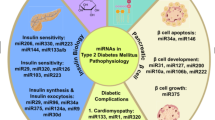
Abstract
MicroRNAs (miRNAs) are non-coding single strand RNAs. MiRNAs are encoded by endogenous genes with a length of about 22 nucleotides. MiRNAs play a vital role in the inhibition of post-transcriptional translation of mRNAs. In recent years, studies have discovered that miRNAs play an essensial role in the pathogenesis of type 2 diabetes. In addition, the discovery of miRNAs in the serum and plasma also provides a potential target for the discovery of disease markers. Taken together, preliminary data suggest a potential role of miRNAs in type 2 diabetes, however more clinical trials need to be studied.
Similar content being viewed by others
Background
Type 2 diabetes and insulin signaling
The incidence of type 2 diabetes has increased year by year. The main reason of type 2 diabetes is insulin resistance. Insulin which maintains the stability of blood glucose levels, is a hormone produced and secreted by islet beta cells. Insulin is caused by an increase of nutrients in the blood, such as glucose. Under normal conditions, insulin acts mainly by binding to the receptors on the membrane, phosphorylating downstream IRS1 and IRS2, and then activating a series of downstream kinases, including PI3K, PDK1 and AKT, which act on a series of downstream proteins such as GSK3β, mTORC1 and the FOXO transcription factor family and so on [1]. It promotes liver glycogen synthesis, inhibits gluconeogenesis, and increases glucose absorption in adipose tissue and muscle tissue [2]. Insulin resistance refers to the insufficient response of target organs to insulin.
MicroRNAs
MiRNAs are small, non-coding RNAs. MiRNAs were first found to regulate development time points in C.elegans. Now, from plants to humans, a lot of miRNAs are discovered, and functions of miRNAs are involved in various fields of biology. The formation of miRNAs begins with primary miRNA (pri-miRNA) in the nucleus [3]. The stem loop structure formed from Pri-mRNA can be identified and cut by the complex formed by Drosha and DGCR8 RNA enzyme III, and precursor miRNA (pre-miRNA) is formed [4]. Pre-miRNA relies on the transport mechanism to enter the cytoplasm and then is cut into a mature 22 nucleotides chain miRNA with the Dicer. The double stranded miRNA is formed after the formation of the AGO protein family, and the chain is melt and integrated into the silencing complex containing AGO (RISC). RISC-miRNA is formed by binding to the target gene sequence of miRNA and then affects target gene expression [5]. Two miRNAs from the opposite side of the pre-miRNA name -3p or -5p. In plants, miRNAs and target sequences are completely complementary, and miRNAs promote the cut and degradation of target genes. In multicellular animals, miRNA and target sequences are not completely matched, and the target gene is mainly inhibited during translation instead of degraded [6].
Under normal physiological conditions, miRNAs may be involved in various pathways to ensure the development process and maintain homeostasis. MiRNAs dysfunction caused by internal factors (genes) or external conditions (environment) can lead to abnormal development and metabolic disorders. The role of miRNAs in the development is relatively detailed, and the role in disease needs further study. In recent years, more and more specific types of miRNAs have been studied in metabolic diseases. This review mainly discusses the function of miRNAs in insulin signaling pathway and glucose homeostasis, and explores the possibility of serum and plasma miRNAs as an endocrine signal.
MicroRNAs and insulin signaling
MiRNAs can regulate the response of target tissues to insulin. For example, the expression of miR-29a and miR-29b are increased in the liver, fat and muscle of diabetic rats, and cell experiments also demonstrated to be associated with insulin resistance [7, 8]. MiR-29a and miR-29b mainly mediate insulin pathway by inhibiting proteins that enhance insulin signaling, including CAV2 [9], INSIG1, and PIK3R1 [7, 10]. MiR-126 can promote insulin resistance by inhibiting IRS1 [11]. In addition, miRNAs can also directly regulate glucose levels in cells, for example, miR-223 can regulate glucose uptake by inhibiting GLUT4 in muscle tissue. MiR-33a and miR-33b can regulate the insulin pathway by IRS2, SIRT6 and AMPKα1 [12, 13]. MiR-130a and miR-204 can improve glucose tolerance by inhibiting GRB10 and GLP1R respectively [14, 15]. MiR-378 and miR-93 lead to insulin resistance by targeting P110a and SIRT7 respectively [16, 17]. In conclusion, miRNAs can regulate the insulin signaling pathway and glucose absorption in target tissues.
Many miRNAs also play a role in other metabolic diseases with abnormal insulin response, including obesity and NAFLD. For example, recently, miR-103 and miR-107 were found to be increased in the liver of ob/ob mice and diet induced obese mice. Inhibition of miR-103 and miR-107 could increase insulin sensitivity. Overexpression of miR-103 and miR-107 may cause imbalance of glucose homeostasis [18]. If miR-103 and miR-107 are studied in nonhuman primates and humans, it can provide the therapeutic target for obesity induced insulin resistance [19].
The other miRNAs were also found to be increased in the obesity model. These miRNAs also regulate the insulin signaling pathway. Similar to miR-103 and miR-107, miR-143 is up-regulated in db/db mice and diet induced obese mice. Overexpression of miR-143 can reduce insulin sensitivity by inhibiting ORP8 [20]. The let7 miRNA family is up-regulated in ob/ob mice and diet induced obese mice [18]. Let7, a tumor suppressor, is widely studied in the field of cancer. However, the recent article has found that let7 plays a role in glucose metabolism. Let7 Overexpression in muscle can lead to insulin resistance by inhibiting IGF1R, INSR and IRS2 [21].
In conclusion, miRNAs can affect insulin signaling pathway and influence glucose homeostasis by affecting insulin signaling and insulin sensitivity. However, in pathological conditions, such as obesity induced insulin resistance, more in vivo tests need to do to prove the role of miRNA as a drug target for treatment.
MicroRNAs and insulin secretion
MiR-375 in pancreas is found to play a role in pancreatic development in zebrafish [22], and has a regulatory effect on pancreatic islet alpha and beta cell volume [23]. MiR-375, on the one hand, can reduce the secretion of insulin by inhibiting myotrophin (Mtpn), and on the other hand, it can affect the insulin signaling pathway by inhibiting the PDPK1 [24]. MiR-124a is found to be co-expressed with miR-375 in cultured cells, and can also act on Mtpn. It also indicates that Mtpn may be coordinated by a variety of miRNAs [25]. MiR-124a is also involved in pancreatic development and insulin secretion by regulating FOXA2 transcription factors and RAB27A [26, 27]. Other kinds of miRNAs have also been found to regulate insulin secretion. For example, miR-9 can regulate insulin secretion by inhibiting OC2 and SIRT1 [28, 29]. MiR-29a and miR-29b can prevent the insulin secretion by inhibiting monocarboxylate transporter 1 (MCT1) [30]. MiR-184 can increased pancreatic beta cell proliferation and mass [31]. In conclusion, all these studies indicate that miRNAs can affect insulin secretion by affecting pancreatic development and insulin exocytosis.
MicroRNAs in serum and plasma
In recent years, studies have discovered that miRNAs exist in serum and plasma, suggesting that serum and plasma miRNAs may become a new biomarker of disease and may regulate target cells as secretory miRNAs [32, 33]. In serum and plasma, miRNAs were initially found in the exocytosis vesicles and particles secreted by donor cells [34]. Later studies showed that miRNA also existed in apolipoprotein assembly bodies, such as combining with miRNA processing enzyme AGO2 [35,36,37]. MiRNAs in serum and plasma are major progress in the discovery of disease markers, and changes in miRNAs are associated with disease status, for example, miR-122 is related to liver injury and non-alcoholic fatty liver [38], miR-223 is related with atherosclerosis [39], miR-126 is associated with type 2 diabetes [40], let7e plays a role in hypertension [41]. MiR-486, miR-146b and miR-15b in the circulation are augmented in T2D patients [42]. MiR-199-3p is increased in plasma of patients with diabetes [43]. MiR-424 is up-regulated in serum of T2D patients by targeting Keap1 and Nrf2 [44]. MiR-146b is decreased in plasma of db/db mice [45]. More miRNA associated with metabolism disease may be found in the future and the underlying mechanisms need to be explored.
MicroRNAs and insulin resistance in type 2 diabetes
The main reason of type 2 diabetes is insulin resistance. As shown in Table 1, microRNAs can regulate insulin resistance by affecting proteins in insulin signaling.
Conclusions
The regulation of insulin pathway and glucose homeostasis by miRNAs suggests that miRNAs plays a regulatory role in type 2 diabetes. The discovery of serum and plasma miRNAs not only provides a new marker for the disease, but also suggests that miRNAs may secrete to the target organ from donor cells.
The discovery of the role of miRNAs not only provides new ways of scientific research, but also provides a new perspective for clinical research. The treatment of type 2 diabetes has been devoted to the inhibition of drug targets (enzymes). Important discoveries in the metabolism of miRNAs provide potential treatments for type 2 diabetes, such as the role of miR-33a and miR-33b in the disease. While other miRNAs, such as miR-103 and miR-107, provide drug targets for treatment intervention.
The role of miRNAs in type 2 diabetes still needs to be explored. For example, multiple target genes are often found in mRNAs, and many miRNAs synergies are likely to inhibit the expression of target genes. Therefore, a new method of combining system biology is needed to study the regulation of miRNA networks. MiRNAs often have multiple target genes and different target genes involved in different processes, and whether miRNAs can regulate glucose metabolism and other processes, such as proliferation, is very important. Finally, in view of the fact that miRNAs usually participates in multiple pathways, the long-term effects of miRNAs as a target therapy need to be considered. These existing problems need to be solved in the research of miRNAs.
References
Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91 Epub 2014/06/06.
Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46(3):200–15 Epub 2011/05/24.
Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4 Epub 2015/08/13.
Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43(6):892–903 Epub 2011/09/20.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. Epub 2009/01/27.
Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136(4):669–87. Epub 2009/02/26.
He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21(11):2785–94 Epub 2007/07/27.
Dooley J, Garcia-Perez JE, Sreenivasan J, Schlenner SM, Vangoitsenhoven R, Papadopoulou AS, et al. The microRNA-29 family dictates the balance between homeostatic and pathological glucose handling in diabetes and obesity. Diabetes. 2016;65(1):53–61 Epub 2015/12/24.
Kim S, Pak Y. Caveolin-2 regulation of the cell cycle in response to insulin in Hirc-B fibroblast cells. Biochem Biophys Res Commun. 2005;330(1):88–96 Epub 2005/03/23.
Pandey AK, Verma G, Vig S, Srivastava S, Srivastava AK, Datta M. miR-29a levels are elevated in the db/db mice liver and its overexpression leads to attenuation of insulin action on PEPCK gene expression in HepG2 cells. Mol Cell Endocrinol. 2011;332(1–2):125–33 Epub 2010/10/15.
Ryu HS, Park SY, Ma D, Zhang J, Lee W. The induction of microRNA targeting IRS-1 is involved in the development of insulin resistance under conditions of mitochondrial dysfunction in hepatocytes. PLoS One. 2011;6(3):e17343 Epub 2011/04/06.
Lu H, Buchan RJ, Cook SA. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res. 2010;86(3):410–20. Epub 2010/01/19.
Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108(22):9232–7 Epub 2011/05/18.
Xiao F, Yu J, Liu B, Guo Y, Li K, Deng J, et al. A novel function of microRNA 130a-3p in hepatic insulin sensitivity and liver steatosis. Diabetes. 2014;63(8):2631–42 Epub 2014/03/29.
Jo S, Chen J, Xu G, Grayson TB, Thielen LA, Shalev A. miR-204 controls glucagon-like peptide 1 receptor expression and agonist function. Diabetes. 2018;67(2):256–64 Epub 2017/11/05.
Liu W, Cao H, Ye C, Chang C, Lu M, Jing Y, et al. Hepatic miR-378 targets p110alpha and controls glucose and lipid homeostasis by modulating hepatic insulin signalling. Nat Commun. 2014;5:5684 Epub 2014/12/05.
Cioffi M, Vallespinos-Serrano M, Trabulo SM, Fernandez-Marcos PJ, Firment AN, Vazquez BN, et al. MiR-93 Controls Adiposity via Inhibition of Sirt7 and Tbx3. Cell reports. 2015;12(10):1594–605 Epub 2015/09/01.
Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474(7353):649–53 Epub 2011/06/10.
Naar AM. MiRs with a sweet tooth. Cell Metab. 2011;14(2):149–50 Epub 2011/08/02.
Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, Bronneke HS, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13(4):434–46 Epub 2011/03/29.
Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94 Epub 2011/10/04.
Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RH. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007;5(8):e203. Epub 2007/08/07.
Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A. 2009;106(14):5813–8. Epub 2009/03/18.
El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes. 2008;57(10):2708–17. Epub 2008/07/02.
Baroukh NN, Van Obberghen E. Function of microRNA-375 and microRNA-124a in pancreas and brain. FEBS J. 2009;276(22):6509–21 Epub 2010/01/28.
Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, et al. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem. 2007;282(27):19575–88. Epub 2007/04/28.
Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol Chem. 2008;389(3):305–12. Epub 2008/01/08.
Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic beta-islets. FEBS J. 2011;278(7):1167–74 Epub 2011/02/04.
Plaisance V, Abderrahmani A, Perret-Menoud V, Jacquemin P, Lemaigre F, Regazzi R. MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. J Biol Chem. 2006;281(37):26932–42 Epub 2006/07/13.
Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol. 2011;31(15):3182–94 Epub 2011/06/08.
Tattikota SG, Rathjen T, McAnulty SJ, Wessels HH, Akerman I, van de Bunt M, et al. Argonaute2 mediates compensatory expansion of the pancreatic beta cell. Cell Metab. 2014;19(1):122–34 Epub 2013/12/24.
Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–32 Epub 2012/01/21.
Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110(3):483–95. Epub 2012/02/04.
Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694 Epub 2008/11/13.
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–8 Epub 2011/03/09.
Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–33 Epub 2011/05/26.
Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38(20):7248–59. Epub 2010/07/10.
Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6(8):e23937 Epub 2011/09/03.
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–33 Epub 2011/03/23.
Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–7 Epub 2010/07/24.
Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124(2):175–84 Epub 2011/06/22.
Cui X, You L, Zhu L, Wang X, Zhou Y, Li Y, et al. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metab Clin Exp. 2018;78:95–105 Epub 2017/10/03.
Li YB, Wu Q, Liu J, Fan YZ, Yu KF, Cai Y. miR199a3p is involved in the pathogenesis and progression of diabetic neuropathy through downregulation of SerpinE2. Mol Med Rep. 2017;16(3):2417–24 Epub 2017/07/06.
Sun L, Li X, Li G, Dai B, Tan W. Actinidia chinensis planch. Improves the indices of antioxidant and anti-inflammation status of type 2 diabetes mellitus by activating Keap1 and Nrf2 via the upregulation of MicroRNA-424. Oxid Med Cell Longev. 2017;2017:7038789. Epub 2017/06/24.
Liu XS, Fan B, Szalad A, Jia L, Wang L, Wang X, et al. MicroRNA-146a mimics reduce the peripheral neuropathy in type 2 diabetic mice. Diabetes. 2017;66(12):3111–21 Epub 2017/09/14.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
JD wrote the article. FG directed the project. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Deng, J., Guo, F. MicroRNAs and type 2 diabetes. ExRNA 1, 36 (2019). https://doi.org/10.1186/s41544-019-0038-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41544-019-0038-5