Psychol Med Settings. 2010;17:230–7. https://doi.org/10.1007/s10880-010-9202-7.
Letourneau NL, Dennis C-L, Benzies K, Duffett-Leger L, Stewart M, Tryphonopoulos PD, Este D, Watson W. Postpartum depression is a family affair: addressing the impact on mothers, fathers, and children. Issues Mental Health Nurs. 2012;33:445–57. https://doi.org/10.3109/01612840.2012.673054.
Luyster FS. Sleep and Health. In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. Springer, New York: New York; 2013. p. 1799–802.
Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, Dulin H, Berkman LF, Buxton OM. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–55.
Marthinsen GN, Helseth S, Fegran L. Sleep and its relationship to health in parents of preterm infants: a scoping review. BMC Pediatr. 2018;18:352. https://doi.org/10.1186/s12887-018-1320-7.
Marthinsen GN, Helseth S, Småstuen M, Bjorvatn B, Bandlien SM, Fegran L. Sleep patterns and psychosocial health of parents of preterm and full-born infants: a prospective, comparative, longitudinal feasibility study. BMC Pregn Childb. 2022;22:546. https://doi.org/10.1186/s12884-022-04862-1.
Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514–27.
Mazza S, Bastuji H, Rey AE. Objective and subjective assessments of sleep in children: comparison of actigraphy, sleep diary completed by children and parents’ estimation. Front Psychiatry. 2020;11:495. https://doi.org/10.3389/fpsyt.2020.00495.
McAndrew S, Acharya K, Westerdahl J, Brousseau DC, Panepinto JA, Simpson P, Leuthner J, Lagatta JM. A prospective study of parent health-related quality of life before and after discharge from the neonatal intensive care unit. J Pediatr. 2019;213:38-45.e33. https://doi.org/10.1016/j.jpeds.2019.05.067.
McGuire E. Maternal and infant sleep postpartum. Breastfeed Rev. 2013;21:38–41.
McMillen IC, Mulvogue HM, Kok JS, Deayton JM, Nowak R, Adamson TM. Circadian rhythms in sleep and wakefulness and in salivary melatonin and cortisol concentrations in mothers of term and preterm infants. Sleep. 1993;16:624–31. https://doi.org/10.1093/sleep/16.7.624.
Medic G, Wille M, Hemels MEH. Short- and long-term health consequences of sleep disruption. Nature Sci Sleep. 2017;9:151–61. https://doi.org/10.2147/NSS.S134864.
Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol. 2010;203:1–7. https://doi.org/10.1016/j.ajog.2010.06.057.
Montgomery-Downs H, Stremler R, Insana S. Postpartum sleep in new mothers and fathers. Open Sleep J. 2013;6:87–97. https://doi.org/10.2174/1874620901306010087.
Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. https://doi.org/10.1097/01.psy.0000030391.09558.a3.
Moura MRS, Araujo CGA, Prado MM, Paro HBMS, Pinto RMC, Abdallah VOS, Mendonca TMS, Silva CHM. Factors associated with the quality of life of mothers of preterm infants with very low birth weight: a 3-year follow-up study. Qual Life Res. 2017;26:1349–60. https://doi.org/10.1007/s11136-016-1456-6.
Natale V, Plazzi G, Martoni M. Actigraphy in the assessment of insomnia: a quantitative approach. Sleep. 2009;32:767–71. https://doi.org/10.1093/sleep/32.6.767.
Paavonen E, Saarenpaa-Heikkila O, Polkki P, Kylliainen A, Porkka-Heiskanen T, Paunio T. Maternal and paternal sleep during pregnancy in the child-sleep birth cohort. Sleep Med. 2017;29:47–56. https://doi.org/10.1016/j.sleep.2016.09.011.
Pagels AA, Stendahl M, Evans M. Patient-reported outcome measures as a new application in the Swedish renal registry: health-related quality of life through RAND-36. Clin Kidney J. 2020;13:442–9. https://doi.org/10.1093/ckj/sfz084.
Pallesen S, Bjorvatn B, Nordhus IH, Sivertsen B, Hjornevik M, Morin CM. A new scale for measuring insomnia: the Bergen insomnia scale. Percept Mot Skills. 2008;107:691–706. https://doi.org/10.2466/pms.107.3.691-706.
Perlis ML, Zee J, Swinkels C, Kloss J, Morgan K, David B, Morales K. The incidence and temporal patterning of insomnia: a second study. J Sleep Res. 2014;23:499–507. https://doi.org/10.1111/jsr.12150.
Polo-Kantola P. Sleep disturbances in pregnancy: Why and how should we manage them? Acta Obstet Gynecol Scand. 2022;101:270–2. https://doi.org/10.1111/aogs.14325.
Post MWM. Definitions of quality of life: what has happened and how to move on. Topics Spinal Cord Injury Rehabil. 2014;20:167–80. https://doi.org/10.1310/sci2003-167.
Redeker N. Sleep health in women of childbearing age. J Womens Health. 2002;2020(29):430–4. https://doi.org/10.1089/jwh.2020.8349.
Redeker NS. Developmental aspects of Normal Sleep. In: Redeker NSMP, editor. Sleep disorders and sleep promotion in nursing practice. New York: Springer publishing company; 2011.
Reed DL, Sacco WP. Measuring sleep efficiency: What should the denominator be? J Clin Sleep Med. 2016;12:263–6. https://doi.org/10.5664/jcsm.5498.
Rezaie L, Fobian AD, McCall WV, Khazaie H. Paradoxical insomnia and subjective-objective sleep discrepancy: A review. Sleep Med Rev. 2018;40:196–202. https://doi.org/10.1016/j.smrv.2018.01.002.
Richter D, Krämer MD, Tang NKY, Montgomery-Downs HE, Lemola S. Long-term effects of pregnancy and childbirth on sleep satisfaction and duration of first-time and experienced mothers and fathers. Sleep. 2019;42:zsz015. https://doi.org/10.1093/sleep/zsz015.
Romano M, Cacciatore A, Giordano R, La Rosa B. Postpartum period: three distinct but continuous phases. J Prenatal Med. 2010;4:22–5.
Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: A critical review. J Psychiatry Neurosci. 2005;30:247–56.
Sawilowsky S. New effect size rules of thumb. J Modern Appl Stat Method. 2009;8:597–9. https://doi.org/10.22237/jmasm/1257035100.
Schaffer L. The impact of guided imagery on sleep quality in mothers of preterm infants. San Diego: University of San Diego; 2012.
Schaffer L, Jallo N, Howland L, James K, Glaser D, Arnell K. Guided imagery: an innovative approach to improving maternal sleep quality. J Perinat Neonatal Nurs. 2013;27:151–9. https://doi.org/10.1097/JPN.0b013e3182870426.
Schappin R, Wijnroks L, Uniken Venema MMAT, Jongmans MJ. Rethinking stress in parents of preterm infants: a meta-analysis. Plos One. 2013;8:1–19. https://doi.org/10.1371/journal.pone.0054992.
Shelton SL, Meaney-Delman DM, Hunter M, Lee S-Y. Depressive symptoms and the relationship of stress, sleep, and well-being among NICU mothers. J Nurs Educ Pract. 2014;4:70. https://doi.org/10.5430/jnep.v4n8p70.
Shrivastava D, Jung S, Saadat M, Sirohi R, Crewson K. How to interpret the results of a sleep study. J Commun Hosp Int Med Perspect. 2014;4:24983–24983. https://doi.org/10.3402/jchimp.v4.24983.
Singer LT, Salvator A, Guo S, Collin M, Lilien L, Baley J. Maternal psychological distress and parenting stress after the birth of a very low-birth-weight infant. JAMA. 1999;281:799–805. https://doi.org/10.1001/jama.281.9.799.
Sivertsen B, Petrie KJ, Skogen JC, Hysing M, Eberhard-Gran M. Insomnia before and after childbirth: The risk of developing postpartum pain-A longitudinal population-based study. Eur J Obstet Gynecol Reprod Biol. 2017;210:348–54. https://doi.org/10.1016/j.ejogrb.2017.01.020.
Sivertsen B, Hysing M, Harvey AG, Petrie KJ. The epidemiology of insomnia and sleep duration across mental and physical health: The SHoT study. Front Psychol. 2021;12:662572–662572. https://doi.org/10.3389/fpsyg.2021.662572.
Sivertsen B, Hysing M, Dorheim SK, Eberhard-Gran M. Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC Pregn Childb. 2015;15:129. https://doi.org/10.1186/s12884-015-0577-1.
Sivertsen B, Lallukka T, Salo P. The economic burden of insomnia at the workplace. An opportunity and time for intervention? Sleep. 2011;34:1151–2. https://doi.org/10.5665/sleep.1224.
Sivertsen B, Lallukka T, Salo P, Pallesen S, Hysing M, Krokstad S, Øverland S. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014;23:124–32. https://doi.org/10.1111/jsr.12102.
Stremler R, Dhukai Z, Pullenayegum E, Weston J, Wong L, Parshuram C. Sleep, sleepiness, and fatigue outcomes for parents of critically ill children. Pediatr Crit Care Med. 2014;15:e56-65. https://doi.org/10.1097/01.pcc.0000436198.15337.15.
Stremler R, Sharkey KM, Wolfson AR. Postpartum Period and Early motherhood. In: Kryger M, Roth TC, Dement WC, editors. Principles and Practice of sleep medicine. 6th ed. Philadelphia: Elsevier; 2017. p. 1547–52.
Tang NKY. Cognitive-behavioral therapy for sleep abnormalities of chronic pain patients. Curr Rheumatol Rep. 2009;11:451. https://doi.org/10.1007/s11926-009-0066-5.
Trimmel K, Eder HG, Böck M, Stefanic-Kejik A, Klösch G, Seidel S. The (mis)perception of sleep: factors influencing the discrepancy between self-reported and objective sleep parameters. J Clin Sleep Med. 2021;17:917–24. https://doi.org/10.5664/jcsm.9086.
Vigod SN, Villegas L, Dennis CL, Ross LE. Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: a systematic review. BJOG. 2010;117:540–50. https://doi.org/10.1111/j.1471-0528.2009.02493.x.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (Sf-36): I. conceptual framework and item selection. Med Care. 1992;30:473–83. https://doi.org/10.1097/00005650-199206000-00002.
Weiss AR, Johnson NL, Berger NA, Redline S. Validity of activity-based devices to estimate sleep. J Clin Sleep Med. 2010;6:336–42.
Witt W, Litzelman K, Spear H, Wisk L, Levin N, McManus B, Palta M. Health-related quality of life of mothers of very low birth weight children at the age of five: results from the newborn lung project statewide cohort study. Int J Qual Life Asp Treat, Care Rehabili- Off J Int Soc Qual Life Res. 2012;21:1565–76. https://doi.org/10.1007/s11136-011-0069-3.
World health organization.Preterm birth, from http://www.who.int/news-room/fact-sheets/detail/preterm-birth . 2018.
Acknowledgements
We would like to express our appreciation to all participating parents, nurses, and hospital ward staff who cooperated in this longitudinal study.
Funding
Open access funding provided by University of Agder This study did not receive any financial support.
Author information
Authors and Affiliations
Contributions
GNM,LF,SH,BB,MS wrote the mail manuscript text and GNM prepared tables and figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Norwegian National Research Ethics Committees (reference no. 2018/1025) and Faculty of Health and Sports Sciences Research Ethics Committee. The study was conducted in accordance with relevant ethical guidelines and regulations. Research departments and respective leaders at the hospitals permitted implementation of the study in hospital wards. All parents gave their informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marthinsen, G.N., Helseth, S., Småstuen, M. et al. A comparison of sleep, insomnia and health-related quality of life between mothers and fathers of preterm versus full-born infants: a longitudinal study from Norway. Sleep Science Practice 8, 8 (2024). https://doi.org/10.1186/s41606-024-00103-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41606-024-00103-w