Abstract
Background
Bacterial pneumonia is among the leading causes of morbidity and mortality worldwide. The extensive misuse and overuse of antibiotics observed during the Corona Virus Disease 2019 (COVID-19) pandemic may have changed the patterns of pathogens causing bacterial pneumonia and their antibiotic susceptibility profiles. This study was designed to establish the prevalence of culture-confirmed bacterial pneumonia and describe their antimicrobial susceptibility profile in adult patients who presented with signs and symptoms of lower respiratory tract infections (LRTIs) during the COVID-19 pandemic.
Methodology
This hospital-based cross-sectional study was conducted from July 2021 to July 2022 at a zonal referral hospital and two district hospitals in Mwanza, Tanzania. Demographic and clinical data were collected using a standardized questionnaire. Sputum samples were processed by conventional culture followed by the identification of isolates and antibiotic susceptibility testing. Descriptive data analysis was performed using STATA version 15.0.
Results
A total of 286 patients with a median age of 40 (IQR 29–60) years were enrolled in the study. More than half of the patients enrolled were females (52.4%, n = 150). The overall prevalence of bacterial pneumonia was 34.3% (n = 98). The majority of the bacterial pathogens isolated were Gram-negative bacteria (GNB) (61.2%, 60/98), with a predominance of Klebsiella spp., 38.8% (38/98), followed by Streptococcus pyogenes (21.4%, 21/98). Multi drug resistant (MDR) bacteria were detected in 72/98 (73.5%) of the isolates. The proportions of GNB-resistant strains were 60.0% (36/60) for ciprofloxacin, 60% (36/60) for amoxicillin, 60% (36/60) for amoxicillin, 68.3% (41/60) for trimethoprim-sulfamethoxazole and 58.3% (35/60) for ceftriaxone.
Conclusion
One-third of the patients with signs and symptoms of LRTIs had laboratory-confirmed bacterial pneumonia with a predominance of Gram negative MDR bacteria. This calls for continuous antimicrobial resistance (AMR) surveillance and antimicrobial stewardship programs in the study setting and other settings in developing countries as important strategies for tackling AMR.
Similar content being viewed by others
Background
Lower respiratory tract infections (LRTIs) affect the trachea and alveolar sacs, resulting in bronchitis, acute exacerbations of chronic obstructive pulmonary disease, acute exacerbation of bronchiectasis and pneumonia [1]. Pneumonia is defined as acute inflammation of the parenchymal structure of the lung and can be classified based on the place of acquisition (community-acquired or hospital-acquired), causative agent (bacterial, viral, fungal, etc.) and mechanism (aspiration or ventilator-associated pneumonia) [2, 3].
Viruses led by Corona Virus Disease 2019 (COVID-19), are the leading causes of pneumonia globally, accounting for more than 770 million cases since 2019 [4]. Furthermore, viral infections predispose patients to secondary bacterial infections by overwhelming the immune system through cytokine storm and immune dysregulation, which may subsequently lead to an increased mortality and morbidity, as evidenced during the COVID-19 pandemic [5,6,7]. Data from South Africa revealed that up to 60% of severe acute respiratory syndrome-2 (SARS-CoV-2) patients who died had secondary bacterial infections [8].
Recent studies have reported an increase in the prevalence of antimicrobial overuse and misuse aimed at the treatment of COVID-19 and its related symptoms [9]. This was due to the lack of immediate and appropriate effective treatments for COVID-19, a viral disease that cannot be treated with antibiotics [10]. Moreover, the reallocation of resources and deviation of the focus from antimicrobial stewardship programs toward COVID-19 mitigation were among the driving factors of the misuse of antibiotics [11, 12]. This leads to increased antimicrobial resistance (AMR) among bacterial pathogens that cause common infections [9].
In the study, wide dispensing of antibiotics without prescriptions was observed during the COVID-19 pandemic for COVID-19-like symptoms [13]. This is due to a lack of antimicrobial stewardship, especially during the COVID-19 pandemic, in Tanzania and other low- and middle-income countries [13].
Globally, studies have documented an increase in AMR after the COVID_19 pandemic, which was associated with the misuse and overuse of antibiotics for the treatment of COVID-19-like symptoms [14]. The picture might be different in Tanzania due to variation in treatment preference during the pandemic because a large pool of individuals opted to use herbal medication and steam therapy as treatment options [15, 16].
A study by Kishimbo et al., 2020 in a similar setting during the pre-COVID-19 pandemic reported a predominance of Gram negative multidrug resistant (MDR) bacteria causing bacterial pneumonia caused by Klebsiella spp. [17].
The COVID-19 pandemic may have changed the patterns of bacteria causing pneumonia while accelerating the progression of AMR and the increase in MDR bacteria in our setting. Therefore, this study was designed to establish the pattern of culture confirmed bacterial pneumonia and its antimicrobial susceptibility pattern among patients with signs and symptoms of LRTI’s during the COVID-19 pandemic.
Methods
Study design, duration, population and setting
This hospital-based cross-sectional study was conducted between July 2021 and July 2022 among adult patients aged ≥ 18 years with signs and symptoms of lower respiratory tract infections (LRTIs) admitted to and admitted to Sengerema District Hospital, Nyamagana District Hospital and Bugando Medical Centre (BMC) in Mwanza, Tanzania.
Sample size estimation and selection criteria
The minimum sample size of 285 was obtained by the Kish-Leslie formula [18] using a prevalence of bacterial pneumonia of 20.4% from a previous study by Kishimbo et al., 2020 [17]. The study enrolled a total of 286 adult patients who presented with a productive cough and at least two of the following symptoms: fever, axillary temperature > 37.5 °C or hypothermia < 36.1 °C, chest pain/discomfort or dyspnea, infiltrates demonstrated on chest radiography, auscultatory findings consistent with pneumonia (altered breath sounds and/or localized rales), shortness of breath or difficulty breathing, nausea or vomiting, loss of taste or smell, sore throat, muscle or body aches.
Data and sample collection
Sociodemographic (e.g., age, sex and place of residence) and clinical (e.g., signs and symptoms) data were collected from the enrolled patients via a standardized questionnaire using Epicollect5 [19]. Patients were instructed to expectorate into sterile, wide-mouthed screw-capped and leak-proof specimen containers. Sputum samples were collected on spot and transported to the microbiology laboratory for processing within 2 h of collection in a cool box.
Laboratory procedures
Gram stain
A portion of the sputum was selected using a sterile wire loop and used to create a thin smear on a clean labeled microscopy slide (BENOYLAB, Jiangsu Benoy Lab Instrument Co., Ltd. Jiangsu China). The smear was air-dried and then heat-fixed on an electric hotplate, followed by Gram staining (Gram Staining Kit, Himedia, Bottal, India) [20]. The quality of the sputa was assessed using the criteria of Bartlett et al.. [21]. A sputum with a Q-score greater than 1 was considered acceptable and termed good quality, while a score of “0” or “–” was considered poor quality [21]. All sputum samples were processed for culture so as not to miss the isolation of bacteria that elicit a low neutrophil response, such as H. influenzae [22].
Culture for the isolation of bacterial pathogens causing bacterial pneumonia
Sputum was directly inoculated onto chocolate agar (OXOID, Hampshire, United Kingdom), blood agar (OXOID, Hampshire, United Kingdom) and MacConkey agar (OXOID, Hampshire, United Kingdom) using a 10 µl loop and incubated for 18–24 h at 35–37 °C. Blood agar and chocolate agar plates were incubated in candle jars to achieve 5–7% CO2 for the isolation of fastidious bacterial pathogens such as H. influenzae [23].
Biochemical and physiological identification testing for identification of isolated bacterial pathogens
The identification of the bacterial pathogens was performed based on the growth characteristics on blood agar, MacConkey agar and chocolate agar, secondary Gram stain, and in-house biochemical tests, such as Christensen urea agar, triple sugar iron (TSI) agar, Simmons citrate agar, sulphur indole motility (SIM) agar, and oxidase for gram-negative bacteria, whereas catalase, coagulase, bile aesculin, optochin, novobiocin, and DNase were used for gram-positive bacteria [20, 23].
Antimicrobial susceptibility testing
A single colony of bacteria from a fresh pure culture plate was emulsified in sterile normal saline to achieve a concentration equivalent to 0.5 McFarland turbidity solution. A sterile cotton swab was then used to obtain bacteria from the suspension, and the swab was then squeezed against the wall of the tube to remove excess fluid before being seeded uniformly onto a Muller-Hinton agar (OXOID, Hampshire, United Kingdom) plate. Antibiotics of the right potency were placed on agar to test for susceptibility patterns using Kirby Bauer’s disc diffusion method [24] as guided by the Clinical Laboratory Standard Institute (CLSI) 30th edition M100 document, 2020 [25]. Antibiotic discs for Gram-positive bacteria included ampicillin (10 µg), cefoxitin (30 µg), trimethoprim-sulfamethoxazole (1.25/23.75 µg), ciprofloxacin (5 µg), erythromycin (15 µg), clindamycin (2 µg), vancomycin (30 µg), linezolid (30 µg), gentamicin (10 µg), clindamycin (2 µg), and tetracycline (30 µg), whereas those for Gram-negative bacteria included ceftriaxone (30 µg), cefepime (30 µg), ciprofloxacin (5 µg), ceftazidime (30 µg), piperacillin-tazobactam (100/10 µg), amoxicillin/clavulanate (20/10 µg), trimethoprim-sulfamethoxazole (1.25/23.75 µg), meropenem (10 µg), amikacin (30 µg), gentamicin (10 µg) and tetracycline (30 µg).
All S. aureus strains with a zone of inhibition on a cefoxitin (30 µg) disc ≤ 21 mm were regarded as methicillin-resistant S. aureus (MRSA); inducible clindamycin resistance was tested by observing the blunting of the zone of inhibition around the clindamycin disc placed adjacent to the erythromycin disc. For Gram-negative bacteria, the extended-spectrum beta-lactamase (ESBL) phenotype was confirmed using the combined disc method with 30 µg cefotaxime and/or 30 µg ceftazidime (with and without 10 µg clavulanic acid) [25].
Quality control
Control strains of Streptococcus pneumoniae (ATCC 49,619), Haemophilus influenzae (ATCC 49,247/49,766), Staphylococcus aureus (ATCC 25,923) and Escherichia coli (ATCC 25,922) were used for quality control of the culture media and the antibiotic discs [25]. MDR was confirmed when resistance to three or more classes of antibiotics was observed among the isolated bacterial pathogens [26].
Data management and analysis
Excel data sheet was extracted from Epi-collect- 5 software® and then, laboratory data were also added into the Microsoft Excel for cleaning and coding. Data was then transferred to STATA version 15 (College Station, Texas, USA) for analysis. Continuous data was summarized using a medium with an inter-quartile range (IQR). Categorical data were summarized using proportions (percent). Pearson chi squared test (or Fisher’s exact where applicable) was used to assess the distribution of categorical variables against culture positivity, and a p-value of less than 0.05 with 95% confidence interval was considered as statistically significant.
Ethical considerations
This study was approved by the CUHAS/BMC Research Ethics and Review Committee with the certificate number CREC/607/2022, and the study also received further clearance from the National Health Research Ethics Review Committee (NATHREC) with the certification number NIMR/HQ/R.8a/Vol.IX/2831. Permission to conduct the study was obtained from the BMC, Sengerema district hospital and Nyamagana district hospital authorities. Informed consent was obtained from all patients.
Results
Sociodemographic characteristics of the study participants
This study enrolled a total of 286 adult patients with signs and symptoms of LRTIs. Half of the enrolled patients were female (150, 52.4%). The median age of the study participants was 40 (IQR 29–60) years, with approximately two-thirds of the study participants being married (188, 65.7%) and 179 (62.6%) employed, as shown in Table 1.
The majority of the patients (203 [71.0%]) had a history of antibiotic use within two weeks prior to specimen collection. Having visited the hospital in the past one year prior to specimen collection was significantly associated with a positive sputum culture (95% CI: p < 0.031), the most commonly reported comorbidity was HIV/AIDS 24 (35.5%). Among all the study participants, 148 (51.7%) claimed to use past experience as a source of information for antibiotic use, as shown in Table 2.
Of the 203 study participants who had used antibiotics two weeks prior to sample collection, 52 (25.6%) had used antibiotics that were not indicated in the Tanzanian standard treatment guidelines (STGs) for the treatment of bacterial pneumonia. Azithromycin, ampicillin-cloxacillin and ciprofloxacin were the most frequently used antibiotics (46.8%, 24% and 16.3%, respectively), as shown in Table 3.
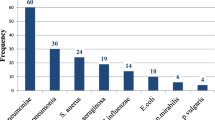
Prevalence of bacterial pneumonia
Out of 286 nonrepetitive sputum samples processed, 34.3% (98/286) had a positive culture for pathogenic bacteria, with a 95% CI of 24-43% (Fig. 1). Of the 286 non-repetitive sputum samples, only 153 (53.5%) were of good quality according to the Bartlett scoring criteria; 96 (62.7%) of the good-quality sputum samples were culture positive for pathogenic bacteria, while only 2 (1.5%) of the poor-quality sputum samples were culture positive (P < 0.001).
Bacterial pathogens causing pneumonia per health care facility
Out of 183 sputum samples from patients attending tertiary hospitals, 64 (35%) had a positive culture for pathogenic bacteria, whereas of the 103 sputum samples from patients attending district hospitals, only 34 (33%) had a positive culture for pathogenic bacteria (p = 0.939) (Table 4).
Resistance pattern for Gram-negative bacteria
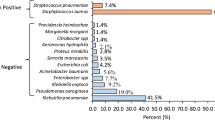
A total of 60 Gram-negative bacteria were subjected to AST; GNB strains with resistance to ampicillin (n = 25)*, 24/25 (96.0%) had the highest proportion of resistance, followed by those with resistance to trimethoprim-sulfamethoxazole (41/60, 68.3%), amoxicillin/clavulanate (38/60, 63.3%), and ciprofloxacin (36/60, 60.0%). Meropenem and amikacin had the lowest proportions of resistance, at 3/60 (5.0%) and 10/60 (16.7%), respectively, as shown in Fig. 2.
Antibiotic resistance proportions of gram-negative bacteria (N = 60). *Ampicillin was not applicable for testing against 35-gram-negative isolates. Keywords: AMP = ampicillin, CIP = ciprofloxacin, SXT = trimethoprim/sulfamethoxazole, CN = gentamicin, TE = tetracycline, CRO = ceftriaxone, TZP = piperacillin/tazobactam, AMC = amoxicillin/clavulanate, MEM = meropenem, AK = amikacin, CAZ = ceftazidime, FEP = cefepime.
Overall, the proportions of antibiotic-resistant E. coli were greater than those of Klebsiella spp., as shown in Table 5. Among the 38 Klebsiella spp., 21 (51.3%) were resistant to ceftazidime and ceftriaxone, while 12/15 (80.0%) were resistant to E. coli, as shown in Table 5.
Antibiotic resistance patterns for Gram-positive bacteria
Among the Gram-positive bacteria (n = 38), 34/38 (89.5%) had resistance to trimethoprim/sulfamethoxazole, 29/38 (76.3%) had resistance to erythromycin, and 22/38 (57.9%) had resistance to tetracycline (Fig. 3).
S. pyogenes showed high proportions of resistance to erythromycin (18/21, 85.7%), tetracycline (76.2%, 16/21) and ciprofloxacin (57.1%, 12/21), whereas for other gram-positive strains, erythromycin (11/17, 64.7%) and ampicillin (10/17, 58.8%) had the highest resistance proportions, as shown in Table 6. All the isolated S. pneumoniae strains were resistant to erythromycin, tetracycline and trimethoprim/sulfamethoxazole. None of the isolated S. pyogenes or S. aureus strains were ICR positive. All S. pneumoniae strains were sensitive to penicillin, and only 16.6% (1/6) of the S. aureus strains were MRSA.
Multidrug-resistant bacteria
A high proportion of multidrug-resistant (MDR) bacteria was detected in this study; of the 98 isolated bacterial pathogens, 72/98 (73.5%) were MDR, with Klebsiella spp. being the predominant contributor. Among those isolated at the district hospital level (n = 34), 17 (50.0%) were MDR, while among those isolated at the tertiary hospital level (n = 64), 55 (86.0%) were MDR (p = 0.0019).
Discussion
In this laboratory-based cross-sectional study, the prevalence of microbiologically confirmed bacterial pneumonia during the COVID-19 pandemic was 34.3%. These findings are significantly greater than those of a previous study before COVID-19 pandemic by Kishimbo et al. in similar settings, which reported a prevalence of 20.4% [27](p < 0.005). This increase may be attributed to the COVID-19 pandemic, which has been reported to predispose patients to secondary bacterial infections by overwhelming their immunity via cytokine overproduction and immune system dysregulation, leading to poor protection against bacterial pneumonia and impeding proper mucociliary clearance of potential pathogenic bacteria through mucociliary killing [28, 29].
Overall, 71% of the participants reported having used antibiotics two weeks prior to specimen collection, with azithromycin, ampicillin-cloxacillin and ciprofloxacin being the most frequently used antibiotics. This may have been accelerated by the ongoing COVID-19 pandemic, which has led to a surge increase in the use of antibiotics as a treatment and management option for COVID-19-like symptoms [30]. The observed use of azithromycin and ampicillin-cloxacillin by the study participants contradicts the current Tanzanian standard treatment guidelines for the treatment of bacterial pneumonia [31]. This is further supported by the fact that 25.6% of the study participants who used antibiotics for the treatment of bacterial pneumonia used antibiotics that were not indicated by the Tanzanian STG. This may have been caused by the current COVID-19 pandemic, which has led to an increase in the overprescription, misuse and overuse of antibiotics in efforts to combat and mitigate the outbreak [32], as supported by findings by Olamijuwon et al., 2021, who reported high rates of mismatched prescription of antibiotics contrary to those of the STG [13].
This study revealed a high proportion of azithromycin use among study participants two weeks prior to specimen collection, at a proportion of 46.8%; this may have been accelerated by the fact that azithromycin was among the antibiotics preferred during the pandemic as a drug of choice in the management of the COVID-19 pandemic [33, 34]. Similar to a previous report by Kishimbo et al., this study revealed the predominance of Gram-negative bacteria led by Klebsiella spp. [27]. These results show that the epidemiology of bacterial pneumonia with regard to its etiology has not changed in our setting, regardless of the presence of COVID-19; however, the findings differ from those in other geographical settings, which have indicated that S. pneumoniae is the leading cause of bacterial pneumonia [35]. This further proves why the use of STG for the treatment of bacterial pneumonia should be structured based on local findings.
Our study revealed that sputum samples with a good Bartlett quality score had a greater probability of having pathogenic bacteria than those with a poor quality score, which was in agreement with the findings of Kishimbo et al [27]. Sputum culture is a good tool for the diagnosis of bacterial pneumonia if collected and processed properly [36]; however, sputum quality determines the quality of the culture results, with good quality sputa having the best culture yield for pathogenic bacteria compared to poor quality sputa [37]. Therefore, clinicians and laboratory scientists should encourage the collection of good-quality sputum for the diagnosis of bacterial pneumonia.
Our findings revealed a high proportion of resistant Gram-negative bacteria to ciprofloxacin, trimethoprim-sulfamethoxazole, ceftriaxone and ceftazidime. These findings are different from those reported by Kishimbo et al. The data reported in this study revealed an increase in the proportion of resistance of the Klebsiella spp. to ciprofloxacin (55.3% vs. 17.4%), gentamicin (31.6% vs. 26.1%), and trimethoprim/sulfamethoxazole (68.4% vs. 43.5%) [27]. The increase in the prevalence of resistance may be attributed to the overuse of antibiotics during the COVID-19 pandemic, as observed in this study, where the majority of the participants used antibiotics within two weeks prior to sample collection [13].
The isolated Gram-positive bacteria showed high proportions of resistance to tetracycline, ciprofloxacin and erythromycin; these findings are different from those reported by Kishimbo et al., who reported comparatively lower proportions of S. pyogenes resistance to erythromycin [27]. The observed misuse and overuse of azithromycin during the COVID-19 pandemic, as reported by Sagenda et al., in a study in which the use of azithromycin in Tanzania increased by 163.7% after COVID-19 [30].
We observed high proportions of MDR pathogens in this study from both district and tertiary hospitals, which may be attributed to the high levels of antibiotic overuse during the COVID-19 pandemic leading to resistance development due to increased antibiotic pressure [38]. Furthermore, there was a greater proportion of MDR pathogens isolated from tertiary hospitals than from district hospitals, possibly because patients attending tertiary hospitals are more exposed to antibiotics than are those attending lower hospitals [39]. These findings are supported by those from Brazil, Italy and the United Kingdom, which reported the influence of COVID-19 on the increase in the overuse and misuse of antibiotics, leading to an increase in MDR among bacterial pathogens [40,41,42].
This study revealed high proportions of strains resistant to erythromycin, ciprofloxacin and 3rd -generation cephalosporins, which are included in the AWaRE WHO classification category of 2021. [43], this calls for continuous surveillance and antimicrobial stewardship programmes.
Limitations
Failure to isolate or detect pathogens known to cause atypical pneumonia, such as Legionella pneumophila and Chlamydia pneumoniae, may have led to underestimation of the true magnitude of bacterial pneumonia. In addition, due to shortcomings of the clinical information in the patients file, the correlation with X rays findings, clinical information and microbiological findings was not done. Furthermore, this study did not include information on the COVID 19 status of participants and only few patients presented with comorbidities data.
Conclusion
One-third of the patients with signs and symptoms of LRTIs had laboratory-confirmed bacterial pneumonia with a predominance of multidrug-resistant Gram-negative bacteria. The observed high proportions of resistance among Gram-negative bacteria to third-generation cephalosporins and ciprofloxacin call for continuous AMR surveillance to obtain data that can guide empiric antibiotic treatment.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AMR:
-
Antimicrobial resistance
- ATCC:
-
American Type Culture Collection
- BMC:
-
Bugando Medical Centre
- CLSI:
-
Clinical Laboratory Standard Institute
- COVID-19:
-
Coronavirus 2019
- CUHAS:
-
Catholic University of Health and Allied Sciences
- ESBL:
-
Extended spectrum beta lactamase
- LRTIs:
-
Lower respiratory tract infections
- MDR:
-
Multidrug resistant
- MRSA:
-
Methicillin-resistant S. aureus
- SARS-CoV-2:
-
Severe acute respiratory syndrome-2
- SIM:
-
Sulphur indole motility
- STG:
-
Standard treatment guideline
- TSI:
-
Triple sugar iron
References
Lower Respiratory Infections. [https://www.templehealth.org/services/conditions/lower-respiratory-tract-infections].
Mackenzie G. The definition and classification of pneumonia. Pneumonia. 2016;8(1):1–5.
Revised W. Classification and treatment of pneumonia in children at health facilities: evidence summaries. Geneva: World Health Organization; 2014.
WHO Coronavirus (COVID-19). Dashboard. In., 18-12-2023 edn: WHO; 2023.
Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, Soucy J-PR, Daneman N. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–9.
Lange A, Lange J, Jaskuła E. Cytokine overproduction and immune system dysregulation in alloHSCT and COVID-19 patients. Front Immunol. 2021;12:658896.
Shafran N, Shafran I, Ben-Zvi H, Sofer S, Sheena L, Krause I, Shlomai A, Goldberg E, Sklan EH. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11(1):12703.
Nunes MC, Hale MJ, Mahtab S, Mabena FC, Dludlu N, Baillie VL, Thwala BN, Els T, Du Plessis J, Laubscher M. Clinical characteristics and histopathology of COVID-19 related deaths in South African adults. PLoS ONE. 2022;17(1):e0262179.
Lai C-C, Chen S-Y, Ko W-C, Hsueh P-R. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021;57(4):106324.
Vinoth R, Kumar RS, Venkateswaramurthy N. Misuse of antibiotic during COVID 19 outbreaks. J Drug Delivery Ther. 2021;11(6–S):181–7.
Spernovasilis NA, Kofteridis DP. COVID-19 and antimicrobial stewardship: what is the interplay? Infect Control Hosp Epidemiol. 2021;42(3):378–9.
Martin E, Philbin M, Hughes G, Bergin C, Talento AF. Antimicrobial stewardship challenges and innovative initiatives in the acute hospital setting during the COVID-19 pandemic. J Antimicrob Chemother. 2021;76(1):272–5.
Olamijuwon E, Konje E, Kansiime C, Kesby M, Keenan K, Neema S, Asiimwe B, Mshana SE, Mushi MF, Loza O. Antibiotic dispensing practices during COVID-19 and implications for antimicrobial resistance (AMR): parallel mystery client studies in Uganda and Tanzania. Antimicrob Resist Infect Control. 2023;12(1):10.
Ng TM, Ong SW, Loo AY, Tan SH, Tay HL, Yap MY, Lye DC, Lee TH, Young BE. Antibiotic therapy in the treatment of COVID-19 pneumonia: who and when? Antibiotics 2022, 11(2):184.
Mshana G, Mchome Z, Aloyce D, Peter E, Kapiga S, Stöckl H. Contested or complementary healing paradigms? Women’s narratives of COVID-19 remedies in Mwanza, Tanzania. J Ethnobiol Ethnomed. 2021;17:1–12.
Kamazima SR, Kakoko D, Kazaura M. Manifold tactics are used to control and prevent pandemics in contemporary Africa: a case of Tanzania’s fight against COVID-19. Int J Adv Sci Res Manage. 2020;5(11):20.
Kishimbo P, Sogone NM, Kalokola F, Mshana SE. Prevalence of gram negative bacteria causing community acquired pneumonia among adults in Mwanza City. Tanzan Pneumonia. 2020;12:1–9.
Kish L. Sampling organizations and groups of unequal sizes. Am Sociol Rev 1965:564–72.
Wongphonboon S. EPICOLLECT5 APPLICATION FOR DATA COLLECTION. J Sci Technol Rajabhat Maha Sarakham Univ. 2019;2(2):18–26.
Engbaek K, Heuck C, Moody A. Manual of basic techniques for a health laboratory. World Health Organization; 2003.
Bartlett JG, Dowell SF, Mandell LA, File TM Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis. 2000;31(2):347–82.
Storisteanu DM, Pocock JM, Cowburn AS, Juss JK, Nadesalingam A, Nizet V, Chilvers ER. Evasion of neutrophil extracellular traps by respiratory pathogens. Am J Respir Cell Mol Biol. 2017;56(4):423–31.
Koneman EW, Allen SD, Janda W, Schreckenberger P, Winn W. Diagnostic microbiology. The nonfermentative gram-negative bacilli Philedelphia: Lippincott-Raven Publishers 1997:253–320.
Biemer JJ. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Annals Clin Lab Sci. 1973;3(2):135–40.
Jorgensen JH, Turnidge JD. Susceptibility test methods: dilution and disk diffusion methods. Man Clin Microbiol 2015:1253–73.
Magiorakos A, Srinivasan A, Carey R, Carmeli Y, Falagas M, Giske C, Harbarth S, Hinndler J. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria. An international expert proposal for interim standard definitions for acquired resistance 2020, 2012:18.
Kishimbo P, Sogone NM, Kalokola F, Mshana SE. Prevalence of gram negative bacteria causing community acquired pneumonia among adults in Mwanza City, Tanzania. Pneumonia. 2020;12(1):1–9.
Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309(3):275–82.
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74.
Sangeda RZ, William SM, Masatu FC, Bitegeko A, Mwalwisi YH, Nkiligi EA, Horumpende PG, Fimbo AM. Antibiotic Utilisation Patterns in Tanzania: A Retrospective Longitudinal Study Comparing Pre-and Post-COVID-19 Pandemic Using Tanzania Medicines and Medical Devices Authority Data. medRxiv 2023:2023.2011. 2027.23299060.
MINISTRY FOR HEALTH CD, GENDER EAC. STANDARD TREATMENT GUIDELINES & NATIONAL ESSENTIAL MEDICINES LIST TANZANIA MAINLAND. In.: TAMISEMI; 2021.
Garg SK. Antibiotic misuse during COVID-19 pandemic: a recipe for disaster. Indian J Crit Care Medicine: Peer-reviewed Official Publication Indian Soc Crit Care Med. 2021;25(6):617.
Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S. Azithromycin in the treatment of COVID-19: a review. Expert Rev anti-infective Therapy. 2021;19(2):147–63.
Abdelmalek SM, Mousa A. Azithromycin misuse during the COVID-19 pandemic: a cross-sectional study from Jordan. Infect Drug Resist 2022:747–55.
Anderson R, Feldman C, Anderson R, Aston S, Rylance J, Song J, Huh K, Chung D, Iannella H, Luna C. The role of Streptococcus pneumoniae in community-acquired pneumonia. In: Seminars in Respiratory and Critical Care Medicine: 2020: Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.; 2020: 455–469.
Murdoch DR, Morpeth SC, Hammitt LL, Driscoll AJ, Watson NL, Baggett HC, Brooks WA, Deloria Knoll M, Feikin DR, Kotloff KL. The diagnostic utility of induced sputum microscopy and culture in childhood pneumonia. Clin Infect Dis. 2017;64(suppl3):S280–8.
Budayanti NS, Suryawan K, Iswari IS, Sukrama DM. The quality of sputum specimens as a predictor of isolated bacteria from patients with lower respiratory tract infections at a tertiary referral hospital, Denpasar, Bali-Indonesia. Front Med. 2019;6:64.
Ndaki PM, Mushi MF, Mwanga JR, Konje ET, Ntinginya NE, Mmbaga BT, Keenan K, Sabiiti W, Kesby M, Benitez-Paez F. Dispensing antibiotics without prescription at community pharmacies and accredited drug dispensing outlets in Tanzania: a cross-sectional study. Antibiotics. 2021;10(8):1025.
Ciofi degli Atti ML, D’Amore C, Ceradini J, Paolini V, Ciliento G, Chessa G, Raponi M. Prevalence of antibiotic use in a tertiary care hospital in Italy, 2008–2016. Ital J Pediatr. 2019;45:1–8.
Polly M, de Almeida BL, Lennon RP, Cortês MF, Costa SF, Guimarães T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am J Infect Control. 2022;50(1):32–8.
Knight GM, Glover RE, McQuaid CF, Olaru ID, Gallandat K, Leclerc QJ, Fuller NM, Willcocks SJ, Hasan R, Van Kleef E. Antimicrobial resistance and COVID-19: intersections and implications. Elife. 2021;10:e64139.
Brogna C, Montano L, Zanolin ME, Bisaccia DR, Ciammetti G, Viduto V, Fabrowski M, Baig AM, Gerlach J, Gennaro I. A retrospective cohort study on early antibiotic use in vaccinated and unvaccinated COVID-19 patients. J Med Virol. 2024;96(3):e29507.
WHO. 2021 AWaRe classification. In.; 2021.
Acknowledgements
We thank the HATUA consortium and medical staffs from BMC, Sengerema and Nyamagana district hospitals for their support and technical assistance during data collection.
Funding
This study was supported by the HATUA/CARE (MR/V036157/1): COVID-19 and Antimicrobial Resistance in East Africa—impact and response funded by UK Research and Innovation (Medical Research Council) and the Department of Health and Social Care (National Institute for Health Research).
Author information
Authors and Affiliations
Contributions
JJ, MFM, KK, WS, MTGH and SEM conceived, designed and executed the study, JJ collected data and samples, JJ, VS, JS and PD performed laboratory analysis, JJ wrote the manuscript which was critically reviewed by all authors. All authors have read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the CUHAS/BMC Research Ethics and Review Committee with the certificate number CREC/607/2022, and the study also received further clearance from the National Health Research Ethics Review Committee (NATHREC) with the certification number NIMR/HQ/R.8a/Vol.IX/2831. Permission to conduct the study was obtained from the BMC, Sengerema district hospital and Nyamagana district hospital authorities. Informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rukyaa, J., Mushi, M.F., Silago, V. et al. Etiology and antimicrobial susceptibility patterns of bacteria causing pneumonia among adult patients with signs and symptoms of lower respiratory tract infections during the COVID-19 pandemic in Mwanza, Tanzania: a cross-sectional study. Pneumonia 16, 16 (2024). https://doi.org/10.1186/s41479-024-00137-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41479-024-00137-9