Abstract
Background
Oral squamous cell carcinoma (OSCC) is the eighth most prevalent cancer worldwide. In recent large-scale studies, by immunohistochemistry and cluster analysis, several markers were associated with patient survival in various tumors. The aim of this study was to analyze the expression profiles of 23 proteins that have been linked to the inhibition (Bcl-2, Bcl-x, Bcl-xL, Bcl-2-related protein A1, BAG-1, and survivin) and promotion (Bak, Bax, Bim/Bod, Bim-Long, Bad, Bid, PUMA, Apaf-1, caspase-2, caspase-3, caspase-6, caspase-7, caspase-8, caspase-9, caspase-10, Smac/DIABLO, and cytochrome c) of apoptosis in OSCC.
Methods
Two-hundred and twenty nine cases of OSCC, arranged in a tissue microarray, were immunohistochemically analyzed, and the results were quantified on an automated imaging system. The data were analyzed using a random forest clustering method.
Results
Overall protein expression patterns defined two chief clusters: an anti-apoptotic cluster (142 cases) and a pro-apoptotic cluster (29 cases). These groups could not be explained by any clinical or pathological characteristic, and overall and disease-free survival did not differ between them.
Conclusions
Although there was no association with survival, the cluster analysis demonstrated specific protein profiles that could be of interest for using targeted therapies: in one of the clusters, the expression of pro-apoptotic proteins was more prominent, demonstrating a pro-apoptotic profile and highlighting the importance of apoptosis during OSCC development.
Similar content being viewed by others
Background
Oral squamous cell carcinoma (OSCC) is the eighth most prevalent cancer worldwide [1, 2]. In Brazil, the National Cancer Institute estimates that 11,140 males and 4350 females develop oral cancer annually [3].
Many studies have identified various changes in gene expression in head and neck squamous cell carcinomas compared with normal oral mucosa, identifying subgroups of head and neck squamous cell carcinomas with profiles that correlate with many aspects of prognosis [4–7].
Molecular profiling by gene array and its translation into surrogate immunohistochemistry profiles is affecting the classification and management of tumors, such as endometrial, breast, gastric cancer, and brain tumors [8–13]. It is important that similar approaches be taken to increase our understanding of OSCC.
The changes that have been observed in such molecular studies affect a wide range of processes that mediate the development of many cancers [4]. One of these processes is apoptosis. The primary function of apoptosis is to eliminate senescent or altered cells that are useless or harmful to a multicellular organism. Altered expression levels of apoptosis-related proteins have been reported in several cancers, including OSCC [14–16]. The development of resistance to apoptosis is a hallmark of malignant cells, enabling them to survive, despite apoptosis-inducing environmental signals and the loss of normal survival signals [17, 18].
Two pathways initiate apoptosis, both of which activate the executioner caspases 3, 6, and 7. The first pathway is the intrinsic, or mitochondrial, pathway, because mitochondria control the initiation of apoptosis. Apoptotic stimuli induce the release of cytochrome c and other apoptotic regulators from the intermembrane space of mitochondria. In the cytosol, cytochrome c, APAF-1, ATP, and the initiator procaspase 9 form the apoptosome, which effects the cleavage of effector caspases. The Bcl-2 family of proteins regulates mitochondrial permeability. The second pathway is known as the extrinsic pathway; it is mediated by various death receptors on the cell surface. These receptors, once activated by specific ligands, initiates the recruitment of FAS-associated death domain protein and procaspases 8 and 10 to the death domain, which forms the death inducing signaling complex and promotes the activation of caspase 8 [16, 19].
The aim of this study was to analyze the expression profiles of 23 proteins that are associated with both apoptosis pathways in a series of 229 cases of OSCC. Hierarchical cluster analysis was performed to identify apoptotic molecular subtypes of OSCC and determine if these different profiles are related to patient outcomes.
Methods
Tissue samples
Paraffin-embedded tissue samples from 229 oral squamous cell carcinoma cases and 10 non-neoplastic samples from oral mucosa tissue adjacent to tumor were obtained from the files of the Department of Pathology of the A.C. Camargo Cancer Center, São Paulo, Brazil. All retrieved cases had been untreated, underwent surgery as the initial treatment at the hospital and have been followed-up for at least 5 years. Clinical and histological details of the cases are provided in Table 1. This study was approved by the AC Camargo Cancer Center Ethics Committee (Protocol number 985/07) and complied with the Helsinki Declaration guidelines.
Tissue microarray (TMA)
To construct the TMA, H&E sections were analyzed, and a representative area of the deepest tumor sheet was marked on the slide. The tissues that corresponded to the selected areas were sampled from the donor block using a tissue microarrayer (Beecher Instruments, Silver Springs, USA). Each sample was arrayed twice with a 1.0-mm-diameter core that was spaced 0.2 mm apart, and two TMAs with different core regions were constructed. After the array was completed, the TMA blocks were sectioned at a thickness of 4 μm.
Immunohistochemistry
The expression of Bcl-2, Bcl-x, Bcl-xL, Bcl-2-related protein A1, BAG-1, Bak, Bax, Bim/Bod, Bim-Long, Bad, Bid, PUMA, Apaf-1, caspase-2, caspase-3, caspase-6, caspase-7, caspase-8, caspase-9, caspase-10, Smac/DIABLO, cytochrome c, and survivin was examined in OSCC tissue samples in a TMA [14,15]. Immunostaining was performed on two tissue slides from different cores of the same sample. The slides were deparaffinized, rehydrated, and subjected to antigen retrieval. Details on the antigen retrieval methods and primary antibodies clones, sources, and titers are listed in Table 2. The sections were incubated in 3% aqueous hydrogen peroxide for 15 min to quench endogenous peroxidase activity and with Protein Block Serum-Free (Dako, CA, USA) for 20 min at room temperature to suppress nonspecific binding of subsequent reagents.
The sections were then incubated with primary antibody for 2 h at room temperature. The antigen-antibody complexes were visualized using the Advance Detection System (Dako, CA, USA) and incubated with 3’3 diaminobenzidine tetrachloride (DAB) (Dako, CA, USA) for 5 min, and the sections were counterstained with Mayer’s hematoxylin, dehydrated, and mounted with a glass coverslip and xylene-based mounting media. The results were quantified on an automated imaging system (ACIS III, Dako, CA, USA), which detects levels of hue, saturation, and luminosity, converting this signal into a numerical measurement of density (staining intensity) that ranges from 0 to 256. Qualitative analysis of the results considered the distribution of the protein (nucleus, cytoplasmic and membrane), the pattern of staining (diffuse or focal) and the intensity of staining (strong, moderate, weak). Negative controls were performed by replacing the primary serum with a non-immune reagent. Positive controls were used per the manufactures’ recommendations.
Cluster analysis
Hierarchical cluster analyses were performed using a random forest clustering method (TMEV, http://mev.tm4.org/), and the results were displayed using Tree-View. This method is an unsupervised learning method that creates molecular classifications, based on distinct global expression profiles, blinded to clinic and pathological covariates. Samples that did not presented interpretable protein expression in more than 80% of the 23 proteins analyzed were excluded from hierarchical cluster analysis.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
TUNEL assay was performed using Apoptag S7100 (Milipore) according to the manufacturer’s protocol.
The slides were deparaffinized, rehydrated, and subjected to proteinase K treatment. The sections were incubated in 3% aqueous hydrogen peroxide for 5 min to quench endogenous peroxidase activity. The sections were then incubated with TdT enzyme working solution for 1 h at 37 °C, followed by incubation with anti-dig conjugate for 30 min at room temperature. The reaction was developed with TSA incubation for 8 min at room temperature. The sections were mounted with a glass coverslip and xylene-based mounting media.
Statistical analysis
The association of the demographic, clinical and pathological characteristics of the patients with protein expression profiles between clusters was analyzed by chi-square test. Overall and disease-free survival probabilities were calculated, based on the Kaplan-Meier method, and log-rank test was used to determine statistical significance. The significance level was 5% for all statistical tests. Statistical analyses were performed using R, version 2.13 (R Development Core Team (2010), Vienna, Austria, www.R-project.org).
Results
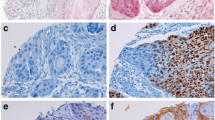
All antiapoptotic (Bcl-2, Bcl-x, Bcl-xL, Bcl-2-related protein A1, BAG1, survivin) and proapoptotic (Bak, Bax, Bim/Bod, Bim-Long, Bad, Bid, PUMA, Apaf-1, caspase-2, caspase-3, caspase-6, caspase-7, caspase-8, caspase-9, caspase-10, Smac/Diablo, cytochrome c) proteins were expressed in the oral squamous cell carcinoma (OSCC) samples (Figs. 1 and 2). The proteins varied in expression, and all were cytoplasmic in neoplastic cells and positive controls, except survivin that was expressed in a cytoplasmatic and/or nuclear pattern. Staining for Bad was robust in well-differentiated tumors. This pattern was also observed with Bcl-xL, which was strong in the fronts of invasive areas. Bcl-2-related protein A1 expression was strong in well-differentiated tumors, concentrated in the cytoplasm of keratinizing neoplastic cells that surrounded keratin pearls. There was no difference in the pattern of distribution of the staining of the other proteins in tumors. All proteins were expressed in non-neoplastic oral squamous mucosa in the middle and lower layers. These results are described in Table 3.
Expression of apoptotic proteins in oral squamous cell carcinoma. a1 Bcl-2 (original magnification X400); a2 Bcl-2 (original magnification X250); b Bcl-x (original magnification X250); c Bcl-xL (original magnification X400); d Bcl-2 related protein a1 (original magnification X400); e BAG-1 (original magnification X400); f1 Bax (original magnification X400); f2 Bak (original magnification X250); g Bim/Bod (original magnification X400); h Bim-Long (original magnification X400); i Bad (original magnification X400); j Bid (original magnification X250); k PUMA (original magnification X400); l APAF (original magnification X400)
Expression of apoptotic proteins in oral squamous cell carcinoma. a caspase 2 (original magnification X250); b caspase 3 (original magnification X250); c caspase 6 (original magnification X250); d caspase 7 (original magnification X400); e caspase 8 (original magnification X400); f caspase 9 (original magnification X250); g caspase 10 (original magnification X250); h Smac/DIABLO (original magnification X250); i cytchrome c (original magnification X250); j survivin (original magnification X400)
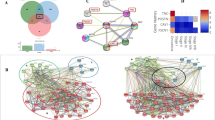
Hierarchical cluster analysis was performed after the exclusion of the samples with values that were missing in more than 20% of the 23 proteins. Therefore, 171 samples were allocated for the analysis. The combined protein expression patterns defined two clusters: cluster A (142 cases) and cluster B (29 cases; Fig. 3).
Two-way hierarchical cluster analysis of 171 oral squamous cell carcinomas based on the expression of 23 proteins. Tumors were grouped into 2 clusters (A and B), based on the protein expression profile. Rows represent proteins, ordered according to their hierarchical distances. Colors in columns represent expression levels: red indicates positive staining, and green represents the absence of staining for each of the antibodies. Within each cluster, samples were ordered based on their correlation distances
Nine proteins (BAG-1, Bcl-2, Bid, Bim/Bod, Bax, caspase-2, caspase-6, caspase-7, Smac/Diablo) were expressed equally between clusters. Apaf-1, caspase-3, caspase-9, cytochrome c, caspase-10, PUMA, survivin, Bad, Bak, Bcl-2-related protein A1, Bcl-x, Bcl-xL, and Bim-Long proteins were preferentially expressed in cluster B (p <0.0001, p < 0.001, p < 0.001, p = 0.001, p = 0.001, p < 0.001, p = 0.007, p = 0.024, p = 0.028, p < 0.001, p < 0.001, p < 0.001 and p = 0.006, respectively), and caspase-8 was predominant in cluster A (p < 0.001) (Fig. 4). Based on the distribution of proteins in the clusters, we defined cluster A as the “anti-apoptotic cluster” and cluster B as the “pro-apoptotic cluster”. Figure 5 shows the occurrence of apoptosis in OSCC samples using TUNEL assay. The apoptotic cells were mainly detected in areas with evidence of keratin pearl formation. The protein expression profile in cases that demonstrated apoptosis by TUNEL assay was similar to “pro-apoptotic cluster”, however no statistical significance was observed.
To determine whether these two clusters represented clinically distinct subgroups of patients, univariate analysis was performed. Significant associations between clusters and clinical/pathological findings, such as location and histological grade, were not observed (Table 4). Five-year overall and disease-free survival rates did not differ between clusters A and B (p = 0.69 and p = 0.68, respectively).
Discussion
Alterations in the expression levels of apoptosis-related proteins have been reported in several cancers, including oral cancer [14–16]. In this study, by tissue microarray (TMA) analysis of 229 oral squamous cell carcinoma (OSCC) cases, the expression profiles of 23 apoptotic proteins were examined and a hierarchical cluster analysis was performed.
More than 60 gene expression-profiling studies of human clinical samples of head and neck squamous cell carcinoma (HNSCC) have been published. Unfortunately, these profiles have failed to effect any clinically relevant application in the management of HNSCC [20, 21]. Several studies have identified gene expression profiles that differ between normal mucosa, premalignant lesions, and invasive carcinoma. Other studies focused on tumor classification, prognostic or predictive value based on molecular characteristics [22–26].
Molecular profiling by gene array and its translation into surrogate immunohistochemistry profiles are being applied to classify and manage various tumors. Cluster analysis has been used, allowing one to make a more objective interpretation of immunoprofiles, based on staining with multiple antibodies, and holding great promise for the immunohistochemical classification of tumors [8–13]. Tissue microarrays have become a widely used tool to screen for protein expression patterns in a large number of tumors and these data could be used to classify tumors and determine whether these putative classes (clusters) are biologically and clinically meaningful [27].
Apoptosis has a significant function in OSCC. Based on the expression of 23 proteins, our group have demonstrated that decreased expression of caspase-3 correlates with lymph node metastasis, lower caspase-7 levels are associated with disease-free survival in oral cancer, high Bim-Long expression is linked to overall survival and that elevated PUMA levels are associated with disease-free survival in oral cancer patients [15, 16]. Elevated expression of Apaf-1 and survivin is associated with the absence of embolization, and the expression of Smac/DIABLO and cytochrome c is not significantly associated to any clinicopathological characteristic (unpublished results).
The contradictory results regarding the prognosis and expression of apoptotic proteins indicate that the induction of apoptosis is complex and that the influence of individual proteins varies between tumors. Further, the balance between anti- and proapoptotic proteins determines cell fate. In this study, a hierarchical cluster analysis was performed to identify protein profiles, which could distinguish different subtypes of OSCC, and how they relate to patient outcomes.
Overall protein expression patterns defined two chief clusters: an anti-apoptotic cluster (142 cases) and a pro-apoptotic cluster (29 cases). These groups could not be explained by any clinical or pathological characteristic, and overall and disease-free survival did not differ between them. Although there was no association with survival, the cluster analysis demonstrated specific protein profiles that could be of interest for using targeted therapies: in one of the clusters, the expression of pro-apoptotic proteins was more prominent, demonstrating a pro-apoptotic profile and highlighting the importance of apoptosis during OSCC development.
First studies in HNSCC focused on discriminatory profiles between normal and tumor samples [28–30]. Chung et al. [22] was the first group to classify HNSCC into prognostic groups based on gene expression profiles of 60 samples from different tumor sites. Zanaruddin et al. [31], using a panel of 11 proteins previously shown to have a prognostic significance in HNSCC, described a 4-protein signature that predicts lymph node metastasis and survival in oral squamous cell carcinoma. Box et al. [32] identified a protein signature associated with EGFR-TKI resistance in head and neck squamous cell carcinoma cell lines.
Walter et al. [33] analyzed gene expression profile of 138 HNSCC samples from different sites and reported the same groups described by Chung et al. [22]. However, they did not observed an association between recurrence-free survival and tumor subtype. Méndez et al. [34] described 314 genes differentially expressed between tumor and normal samples. However, considering patient’s outcome only one gene was differentially expressed between metastatic and non-metastatic tumors.
These apparent conflicting results regarding prognostic findings presented by different studies might be due to the use of different array platforms, analytical procedures, tumor sample selection, number of patients evaluated, and different endpoints evaluated [7, 20, 21].
One of the limitations of this study is the lack of data on other critical cellular pathways, besides apoptosis. Another possible limitation of this study is the use of Tissue microarrays, due to intra-tumor heterogeneity of protein expression. However, increasing the number of cores collected from the sample and/or increasing the core diameter can circumvent this limitation [35, 36]. Furthermore, the use of an automated scoring system enables a more objective and quantitative acquisition of staining result measurements due to its higher accuracy, sensitivity and better reproducibility of data [36, 37].
Despite these limitations, this study was able to demonstrate different biological behavior in OSCC. Further validation using another cohort and in vivo studies are necessary to evaluate if the cluster analysis could be predictive of response to therapeutic interventions.
In summary, the expression 23 apoptosis-related proteins was evaluated on 229 OSCC samples arranged on a tissue microarray. Although hierarchical cluster analysis identified two clusters, no significant associations between the clusters and clinical and pathological findings were observed. Based on the expression profiles of the clusters, a pro-apoptotic cluster and an anti-apoptotic cluster were defined, suggesting that apoptosis is linked to tumor behavior in OSCC.
Conclusions
Our results suggested that apoptosis is present in OSCC, but other mechanisms of cell growth outcome those of cell-death. This information may be of value in establishing new approaches to investigate the complex molecular roads that lead to uncontrolled cell proliferation in malignant neoplasms.
Abbreviations
- DAB:
-
3’3 diaminobenzidine tetrachloride
- OSCC:
-
Oral squamous cell carcinoma
- TMA:
-
Tissue microarray
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
Massano J, Regateiro FS, Januário G, et al. Oral Squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Ministério da Saúde, Instituto Nacional de Câncer. Estimativa 2016: incidência de câncer no Brasil/Instituto Nacional de Câncer. Rio de Janeiro: INCA; 2016.
Hunter K, Parkinson EK, Thakker N. An overview of the molecular pathology of head and neck cancer, and its clinical implications. Periodontol. 2011;57:132–49.
Viet CT, Schmidt BL. Understanding oral cancer in the genome era. Head Neck. 2010;32:1246–68.
Nagaraj NS. Evolving ‘omics’ technologies for diagnostics of head and neck cancer. Brief Funct Genomic Proteomic. 2009;8:49–59.
Mahfouz ME, Rodrigo JP, Takes RP, et al. Current potential and limitations of molecular diagnostic methods in head and neck cancer. Eur Arch Otorhinolaryngol. 2010;267(6):851–60.
Alkushi A, Irving J, Hsu F, et al. Immunoprofile of cervical and endometrial adenocarcinomas using a tissue microarray. Virchows Arch. 2003;442:271–7.
Jacquemier J, Ginestier C, Rougemont J, et al. Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res. 2005;65:767–79.
Clark SE, Warwick J, Carpenter R, et al. Molecular subtyping of DCIS: heterogeneity of breast cancer reflected in pre-invasive disease. Br J Cancer. 2011;104:120–7.
Begnami MD, Fregnani JH, Brentani H, et al. Identification of protein expression signatures in gastric carcinomas using clustering analysis. J Gastroenterol Hepatol. 2012;27:378–84.
Lee HS, Cho SB, Lee HE, et al. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13:4154–63.
Ikota H, Kinjo S, Yokoo H, et al. Systematic immunohistochemical profiling of 378 brain tumors with 37 antibodies using tissue microarray technology. Acta Neuropathol. 2006;111:475–82.
Schoelch ML, Le QT, Silverman Jr S, et al. Apoptosis-associated proteins and the development of oral squamous cell carcinoma. Oral Oncol. 1999;35:77–85.
Coutinho-Camillo CM, Lourenço SV, Nishimoto IN, et al. Expression of Bcl-2 family proteins and association with clinicopathological characteristics of oral squamous cell carcinoma. Histopathology. 2010;57:304–16.
Coutinho-Camillo CM, Lourenço SV, Nishimoto IN, et al. Caspase expression in oral squamous cell carcinoma. Head Neck. 2011;33:1191–8.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–90.
Lallemant B, Evrard A, Chambon G, et al. Gene expression profiling in head and neck squamous cell carcinoma: Clinical perspectives. Head Neck. 2010;32:1712–9.
Braakhuis BJ, Brakenhoff RH, Leemans CR. Gene expression profiling in head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2010;18:67–71.
Chung CH, Parker JS, Karaca G, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5(5):489–500.
Lohavanichbutr P, Houck J, Fan W, et al. Genome wide gene expression profiles of HPV-positive and HPV-negative oropharyngeal cancer: potential implications for treatment choices. Arch Otolaryngol Head Neck Surg. 2009;135(2):180–8.
Zhou X, Temam S, Oh M, et al. Global expression-based classification of lymph node metastasis and extracapsular spread of oral tongue squamous cell carcinoma. Neoplasia. 2006;8(11):925–32.
Dumur CI, Ladd AC, Wright HV, et al. Genes involved in radiation therapy response in head and neck cancers. Laryngoscope. 2009;119(1):91–101.
Tan M, Myers JN, Agrawal N. Oral cavity and oropharyngeal squamous cell carcinoma genomics. Otolaryngol Clin North Am. 2013;46(4):545–66.
Shi T, Seligson D, Belldegrun AS, et al. Tumor classification by tissue microarray profiling: random forest clustering applied to renal cell carcinoma. Mod Pathol. 2005;18(4):547–57.
El-Naggar AK, Kim HW, Clayman GL, et al. Differential expression profiling of head and neck squamous carcinoma: significance in their phenotypic and biological classification. Oncogene. 2002;21(53):8206–19.
Hwang D, Alevizos I, Schmitt WA, et al. Genomic dissection for characterization of cancerous oral epithelium tissues using transcription profiling. Oral Oncol. 2003;39(3):259–68.
Belbin TJ, Singh B, Barber I, et al. Molecular classification of head and neck squamous cell carcinoma using cDNA microarrays. Cancer Res. 2002;62(4):1184–90.
Zanaruddin SN, Saleh A, Yang YH, et al. Four-protein signature accurately predicts lymph node metastasis and survival in oral squamous cell carcinoma. Hum Pathol. 2013;44:417–26.
Box C, Mendiola M, Gowan S, et al. A novel serum protein signature associated with resistance to epidermal growth factor receptor tyrosine kinase inhibitors in head and neck squamous cell carcinoma. Eur J Cancer. 2013;49(11):2512–21.
Walter V, Yin X, Wilkerson MD, Cabanski CR, Zhao N, Du Y, Ang MK, Hayward MC, Salazar AH, Hoadley KA, Fritchie K, Sailey CJ, Weissler MC, Shockley WW, Zanation AM, Hackman T, Thorne LB, Funkhouser WD, Muldrew KL, Olshan AF, Randell SH, Wright FA, Shores CG, Hayes DN. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One. 2013;8(2):e56823.
Méndez E, Cheng C, Farwell DG, et al. Transcriptional expression profiles of oral squamous cell carcinomas. Cancer. 2002;95(7):1482–94.
Avninder S, Ylaya K, Hewitt SM. Tissue microarray: a simple technology that has revolutionized research in pathology. J Postgrad Med. 2008;54(2):158–62.
Ilyas M, Grabsch H, Ellis IO, et al. Guidelines and considerations for conducting experiments using tissue microarrays. Histopathology. 2013;62(6):827–39.
Hilbe W, Gächter A, Duba HC, et al. Comparison of automated cellular imaging system and manual microscopy for immunohistochemically stained cryostat sections of lung cancer specimens applying p53, ki-67 and p120. Oncol Rep. 2003;10(1):15–20.
Acknowledgements
Not applicable.
Funding
Grants 1998/14335-2, 2007/50608-4, and 2008/57887-9, São Paulo Research Foundation (FAPESP).
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Authors’ contributions
CMCC designed the research study, performed the research, analyzed the data and wrote the paper; SVL designed the study and wrote the paper; RDP analyzed the data; ASD analyzed the data; THNT performed the research, analyzed the data; LPK designed the research study and revised the paper; FAS designed the research study and revised the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the AC Camargo Cancer Center Ethics Committee (Protocol number 985/07) and complied with the Helsinki Declaration guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Coutinho-Camillo, C.M., Lourenço, S.V., Puga, R.D. et al. Profile of apoptotic proteins in oral squamous cell carcinoma: A cluster analysis of 171 cases. Appl Cancer Res 37, 2 (2017). https://doi.org/10.1186/s41241-016-0008-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41241-016-0008-2