Abstract
Germline CYLD mutation is associated with the development of a rare inheritable syndrome, called the CYLD cutaneous syndrome. Patients with this syndrome are distinctly presented with multiple tumors in the head and neck region, which can grow in size and number over time. Some of these benign head and neck tumors can turn into malignancies in some individuals. CYLD has been identified to be the only tumor suppressor gene reported to be associated with this syndrome thus far. Here, we summarize all reported CYLD germline mutations associated with this syndrome, as well as the reported paired somatic CYLD mutations of the developed tumors. Interestingly, whole-exome sequencing (WES) studies of multiple cancer types also revealed CYLD mutations in many human malignancies, including head and neck cancers and several epithelial cancers. Currently, the role of CYLD mutations in head and neck carcinogenesis and other cancers is poorly defined. We hope that this timely review of recent findings on CYLD genetics and animal models for oncogenesis can provide important insights into the mechanism of head and neck tumorigenesis.
Similar content being viewed by others
Introduction
Understanding of genetic diseases that are closely linked to tumor development can provide important insights into the biology of human tumorigenesis and treatment. To date, only a handful of human genetic diseases are uniquely associated with predisposition of head and neck tumor formation. In this focused review, we will provide an up-to-date summary of the cylindromatosis (CYLD) gene defects in a genetic disease called the CYLD cutaneous syndrome. This genetic syndrome is, in particular, characterized by multiple tumor formation in the head and neck region often with early age onset. Some of these tumors will remain benign, while some can turn malignant. Interestingly, CYLD genetic aberrations have recently been reported by recent whole-exome sequencing (WES) studies in head and neck cancers, and some other cancers, thus revealing its potential involvement in human carcinogenesis. Therefore, it is timely to review the genomic aberrations of CYLD in this particular genetic disease, which will deepen our understanding of human tumorigenesis, in particular, of the head and neck.
The CYLD gene
The CYLD gene (chr 16q12.1) codes for a 107 kDa cytoplasmic deubiquitinating (DUB) enzyme, which removes ubiquitin molecules from various signaling proteins, and regulates the activities of many cellular and signaling processes. This gene was first discovered and cloned in 2000 by Bignell et al. with prior evidence suggesting the existence of a potential tumor suppressor gene on chr 16q12-q13 linked to a peculiar cutaneous disease characterized by multiple tumors in the head and neck region [1]. Subsequent functional studies revealed multiple roles of CYLD in the regulation of inflammation, immunity, cell cycle progression, spermatogenesis, osteoclastogenesis, ciliogenesis, migration and potentially tumorigenesis [1–4]. To date, several major signaling pathways have been found to be linked with or regulated by CYLD, which include the Nuclear Factor-kB (NF-kB), Wnt/β-catenin and c-Jun NH(2)-terminal kinase (JNK) pathways, and potentially others [5–7]. Genetic alterations of CYLD could result in aberrant activation or inhibition of these signaling pathways, which may contribute to disease pathology.
The CYLD cutaneous syndrome
In 1842, a rare cutaneous disease was first described in a female patient, named Frances Massenger, who developed multiple tumors in the head, neck and face. In addition to her early disease onset at age 14, multiple family members of this patient also had a history of head and neck tumors [8], which strongly implied a potential underlying genetic cause of this rare disease. Over a century later in 1995, Biggs et al. discovered the locus of the susceptibility gene on chromosome 16q12-q13 by linkage analysis of the members of two affected families, revealing the potential loss of a likely tumor suppressive gene associated with this rare syndrome [9]. The following year, Biggs et al. provided further evidence to suggest that CYLD (referred to as Cyld1) may be the only tumor suppressor gene involved in the CYLD cutaneous syndrome [10]. A subsequent larger study with 21 affected families ultimately helped to identify the gene associated with this syndrome to be the CYLD gene on chromosome 16q12 and detected, for the first time, germline and somatic mutations of CYLD in affected patients [1]. The gene was cloned by fine-mapping and positional cloning and it was confirmed that CYLD germline mutations are associated with and are the underlying cause of this cutaneous syndrome in humans [1].
The term, CYLD cutaneous syndrome, was proposed recently by Rajan et al. [11] to describe this rare inheritable condition that is known to be caused by germline mutations of the CYLD gene based on genetic evidence [9]. The occurrence rate of CYLD germline defects is ~1:100,000 based on the UK data [12]. Patients with this syndrome are clinically characterized with multiple tumors of the skin appendages often in the head and neck region (i.e. skin lesions derived from the epidermal appendages, hair follicles, sweat apparatus, etc.). The CYLD syndrome encompasses three previously known appendageal tumor predisposition syndromes: familial cylindromatosis (FC, or Turban tumor syndrome; OMIM 132700), multiple familial trichoepithelioma 1 (MFT1; also called epithelioma adenoides cysticum, EAC, or Brooke-Fordyce trichoepitheliomas; OMIM 601606), and Brooke-Spiegler syndrome (BSS or BRSS; OMIM 605041), which are believed to be allelic disorders with overlapping phenotypes associated with CYLD mutations. The clinical manifestations of these CYLD-associated syndromes as well as the images for the head and neck, and facial manifestations have been recently reviewed [13]. All three tumor predisposition syndromes are autosomal dominant disorders, in which a germline CYLD mutation was inherited, and a second, non-inherited CYLD mutation or loss of heterozygosity (LOH) occurs in cells for tumor formation. FC is typically presented with multiple cylindromas (i.e. benign tumors with differentiation towards apocrine sweat glands that increase in number and size over age). These multiple cylindromas growing in the scalp may coalesce and cover the entire scalp like a turban (thus FC is also called the Turban tumor syndrome). MFT1 is characterized by multiple trichoepitheliomas (i.e. skin tumors on the face with histologic dermal aggregates of basaloid cells with connection to or differentiation toward hair follicles), which can turn into basal cell carcinoma [14]. BSS, mostly with early adulthood onset, is classically characterized by multiple skin appendage tumors including cylindroma, trichoepithelioma, and spiradenoma (eccrine spiradenomas or cystic epitheliomas of the sweat gland, usually solitary, deep-seated dermal nodule typically located in the head and neck region [15]). Since members of a single family can manifest as FC, MFT1 or BSS with CYLD aberrations, many consider these three diseases as a phenotypic spectrum of a single disease entity with underlying CYLD mutation. These tumors can be painful, itchy and irritating, and in some cases, turn to malignancies. Due to the very disfiguring nature of these head and neck, facial tumors, surgical removal and often repeated surgeries are performed on these individuals to limit tumor growth over their life-time. The psychological impacts due to the disfiguring appearance of affected individuals may lead to depression and social withdrawal [16].
To date, the CYLD cutaneous syndrome has been reported in various ethnic backgrounds, with age onset as early as 5, to 40 years old. The average age onset is around teenage (~16 years old) [11]. Such an early age onset of multiple tumor formation distinctly in the head and neck region strongly imply a potential critical role of CYLD mutations in promoting head and neck tumorigenesis.
CYLD Germline and somatic mutations in individuals with the CYLD cutaneous syndrome
As of today, a total of 107 germline CYLD mutations have been reported in patients developing FC, BSS and MFT1 (Table 1). Most reported mutations reside between exons 9 and 20 of the CYLD gene. The current data revealed several hotspot mutation sites of CYLD: 1112C > A (S371*), 2272C > T (R758*) and 2806C > T (R936*) in 14, 10 and 13 independent families, respectively [17–19] (Fig. 1). Note that all three hotspot mutations are nonsense mutations, which are likely to produce truncated forms of the CYLD protein, potentially representing loss-of-function of the CYLD protein. In fact, the majority of CYLD germline mutations are deleterious mutations, including frameshift (44 %), splice-site (11 %), nonsense mutations (25 %), germline deletions (2.7 %) followed by missense mutations (11 %) and silent mutations (1 %) (Table 1). Note that a few studies reported the absence of detectable CYLD germline mutation in a small number of affected individuals [20, 21]. It is possible that some CYLD alterations may have been missed as these previous studies examined only certain exons/regions CYLD using direct sequencing, or probe-based fluorescence in-situ hybridization (FISH) or linkage analysis. Thus far, no single study has sequenced the entire CYLD gene including the regulatory and intronic regions, which can also be potentially altered but missed by targeted sequencing. Note that sporadic occurrences of the syndrome have also been reported. In those cases, only the affected individual, but not their family members, will carry a germline CYLD mutation and present with the syndrome phenotype [22, 23].
Reported CYLD germline mutations in patients with the CYLD cutaneous syndrome [1, 11, 17, 19–23, 25, 26, 81, 120]. The frequency of familial cases of CYLD cutaneous syndrome with germline CYLD mutations, and the corresponding amino acid positions affected by these mutations are indicated (as detailed in Table 1 and predicted using the Integrative Genomics Viewer (IGV) software, the Broad Institute, USA). The CYLD protein contains three CAP-GLY domains (aa 155–198, 253–286, 492–535), a UCH catalytic domain (aa 591–950) and a Zinc binding region (aa 778–842) within in the catalytic domain based on the NCBI number NP_056062.1
Theoretically, it is possible that other genetic events, besides CYLD, may be involved. Candidates like Patched 1 (PTCH1) has been proposed earlier, but later disputed to be a potential candidate for the CYLD cutaneous syndrome [21, 24, 25]. As next-generation sequencing (NGS) can now be easily employed to study various diseases, it is likely that whole-exome or even whole-genome studies of these head and neck tumors from affected individuals can reveal previously unidentified genetic changes associated with the disease, in addition to CYLD.
Patients with the CYLD cutaneous syndrome inherit one copy of the mutated CYLD gene, while LOH or mutation of the second copy of the CYLD gene occur somatically for tumor formation. Several studies investigated the actual genetic change of CYLD in the developed tumors versus that of the germline aberrations in affected individuals. A total of 15 such cases have been reported thus far. As shown in Table 2, tumors from each of the 15 cases all harbored additional CYLD aberration(s) different from the original germline CYLD mutation. In some cases, somatic CYLD changes among different tumors of the same individual can also be different. In general, nonsense CYLD mutations seem to be the most common germline event, while LOH or loss-of function CYLD mutations (nonsense, or frameshift mutations) were frequently detected as somatic events (Table 2). This genetic pattern is supportive of the 2-hit hypothesis of tumorigenesis, similar to that of the retinoblastoma 1 (RB1) gene alterations for the development of retinoblastoma. Not only genetic heterogeneity was observed among tumors from the same individual, the pathologies of these tumors can also vary from benign to malignant in some cases. It is likely that CYLD alteration is an early event for head and neck tumorigenesis, and potentially supportive of later malignant transformation over time.
CYLD aberrations with benign tumor formation or malignant transformation?
Most clinical reports on the CYLD cutaneous syndrome indicate that the majority of tumors developed in the head and neck region are benign in nature, with progressive growth in size and number over one’s lifetime. However, emerging evidence is supportive of malignant transformation of these usually benign tumors into malignancies in some affected individuals, perhaps even in situ, arising from the original benign tumors [26]. In fact, the very first case report of such cutaneous syndrome (though with unclear genetics), had extensively documented multiple tumor formation in the patient's peritoneum, reminiscent of the patient’s head and neck tumors. The patient who later manifested a state of cachexia did suggest a “malignancy” as indicated in the report [8]. Yet, it remains unclear if these tumors in the peritoneum were originated in situ or were actually metastatic lesions from the head and neck tumors.
Due to the rarity of the syndrome, and repeated surgeries for most patients (for cosmetic reasons), documentation of malignant transformation of these seemingly benign tumors is scarce. Recently, Kazakov et al. reported multiple cases with histological evidences suggesting that the malignant lesions seemed to develop or transform in situ at the original “benign” tumors of the cutaneous syndrome patients [26]. A histological study showed that in an invasive carcinoma, the basal cell adenocarcinoma (BCAC) of the salivary gland that was developed in the affected individual, there remained a residuum of spiradenoma which merged with the invasive carcinoma by histology. Similar findings in another affected individual showed that the benign tumor had developed into an invasive lesion in the skull with a BCAC histology. Invasive adenomas of various histologies have been identified in several affected individuals as well. How did these malignant transformations occur in situ? Did the tumors acquire additional genetic aberrations that caused or supported malignant transformation? Or were the CYLD genetic aberrations (two copies of CYLD mutated or loss) sufficient to drive such a malignant transformation over time if the tumors had not been excised early enough by surgery?
As demonstrated by chemically-induced colon and liver cancer models with CYLD −/− mice [16, 27], it seems that phenotypically invasive or potentially metastatic tumors can develop with a CYLD deficient background in vivo. This may imply that CYLD loss, together with a strong cancer inducing agent or DNA mutagen, can turn normal cells to tumors with the potential to further transform into malignancies. This notion is further supported by findings from Alameda et al. that expression of a catalytically inactive form of CYLD in a Ha-ras-mutated tumorigenic epidermal cell line (PDVC57) significantly promoted in vitro cell proliferation, migration (with changes to a mesenchymal phenotype), anchorage-independent growth, as well as pronounced in vivo tumor growth and angiogenesis with upregulation of vascular endothelial growth factor-A (VEGF-A) expression [28]. Using a subcutaneous tumor model, the authors demonstrated that the CYLD mutant tumors not only grew faster and larger in size, but also showed a more aggressive, poorly differentiated phenotype when compared to the control tumors which bore a less aggressive, differentiated phenotype. It was hypothesized that the presence of Ha-ras mutation in this cell model, PDVC57, together with CYLD mutation, may be responsible for such an aggressive phenotype, which is in contrast with the observed benign skin tumors developed in CYLD −/− mice as previously reported by Massoumi et al. [29]. These findings may suggest that CYLD may cooperate with other oncogenic events, in this case Ha-ras mutation, to promote malignant transformation. Thus, future investigations on CYLD gene interaction may further define the biological importance of CYLD in head and neck carcinogenesis and progression.
CYLD Mutations in head and neck cancers, and other human malignancies
CYLD has been suggested to be a tumor suppressor gene, as supported by evidences from the first genetic susceptibility study for the CYLD cutaneous syndrome [1]. It is known that deleterious loss of an important tumor suppressor gene in germline settings can confer cancer predisposition in an inherited manner. A well-known comparable example is the Li–Fraumeni syndrome, a rare cancer predisposition hereditary disease caused by germline tumor protein 53 (TP53) mutations and the affected individuals often develop various cancers at young age. Although our current understanding of CYLD is insufficient, the very first reported case of such a cutaneous syndrome in Frances Massenger (1842) who first developed multiple scalp and face tumors, and later, multiple abdominal/peritoneal tumors reminiscent of the ones in her head and neck, and subsequently died with symptoms of cancer cachexia did suggest a potential link of the cutaneous syndrome to malignant conditions [8]. Several female family members also had a history of head and neck tumors (grandmother, mother, and sister), and breast tumors (sister), suggesting the inheritable nature of the syndrome linked to human malignancies. In fact, a recent study by Kazakov et al. reported a total of 5 patients with BSS, who were found to develop malignancies arising from pre-existing tumors in the head and neck region [26]. Further microscopic analyses of the tumors confirmed the presence of “residuum of a pre-existing benign neoplasm” indicative of in situ development of malignancies from the apparently benign lesions. A handful of malignant cases developed in patients with BSS have also been reported by others [30–49]. These malignancies included salivary gland type basal cell adenocarcinoma-like pattern, low-grade (BCAC-LG), and high grade (BCAC-HG), invasive adenocarcinomas (IACs), squamous cell carcinomas (SCCs), anaplastic neoplasms and sarcomatoid (metaplastic) carcinomas [34, 50–59].
Although it remains unclear how CYLD genomic aberrations precisely drive multiple head and neck tumor formation, and potentially, malignant progression, CYLD somatic mutations have been reported in a subset of head and neck squamous cell carcinoma (HNSCC) patients as revealed by recent WES efforts of The Cancer Genome Atlas (TCGA, USA). HNSCC is the most common type of head and neck cancer, ranking the sixth most common cancer worldwide. A total of 8 CYLD somatic mutations (8/279 patient cases) have been identified in primary HNSCC tumors by WES [60]. These include: F110L, V180Cfs*23, N300S, S361Lfs*47, S371*, T575S, D618A, and K680*. Among which, the S371* mutation has been found to be a hotspot germline mutation in patients with the CYLD cutaneous syndrome as mentioned above. Yet, the functional role of these CYLD mutations in HNSCC development remains unknown. Among the 8 CYLD-mutated HNSCC tumors, 4 were Human Papilloma virus (HPV)-negative (all smokers; age onset is 71.75 ± 3.77 years old) and the remaining 4 were HPV-positive (with 1 smoker only; age onset is 54.00 ± 6.82 years old). All HPV-negative CYLD-mutated tumors were also TP53 mutated, while as expected, the HPV-positive counterparts were all TP53 wildtype. Although all patients carrying the CYLD-mutated HNSCC tumors had advanced disease at the time of diagnosis [Stage III (2/8 cases) and Stage IV (6/8 cases)], the published TCGA cohort with only 8 CYLD-mutated cases was not able to reveal any CYLD-mutation and overall patient survival correlation (data not shown).
Besides the published HNSCC TCGA dataset, a recent study has identified a high incidence of CYLD aberrations in a rare salivary gland tumor, namely the dermal analogue tumor, which can be of sporadic or familial origins. Dermal analogue tumor is a subtype of basal cell monomorphic adenoma with remarkable histological and clinical resemblance to cylindromas. Choi et al. reported that as high as 80.9 % (17/21) of the sporadic cases, and 75 % of familial cases (9/12 tumors from two sisters) harbored LOH near the CYLD gene locus (16q12-13) [51]. These findings suggest that both skin adnexal tumors, which are commonly associated with the CYLD cutaneous syndrome, and dermal analogue tumors may share a common genetic basis, namely CYLD genetic alteration.
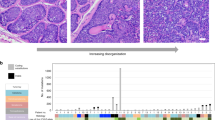
Besides HNSCC, the TCGA WES efforts also revealed other human cancers with a ≥3 % mutation rate of CYLD. These include (arranged in descending order of percent cases mutated in each cohort and the actual number shown in the legend; Additional file 1: Figure S1): uterine corpus endometrial carcinoma (5.2 %; 13/248 cases), lung squamous cell carcinoma (4.5 %; 8/177 cases), stomach adenocarcinoma (3.8 %; 15/395 cases) and lung adenocarcinoma (3 %; 7/230 cases). An additional 15 cancer types harbor somatic CYLD mutations at ~1-3 % rates. These are cancers of the skin, esophagus, colon, glioma, pancreas, liver and cervix, as well as intrahepatic cholangiocarcinoma, small cell lung cancer, large B cell lymphoma, thymoma, chromophobe renal cell carcinoma, multiple myeloma, uveal melanoma, glioblastoma (TCGA, USA; www.cbioportal.org; [61, 62]). Interestingly, two of the germline CYLD hotspot mutations (S371* and R758*) in CYLD cutaneous syndrome patients are also found in primary tumors of HNSCC, lung and stomach. Yet, the roles of these CYLD mutations in these solid tumors remain undetermined. It is possible that CYLD alterations may be involved in the tumorigenesis of many other cancers, in addition to head and neck cancers.
CYLD signaling
Important cellular processes are known to be regulated by ubiquitination and deubiquitination of cellular proteins. Ubiquitination of a protein can determine and regulate its stability, and even its signaling functions [63]. Ubiquitins (Ubs) are small proteins (8.5 kDa) with seven lysine (K) residues (K6, K11, K27, K29, K33, K48 and K63). Ubiquitination of different K residues can serve different biological functions. For instance, K48-linked ubiquitin chains on a target protein directs the protein for proteosome degradation, while K63 links can promote protein-protein interactions and signaling activation [2].
The CYLD protein has three cytoskeletal-associated protein-glycine-conserved (CAP-GLY) domains and a UCH catalytic domain with a zinc-motif [1] (Fig. 1). The CAP-GLY domains combined with proline-rich regions are responsible for microtubule and target protein binding, while the UCH domain mediates deubiquitination, and the zinc-motif allows for CYLD folding and domain interaction [1]. CYLD is highly specific for K63 ubiquitin chains [64], however has also been demonstrated to mediate K48 deubiquitination of target proteins [65]. Target proteins of CYLD include B-cell lymphoma 3 (Bcl-3), Histone-deacetylase 6 (HDAC6), Transient receptor potential cation channel A1 (TRPA1), NF-kB essential modulator (NEMO), TRAF interacting protein (TRIP), transforming growth factor-β-activated kinase 1 (TAK1), receptor-interacting protein 1 (RIP1), retinoic acid-inducible gene-1 (RIG1) and TNF-receptor associated factor (TRAF) proteins, etc. [66]. Through deubiquitination of these signaling proteins, CYLD has been shown to regulate major signaling pathways including the NF-kappaB (NF-kB) (canonical and non-canonical), Wnt/β-catenin and c-Jun NH(2)-terminal kinase (JNK) pathways (Fig. 2) [5–7, 67]. Several studies showed that the tumor suppressor CYLD inhibits NF-kB as well as the p38 MAPK pathway activation by deubiquitinating several upstream regulatory signaling molecules of these pathways, thus suppressing these signaling pathways [68]. Alternatively, CYLD has been shown to be negatively regulated by the Notch [69] and Sonic Hedgehog (Shh) [70] signaling pathways in T-cell leukemia and skin cancer, respectively (Fig. 2). As of today, among all currently identified target proteins of CYLD, many are signaling regulators of the NF-kB pathway (e.g. the TRAF proteins, NEMO, TRIP, RIP1, TAK1 and Bcl-3). Therefore, it is believed that genomic aberrations of CYLD may alter NF-kB signaling activity, which may also contribute to the pathophysiology of the CYLD cutaneous syndrome and tumor formation.
Although it is unclear if other non-NF-kB signaling pathways are potentially involved, recent evidences revealed such a possibility. CYLD has recently been shown to promote ciliogenesis, a process that is plausibly associated with tumorigenesis. The primary cilium is a cell surface antenna-like structure sensing chemical and mechanical signals from the environment on almost all mammalian cells. Since the formation of the primary cilium is coordinately regulated with the cell cycle progression via its connection with the centrosome, it has been hypothesized that regulators of ciliogenesis may also control cell proliferation and tissue homeostasis, and defects in primary cilium formation or function may contribute to tumorigenesis due to “non-communicative and unrestrained growth” [71–73]. In fact, in addition to this CYLD tumor suppressor, several key tumor suppressors and oncogenes such as the VHL, PDGFR-α, and Shh/Patched 1 (Shh/Ptch1) were recently identified to regulate ciliogenesis [3, 4, 74]. Eguether et al. demonstrated that both the centrosomal localization (via interaction with a centrosomal protein CAP350) and deubiquitination activity of CYLD were required for its ciliogenic activity, independent of NF-kB [3]. Note that another NF-kB-independent and ciliogenic signaling pathway, the Shh/Ptch1 pathway, which is the most critical signaling pathway regulating cell proliferation and differentiation of basal cell carcinoma (a type of skin cancer arising from epidermal stem cell of the hair follicles) [75], has been recently identified as an upstream regulator of CYLD expression (Fig. 2). It remains to be investigated if this Shh/Ptch1-CYLD link is relevant for ciliogenesis as well as tumorigenesis of the skin, which can be pathologically related to this CYLD Cutaneous syndrome.
CYLD-associated signaling pathways. NF-kB, Wnt/β-catenin, and JNK pathways have been shown to be regulated by CYLD. The canonical NF-kB signaling pathway has been shown to be regulated by CYLD through deubiquitination of target substrates such as RIP1, the TAK1 complex and NEMO [2]. In the non-canonical NF-kB signaling pathway, deubiquitination of Bcl-3 by CYLD results in the inhibition of cyclin D1 gene expression [29]. Wnt/β-catenin signaling has been shown to be regulated by CYLD, via deubiquitination of the (disheveled) DVL protein [6]. The JNK signaling pathway has been demonstrated to be regulated by CYLD activity through unknown mechanisms likely involving TRAF2 and MKK7 [7]. In addition, the Notch/Hes1 pathway and the Hedgehog signaling have been shown to regulate transcription of CYLD, via suppression of CYLD transcription by Hes1 and snail1, respectively [69, 70]. Blue arrows indicate nuclear translocation of the proteins. The lower grey box shows the published signaling changes and likely consequences of CYLD deficiencies due to CYLD knockout, CYLD silencing by siRNA or shRNA or CYLD mutation. Red arrows indicate that the nuclear translocation of the indicated proteins was found to be increased. Potential therapeutic targets due to CYLD aberrations are highlighted in red within the lower grey box
CYLD and potential mechanisms of multiple head and neck tumor development
Although the genetic link between CYLD defects and the CYLD cutaneous syndrome has been identified, there remain many interesting questions to be answered regarding this peculiar syndrome. How do CYLD germline mutations give rise to “multiple” tumor formation, in particular, in the head and neck region in these patients? Furthermore, what are the molecular mechanisms underlying the progression of benign tumor lesions to malignancies in some patients?
Loss of CYLD links to multiple tumor development?
Almost all CYLD cutaneous syndrome patients do carry a germline mutation of CYLD which is inheritable. Interestingly, CYLD somatic mutations have also been identified in sporadic cases of cylindroma [1] and spiradenoma patients [76]. This evidence suggests that CYLD aberration is associated with the disease phenotype of multiple head and neck tumors. Thus far, CYLD is the only tumor suppressor gene identified to be linked with the disease. Genetically-engineered mouse models have been generated to study the function of CYLD in mammalian settings. A study by Massoumi et al. demonstrated that CYLD knockout mice (with disruption of ATG start codon) were much more susceptible to chemically-induced cutaneous squamous papilloma formation upon a single dose of 7,12-dimethybenza(a)anthracene (DMBA) followed by 12-Otetradecanoylphorbol-13-acetate (TPA) treatment [29]. All CYLD −/− mice developed skin tumors (papillomas) after 11 weeks vs. only 50–60 % of tumor incidence in CYLD +/+ mice at a later time of 16 weeks. Importantly, mice with homozygous as well as heterozygous loss of CYLD (i.e. CYLD −/− and CYLD +/− mice) both developed multiple tumor phenotype on the skin much earlier than the CYLD +/+ mice. By week 16, CYLD −/− and CYLD +/− mice harbored ~30 and 15 tumors/mouse, as compared to only 5 tumors per mouse in the CYLD +/+ group. These results indicated that the loss of a single copy of CYLD gene was sufficient to confer a “multiple tumor phenotype” upon chemical insults in mice (although the tumor-bearing phenotype is more severe when both copies of CYLD were lost). Further, the average tumor size of papilloma developed in the CYLD −/− mice were >2.8 times of those found in the CYLD +/+ mice, implicating a potential CYLD gene dose effect on tumor cell proliferation. Despite the fact that spontaneous tumor development was not observed in the CYLD −/− mice, loss of CYLD (either one or both copies) did confer a “tumor susceptible phenotype” reminiscent of patients with the CYLD cutaneous syndrome. It was further noted that the tumor number and size in CYLD −/− and CYLD +/− mice did grow over time after the initial DMBA/TPA insult, which is also reminiscent of the tumor characteristics reported in patients with the syndrome [1, 29]. Yet, all the tumors developed in the CYLD −/− and CYLD +/− backgrounds were hyperplastic lesions with no signs of malignancy [29]. It is likely that the loss of this CYLD tumor suppressor gene makes the entire epithelium of the skin highly prone to tumor initiation by chemicals or environmental insults in the “affected site”, skin in this model, thus multiple tumors can develop in this “primed soil”.
This is further supported by another CYLD knockout mice study, in which multiple tumors were developed in the colon of the CYLD −/− mice in a chemical-induced colitis-associated cancer (CAC) model [27], with which a DNA mutagen (azoxymethane; AOM) and an inflammation-inducing chemical (dextran sulphate sodium; DSS) were used in the drinking water to target the colon epithelium of the animals. The study demonstrated that as early as second round of DSS treatment, the CYLD −/− mice developed multiple measurable broad-based adenocarcinomas (i.e. flattened, or called sessile) in the colonic epithelium, as compared to almost no tumor in the CYLD +/+ mice. In humans, it is noted that sessile polys or adenomas are pre-cancerous lesions in the colon [77]. Further investigation demonstrated that CYLD could limit inflammation and tumorigenesis by regulating ubiquitination [27]. Similar multi-tumor phenotype was also observed in a diethylnitrosamine (DEN)-induced carcinogenic liver injury model, in which significantly more, larger and multiple tumors with invasive or metastatic potential (displaying trabecular sinusoidal structures related to initial stage of invasion and metastasis in human hepatocellular carcinoma) were observed in the livers of the CYLD −/− mice as compared to that of the CYLD +/+ mice [68]. The observation that multiple papillomas, colon adenocarcinomas, and liver tumors were easily induced upon treatment with chemical insults or DNA mutagens in CYLD knockout mice did strongly imply a generalized tumor susceptibility nature of the affected epithelium or tissue due to CYLD mutation or CYLD loss. However, it remains unclear as to why some tissues seem to develop potentially malignant tumors (e.g. liver, and colon), while some tissues tend to develop more benign tumors (e.g. skin papilloma) in vivo. Thus, it is important to determine if CYLD aberrations do confer any tissue-specific oncogenic activity in various human cancer types.
Why do these tumors develop predominantly in the head and neck region?
The next question is why these tumors mostly developed in the head and neck, and face of the affected individuals? The possible reason(s) may lie in the fact that these areas are always exposed to strong chemical or DNA-damaging insults. It is possible that frequent exposure to UV, a strong DNA-damaging insult can serve as a tumor inducer or potentiating agent for tumor development in the epithelium of the head and neck, and the face. It has been shown by Massoumi et al. that UV light could trigger cellular proliferation of CYLD −/− keratinocytes, as well as cyclin D1 expression [29]. The study proposed a model that in the presence of UV light and in conjunction with CYLD loss, Bcl-3 will translocate into the nucleus, complexed with p50 to induce cyclin D1 expression, thus cellular proliferation, while the presence of intact CYLD will inhibit Bcl-3 nuclear translocation and growth.
Another equally important possibility is the likely origin(s) of tumor from the hair stem cells as previously suggested for cylindromas [78]. As the region of head and neck, and the face harbor many stem cell -containing hair follicles in the sebaceous and sweat glands, CYLD genetic aberrations may affect the proliferation control, or inflammatory status of the stem cell niches, thus resulting in predominant head and neck tumor formation. Evidence for this can be noted as these tumors never grow from the hair-less parts of the body (e.g. the palms and soles), but only in the hairy parts of the body. It is also possible that hair follicle stem cells that harbor CYLD alterations may acquire additional genetic changes over one’s lifetime thus resulting in tumor formation. However, since the origin of these tumors of the CYLD cutaneous syndrome patients is still of debate, this hypothesis remains to be proven. Another possibility that remains to be proven is that, maybe, CYLD is specifically and functionally associated with developmental control or growth regulation of the head and neck or hair follicles in humans. Thus, germline defects of CYLD in patients with the CYLD cutaneous syndrome are mainly presented with head and neck tumors or tumors in regions with lots of hair follicles.
As CYLD somatic mutations occur in HNSCC tumors, and CYLD aberrations seem to be the key genetic driver for multiple head and neck tumor formation in patients with this cutaneous syndrome, an unanswered question is whether CYLD aberration alone is sufficient to directly drive head and neck tumor formation. Do additional genetic or chemical insults associated with head and neck carcinogenesis, such as smoking, drinking, or HPV infection, promote tumorigenesis in CYLD-mutated head and neck cancers? Is the immune system involved as well, since CYLD is also implicated in the regulation of immunity? All these questions remain to be addressed.
Conclusions
The genetics of the CYLD cutaneous syndrome underlies the formation of multiple tumors in the head and neck epithelium. Current treatments are limited, except for repeated surgical removal of the tumors when needed. Inhibition of NF-kB signaling can potentially be a treatment option. Yet, a prior clinical trial on the topical use of salicylic acid showed some efficacies in some affected individuals only (2/12 cases) [79]. A recent study showed that CYLD mutations can cause activation of the tropomyosin kinase (TRK) signaling in tumors of affected individuals [80]. Further, inhibition of TRK signaling in CYLD-mutant tumor models demonstrated the potential efficacies of TRK targeting. Thus TRK inhibitors can be a potential treatment strategy for these patients. It is important to understand more about the genetics and biology of these CYLD-mutant tumors, which may point to new treatment or prevention of these disfiguring tumors. Further understanding of the role of CYLD in head and neck epithelial biology may also identify mechanisms of tumorigenesis and progression of head and neck cancers, as well as other human malignancies.
Abbreviations
- DMBA:
-
7,12-dimethybenza(a)anthracene
- TPA:
-
12-O-tetradecanoylphorbol-13-acetate
- BCAC:
-
Basal cell adenocarcinoma
- BCAC-HG:
-
Basal cell adenocarcinoma-like pattern high grade
- BCAC-LG:
-
Basal cell adenocarcinoma-like pattern low-grade
- BCC:
-
Basal cell carcinoma
- Bcl-3:
-
B-cell lymphoma 3
- BSS:
-
Brooke-Spiegler Syndrome
- CAP350:
-
Centrosome-Associated Protein 350
- cIAP1/2:
-
Cellular inhibitor of apoptosis 1 and 2
- JNK:
-
c-Jun NH(2)-terminal kinase
- CCD:
-
Clear cell differentiation
- CYLD:
-
Cylindromatosis
- CAP-GLY:
-
Cytoskeletal-associated proteinglycine-conserved
- DUB:
-
Deubiquitinating
- DSS:
-
Dextran sulphate sodium
- DEN:
-
Diethylnitrosamine
- Dvl:
-
Dishevelled
- FC:
-
Familial Cylindromatosis
- FISH:
-
Fluorescence in-situ hybridization
- HNSCC:
-
Head & neck squamous cell carcinoma
- Hes1:
-
Hes Family BHLH Transcription Factor 1
- HDAC6:
-
Histone-deacetylase 6
- HPV:
-
Human Papilloma virus
- T:
-
Individual tumor
- IACs:
-
Invasive adenocarcinomas
- IKKα/IKKβ:
-
IkB Kinase α and β
- LOH:
-
Loss of heterozygosity
- LRP6:
-
Low-density lipoprotein receptor-related protein 6
- LEF/TCF:
-
Lymphoid enhancer factor/T-cell factor
- K:
-
Lysine
- MEFs:
-
Mouse embryonic fibroblasts
- Md:
-
Mild
- MKK7:
-
Mitogen-Activated Protein Kinase 7
- MFT1:
-
Multiple Familial Trichoepithelioma 1
- NGS:
-
Next generation sequencing
- NIK:
-
NF-kappa-B inducing kinase
- NEMO:
-
NF-kB essential modulator
- NF-kB:
-
Nuclear Factor-kB
- PTCH1:
-
Patched 1
- PDGFR-α:
-
Platelet-derived growth factor receptor
- RIP1:
-
Receptor-interacting protein 1
- RB1:
-
Retinoblastoma 1
- RIG1:
-
Retinoic acid-inducible gene-1
- S:
-
Severe
- SMO:
-
Smoothened
- snail1:
-
Snail family transcriptional repressor 1
- Shh:
-
Sonic Hedgehog
- Shh/Ptch1:
-
Shh/Patched 1
- SCCs:
-
Squamous cell carcinomas
- SUFU:
-
Suppressor of Fused
- TAB1:
-
TGF-beta activated kinase 1
- TAK1:
-
TGF-β-activated kinase 1
- TRAF:
-
TNF receptor associate factor
- TRADD:
-
TNFRSF1AAssociated Via Death Domain
- TRIP:
-
TRAF interacting protein
- TRPA1:
-
Transient receptor potential cation channel A1
- TRK:
-
Tropomyosin kinase
- TNFR:
-
Tumor necrosis factor receptor
- TNF-α:
-
Tumor necrosis factor-α
- TP53:
-
Tumor protein 53
- UCH:
-
Ubiquitin C-terminal Hydrolase
- Ubs:
-
Ubiquitins
- VEGF-A:
-
Vascular endothelial growth factor-A
- VS:
-
Very severe
- WES:
-
Whole-exome sequencing
References
Bignell GR, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25(2):160–5.
Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17(1):25–34.
Eguether T, et al. The deubiquitinating enzyme CYLD controls apical docking of basal bodies in ciliated epithelial cells. Nat Commun. 2014;5:4585.
Yang Y, et al. CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res. 2014;24(11):1342–53.
Kovalenko A, et al. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424(6950):801–5.
Tauriello DV, et al. Loss of the tumor suppressor CYLD enhances Wnt/beta-catenin signaling through K63-linked ubiquitination of Dvl. Mol Cell. 2010;37(5):607–19.
Reiley W, Zhang M, Sun SC. Negative regulation of JNK signaling by the tumor suppressor CYLD. J Biol Chem. 2004;279(53):55161–7.
Ancell H. History of a remarkable case of tumours, developed on the head and face; accompanied with a similar disease in the abdomen. Med Chir Trans. 1842;25:227–306. 11.
Biggs PJ, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11(4):441–3.
Biggs PJ, et al. The cylindromatosis gene (cyld1) on chromosome 16q may be the only tumour suppressor gene involved in the development of cylindromas. Oncogene. 1996;12(6):1375–7.
Rajan N, et al. Tumor mapping in 2 large multigenerational families with CYLD mutations: implications for disease management and tumor induction. Arch Dermatol. 2009;145(11):1277–84.
Dubois A, et al. CYLD GeneticTesting for Brooke-Spiegler Syndrome, Familial Cylindromatosis and Multiple Familial Trichoepitheliomas. PLoS Curr, 2015. 7
Rajan N, Ashworth A. Inherited cylindromas: lessons from a rare tumour. Lancet Oncol. 2015;16(9):e460–9.
Johnson SC, Bennett RG. Occurrence of basal cell carcinoma among multiple trichoepitheliomas. J Am Acad Dermatol. 1993;28(2 Pt 2):322–6.
Scheinfeld N, et al. Identification of a recurrent mutation in the CYLD gene in Brooke-Spiegler syndrome. Clin Exp Dermatol. 2003;28(5):539–41.
Parren LJ, et al. A novel therapeutic strategy for turban tumor: scalp excision and combined reconstruction with artificial dermis and split skin graft. Int J Dermatol. 2014;53(2):246–9.
Li ZL, et al. Germline mutation analysis in the CYLD gene in Chinese patients with multiple trichoepitheliomas. Genet Mol Res. 2014;13(4):9650–5.
Farkas K, et al. The CYLD p.R758X worldwide recurrent nonsense mutation detected in patients with multiple familial trichoepithelioma type 1, Brooke-Spiegler syndrome and familial cylindromatosis represents a mutational hotspot in the gene. BMC Genet. 2016;17(1):36.
Nagy N, et al. A mutational hotspot in CYLD causing cylindromas: a comparison of phenotypes arising in different genetic backgrounds. Acta Derm Venereol. 2013;93(6):743–5.
Grossmann P, et al. Novel and recurrent germline and somatic mutations in a cohort of 67 patients from 48 families with Brooke-Spiegler syndrome including the phenotypic variant of multiple familial trichoepitheliomas and correlation with the histopathologic findings in 379 biopsy specimens. Am J Dermatopathol. 2013;35(1):34–44.
Kazakov DV, et al. Multiple (familial) trichoepitheliomas: a clinicopathological and molecular biological study, including CYLD and PTCH gene analysis, of a series of 16 patients. Am J Dermatopathol. 2011;33(3):251–65.
Ponti G, et al. Brooke-Spiegler syndrome tumor spectrum beyond the skin: a patient carrying germline R936X CYLD mutation and a somatic CYLD mutation in Brenner tumor. Future Oncol. 2014;10(3):345–50.
Nasti S, et al. Five novel germline function-impairing mutations of CYLD in Italian patients with multiple cylindromas. Clin Genet. 2009;76(5):481–5.
Salhi A, et al. Multiple familial trichoepithelioma caused by mutations in the cylindromatosis tumor suppressor gene. Cancer Res. 2004;64(15):5113–7.
Zheng G, et al. CYLD mutation causes multiple familial trichoepithelioma in three Chinese families. Hum Mutat. 2004;23(4):400.
Kazakov DV, et al. Morphologic diversity of malignant neoplasms arising in preexisting spiradenoma, cylindroma, and spiradenocylindroma based on the study of 24 cases, sporadic or occurring in the setting of Brooke-Spiegler syndrome. Am J Surg Pathol. 2009;33(5):705–19.
Zhang J, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116(11):3042–9.
Alameda JP, et al. An inactivating CYLD mutation promotes skin tumor progression by conferring enhanced proliferative, survival and angiogenic properties to epidermal cancer cells. Oncogene. 2010;29(50):6522–32.
Massoumi R, et al. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125(4):665–77.
Antonescu CR, Terzakis JA. Multiple malignant cylindromas of skin in association with basal cell adenocarcinoma with adenoid cystic features of minor salivary gland. J Cutan Pathol. 1997;24(7):449–53.
Gerretsen AL, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72(5):1618–23.
Beideck M, Kuhn A. Malignant transformation of cutaneous cylindromas. 2 case reports and a review of the literature. Z Hautkr. 1985;60(1–2):73–8.
Braun-Falco M, Hein R, Ring J. Cylindrospiradenomas in Brooke-Spiegler syndrome. Hautarzt. 2001;52(11):1021–5.
De Francesco V, et al. Carcinosarcoma arising in a patient with multiple cylindromas. Am J Dermatopathol. 2005;27(1):21–6.
Durani BK, et al. Malignant transformation of multiple dermal cylindromas. Br J Dermatol. 2001;145(4):653–6.
Hammond DC, Grant KF, Simpson WD. Malignant degeneration of dermal cylindroma. Ann Plast Surg. 1990;24(2):176–8.
Iyer PV, Leong AS. Malignant dermal cylindromas. Do they exist? A morphological and immunohistochemical study and review of the literature. Pathology. 1989;21(4):269–74.
Korting GW, Hoede N, Gebhardt R. Malignant degeneration of Spiegler’s tumor. Dermatol Monatsschr. 1970;156(3):141–7.
Kostler E, et al. Psoriasis and Brooke-Spiegler syndrome with multiple malignancies. J Eur Acad Dermatol Venereol. 2005;19(3):380–1.
Lausecker H. Beitrag zu den Naevo-epitheliomen. Arch Dermatol Syph. 1952;194(6):639–62.
Lotem M, et al. Multiple dermal cylindroma undergoing a malignant transformation. Int J Dermatol. 1992;31(9):642–4.
Lyon JB, Rouillard LM. Malignant degeneration of turban tumour of scalp. Trans St Johns Hosp Dermatol Soc. 1961;46:74–7.
Pierard-Franchimont C, Pierard GE. Development and neoplastic progression of benign and malignant cutaneous cylindroma. Ann Dermatol Venereol. 1984;111(12):1093–8.
Pingitore R, Campani D. Salivary gland involvement in a case of dermal eccrine cylindroma of the scalp (turban tumor). Report of a case with lung metastases. Tumori. 1984;70(4):385–8.
Pizinger K, Michal M. Malignant cylindroma in Brooke-Spiegler syndrome. Dermatology. 2000;201(3):255–7.
Rockerbie N, et al. Malignant dermal cylindroma in a patient with multiple dermal cylindromas, trichoepitheliomas, and bilateral dermal analogue tumors of the parotid gland. Am J Dermatopathol. 1989;11(4):353–9.
Tsambaos D, Greither A, Orfanos CE. Multiple malignant Spiegler tumors with brachydactyly and racket-nails. Light and electron microscopic study. J Cutan Pathol. 1979;6(1):31–41.
Volter C, et al. Cylindrocarcinoma in a patient with Brooke-Spiegler syndrome. Laryngorhinootologie. 2002;81(3):243–6.
Zontschew P. Cylindroma capitis mit maligner Entartung. Zentralbl Chir. 1961;86:1875–9.
Chou SC, Lin SL, Tseng HH. Malignant eccrine spiradenoma: a case report with pulmonary metastasis. Pathol Int. 2004;54(3):208–12.
Dabska M. Malignant transformation of eccrine spiradenoma. Pol Med J. 1972;11(2):388–96.
Engel CJ, et al. Eccrine spiradenoma: a report of malignant transformation. Can J Surg. 1991;34(5):477–80.
Fernandez-Acenero MJ, et al. p53 expression in two cases of spiradenocarcinomas. Am J Dermatopathol. 2000;22(2):104–7.
Galadari E, Mehregan AH, Lee KC. Malignant transformation of eccrine tumors. J Cutan Pathol. 1987;14(1):15–22.
Ishikawa M, et al. Malignant eccrine spiradenoma: a case report and review of the literature. Dermatol Surg. 2001;27(1):67–70.
Leonard N, Smith D, McNamara P. Low-grade malignant eccrine spiradenoma with systemic metastases. Am J Dermatopathol. 2003;25(3):253–5.
McCluggage WG, et al. Malignant eccrine spiradenoma with carcinomatous and sarcomatous elements. J Clin Pathol. 1997;50(10):871–3.
McKee PH, et al. Carcinosarcoma arising in eccrine spiradenoma. A clinicopathologic and immunohistochemical study of two cases. Am J Dermatopathol. 1990;12(4):335–43.
Swanson PE, et al. Eccrine sweat gland carcinoma: an histologic and immunohistochemical study of 32 cases. J Cutan Pathol. 1987;14(2):65–86.
Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82.
Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4.
Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807.
Komander D, et al. The structure of the CYLD USP domain explains its specificity for Lys63-linked polyubiquitin and reveals a B box module. Mol Cell. 2008;29(4):451–64.
Reiley WW, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7(4):411–7.
Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem Sci. 2010;35(7):392–9.
Ke H, et al. CYLD inhibits melanoma growth and progression through suppression of the JNK/AP-1 and beta1-integrin signaling pathways. J Invest Dermatol. 2013;133(1):221–9.
Reiley WW, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204(6):1475–85.
Espinosa L, et al. The Notch/Hes1 pathway sustains NF-kappaB activation through CYLD repression in T cell leukemia. Cancer Cell. 2010;18(3):268–81.
Massoumi R. CYLD: a deubiquitination enzyme with multiple roles in cancer. Future Oncol. 2011;7(2):285–97.
Castresana, JS. Cancer as a Ciliopathy: The Primary Cilium as a New Therapeutic Target. Carcinogenesis & Mutagenesis, 2015. 6(6).
Moser JJ, Fritzler MJ, Rattner JB. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer. 2009;9:448.
Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res. 2006;66(13):6463–7.
Yang Y, Zhou J. CYLD - a deubiquitylase that acts to fine-tune microtubule properties and functions. J Cell Sci. 2016;129(12):2289–95.
Bonilla X, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48(4):398–406.
Dijkhuizen T, et al. Cytogenetics of a case of eccrine spiradenoma. Hum Pathol. 1992;23(9):1085–7.
Deutsch, J., Sessile Serrated Adenomas and Melanosis Coli Visible Human Journal of Endoscopy, 2014. 13(1)
Massoumi R, et al. Cylindroma as tumor of hair follicle origin. J Invest Dermatol. 2006;126(5):1182–4.
Oosterkamp HM, et al. An evaluation of the efficacy of topical application of salicylic acid for the treatment of familial cylindromatosis. Br J Dermatol. 2006;155(1):182–5.
Rajan N, et al. Dysregulated TRK signalling is a therapeutic target in CYLD defective tumours. Oncogene. 2011;30(41):4243–60.
Saggar S, et al. CYLD mutations in familial skin appendage tumours. J Med Genet. 2008;45(5):298–302.
Bowen S, et al. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124(5):919–20.
Lv H, et al. Three mutations of CYLD gene in Chinese families with multiple familial trichoepithelioma. Am J Dermatopathol. 2014;36(7):605–7.
Linos K, et al. Recurrent CYLD nonsense mutation associated with a severe, disfiguring phenotype in an African American family with multiple familial trichoepithelioma. Am J Dermatopathol. 2011;33(6):640–2.
van den Ouweland AM, et al. Identification of a large rearrangement in CYLD as a cause of familial cylindromatosis. Fam Cancer. 2011;10(1):127–32.
Almeida S, et al. Five new CYLD mutations in skin appendage tumors and evidence that aspartic acid 681 in CYLD is essential for deubiquitinase activity. J Invest Dermatol. 2008;128(3):587–93.
Blake PW, Toro JR. Update of cylindromatosis gene (CYLD) mutations in Brooke-Spiegler syndrome: novel insights into the role of deubiquitination in cell signaling. Hum Mutat. 2009;30(7):1025–36.
Ying ZX, et al. A novel mutation of CYLD in a Chinese family with multiple familial trichoepithelioma. J Eur Acad Dermatol Venereol. 2012;26(11):1420–3.
Liang YH, et al. Novel substitution and frameshift mutations of CYLD in two Chinese families with multiple familial trichoepithelioma. Br J Dermatol. 2008;158(5):1156–8.
Sima R, et al. Brooke-Spiegler syndrome: report of 10 patients from 8 families with novel germline mutations: evidence of diverse somatic mutations in the same patient regardless of tumor type. Diagn Mol Pathol. 2010;19(2):83–91.
Nagy N, et al. Phenotype-genotype correlations for clinical variants caused by CYLD mutations. Eur J Med Genet. 2015;58(5):271–8.
Ly H, Black MM, Robson A. Case of the Brooke-Spiegler syndrome. Australas J Dermatol. 2004;45(4):220–2.
Kazakov DV, et al. Brooke-Spiegler syndrome: report of a case with a novel mutation in the CYLD gene and different types of somatic mutations in benign and malignant tumors. J Cutan Pathol. 2010;37(8):886–90.
Pinho AC, et al. Brooke-Spiegler Syndrome - an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9(3):67–70.
Zuo YG, et al. A novel mutation of CYLD in a Chinese family with multiple familial trichoepithelioma and no CYLD protein expression in the tumour tissue. Br J Dermatol. 2007;157(4):818–21.
Tantcheva-Poor I, et al. Report of Three Novel Germline CYLD Mutations in Unrelated Patients with Brooke-Spiegler Syndrome, Including Classic Phenotype, Multiple Familial Trichoepitheliomas and Malignant Transformation. Dermatology. 2016;232(1):30–7.
Huang TM, Chao SC, Lee JY. A novel splicing mutation of the CYLD gene in a Taiwanese family with multiple familial trichoepithelioma. Clin Exp Dermatol. 2009;34(1):77–80.
Reuven B, et al. Multiple trichoepitheliomas associated with a novel heterozygous mutation in the CYLD gene as an adjunct to the histopathological diagnosis. Am J Dermatopathol. 2013;35(4):445–7.
Heinritz W, et al. A case of Brooke-Spiegler syndrome with a new mutation in the CYLD gene. Br J Dermatol. 2006;154(5):992–4.
Kacerovska D, et al. A novel germline mutation in the CYLD gene in a Slovak patient with Brooke-Spiegler syndrome. Cesk Patol. 2013;49(2):89–92.
Hu G, et al. A novel missense mutation in CYLD in a family with Brooke-Spiegler syndrome. J Invest Dermatol. 2003;121(4):732–4.
Liang YH, et al. Two novel CYLD gene mutations in Chinese families with trichoepithelioma and a literature review of 16 families with trichoepithelioma reported in China. Br J Dermatol. 2005;153(6):1213–5.
Poblete Gutierrez P, et al. Phenotype diversity in familial cylindromatosis: a frameshift mutation in the tumor suppressor gene CYLD underlies different tumors of skin appendages. J Invest Dermatol. 2002;119(2):527–31.
Oiso N, et al. Mild phenotype of familial cylindromatosis associated with an R758X nonsense mutation in the CYLD tumour suppressor gene. Br J Dermatol. 2004;151(5):1084–6.
Hester CC, et al. A new Cylindromatosis (CYLD) gene mutation in a case of Brooke-Spiegler syndrome masquerading as basal cell carcinoma of the eyelids. Ophthal Plast Reconstr Surg. 2013;29(1):e10–1.
Zhang XJ, et al. Identification of the cylindromatosis tumor-suppressor gene responsible for multiple familial trichoepithelioma. J Invest Dermatol. 2004;122(3):658–64.
Hunstig F, et al. A case of Brooke-Spiegler syndrome with a novel mutation in the CYLD gene in a patient with aggressive non-Hodgkin's lymphoma. J Cancer Res Clin Oncol. 2016;142(4):845–8.
Chen M, et al. Mutation analysis of the CYLD gene in two Chinese families with multiple familial Trichoepithelioma. Australas J Dermatol. 2011;52(2):146–7.
Malzone MG, et al. Brooke-Spiegler syndrome presenting multiple concurrent cutaneous and parotid gland neoplasms: cytologic findings on fine-needle sample and description of a novel mutation of the CYLD gene. Diagn Cytopathol. 2015;43(8):654–8.
Guardoli D, et al. A novel CYLD germline mutation in Brooke-Spiegler syndrome. J Eur Acad Dermatol Venereol. 2015;29(3):457–62.
Shiver M, et al. A novel CYLD gene mutation and multiple basal cell carcinomas in a patient with Brooke-Spiegler syndrome. Clin Exp Dermatol. 2016;41(1):98–100.
Melly L, Lawton G, Rajan N. Basal cell carcinoma arising in association with trichoepithelioma in a case of Brooke-Spiegler syndrome with a novel genetic mutation in CYLD. J Cutan Pathol. 2012;39(10):977–8.
Zhang G, et al. Diverse phenotype of Brooke-Spiegler syndrome associated with a nonsense mutation in the CYLD tumor suppressor gene. Exp Dermatol. 2006;15(12):966–70.
Amaro C, et al. Multiple trichoepitheliomas--a novel mutation in the CYLD gene. J Eur Acad Dermatol Venereol. 2010;24(7):844–6.
Scholz IM, et al. New mutation in the CYLD gene within a family with Brooke-Spiegler syndrome. J Dtsch Dermatol Ges. 2010;8(2):99–101.
Oranje AP, et al. Multiple familial trichoepithelioma and familial cylindroma: one cause! J Eur Acad Dermatol Venereol. 2008;22(11):1395–6.
Nagy N, et al. A novel missense mutation of the CYLD gene identified in a Hungarian family with Brooke-Spiegler syndrome. Exp Dermatol. 2012;21(12):967–9.
Espana A, et al. A novel missense mutation in the CYLD gene in a Spanish family with multiple familial trichoepithelioma. Arch Dermatol. 2007;143(9):1209–10.
Furuichi M, et al. Blaschkoid distribution of cylindromas in a germline CYLD mutation carrier. Br J Dermatol. 2012;166(6):1376–8.
Young AL, et al. CYLD mutations underlie Brooke-Spiegler, familial cylindromatosis, and multiple familial trichoepithelioma syndromes. Clin Genet. 2006;70(3):246–9.
Vanecek T, et al. Large germline deletions of the CYLD gene in patients with Brooke-Spiegler syndrome and multiple familial trichoepithelioma. Am J Dermatopathol. 2014;36(11):868–74.
Wang FX, et al. A novel missense mutation of CYLD gene in a Chinese family with multiple familial trichoepithelioma. Arch Dermatol Res. 2010;302(1):67–70.
Acknowledgements
Not applicable.
Funding
VWYL was supported by the School of Biomedical Sciences Start-up Fund, Faculty of Medicine, Chinese University of Hong Kong, the Theme-based Research Grant (T12-401/13-R) and General Research Fund (#17114874), Research Grants Council (RGC), Hong Kong Government, Hong Kong. KRV and HLN were supported by the Hong Kong PhD Fellowship Scheme, RGC, Hong Kong Government, and HKU SPACE Research Fund, University of Hong Kong, respectively. There is no other source of funding directly related to this manuscript.
Availability of data and materials
This is a review article and there is no raw data related to this manuscript for data sharing.
Authors’ contributions
KRV contributed to manuscript writing. HLN did CYLD mutational analyses. VWYL conceived the idea, and contributed to the writing. All authors read and approved the final manuscript.
Competing interests
VWYL served as a Consultant for Novartis Pharmaceuticals (HK) Ltd. All other authors declare no conflict of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
Graph showing the mutation frequencies of CYLD gene in major cancer types. Data were extracted from the cBioPortal database (www.cbioportal.org; dated 3rd August, 2016). The CYLD mutation frequencies of 15 most updated TCGA Provisional cancer cohorts, and five other important cancer types with CYLD mutation rates of >1–3 % rates were shown, with actual number of mutated cases shown in this legend. Abbreviations: Uterine (TCGA Provisional): Uterine Corpus Endometrial Carcinoma 13/248 cases (5.2 %), Lung squ (TCGA Provisional): Lung Squamous Cell Carcinoma 8/177 cases (4.5 %), Stomach (TCGA Provisional): Stomach Adenocarcinoma 15/395 cases (3.8 %), Lung adeno (TCGA Provisional): Lung Adenocarcinoma 7/230 cases (3 %), Head & neck (TCGA Provisional): Head and Neck Squamous Cell Carcinoma 15/512 cases (2.9 %), Cholangiocarcinoma (JHU, 2013): Intrahepatic Cholangiocarcinoma 1/40 (2.5 %), Small Cell Lung (JHU, 2012): Small Cell Lung Cancer 1/42 (2.4 %), Melanoma (TCGA Provisional): Skin Cutaneous Melanoma 8/368 cases (2.2 %), Esophagus (TCGA Provisional): Esophageal Carcinoma 4/185 cases (2.2 %), DLBC (TCGA Provisional): Lymphoid Neoplasm Diffuse Large B-cell Lymphoma 1/48 case (2.1 %), Colorectal (TCGA Provisional): Colorectal Adenocarcinoma 4/223 cases (1.8 %), Glioma (UCSF, 2014): Low-Grade Gliomas 1/61 (1.6 %), Thymoma (TCGA Provisional): Thymoma 2/123 cases (1.6 %), chRCC (TCGA Provisional): Kidney Chromophobe 1/66 case (1.5 %), MM (Broad, 2014): Multiple Myeloma 3/205 (1.5 %), Pancreas (TCGA Provisional): Pancreatic Adenocarcinoma 2/150 cases (1.3 %), Uveal melanoma (TCGA Provisional): Uveal melanoma 1/80 case (1.3 %), GBM (TCGA, 2008): Glioblastoma 1/91 (1.1 %), Liver (TCGA Provisional): Liver Hepatocellular Carcinoma 4/373 cases (1.1 %), Cervical (TCGA Provisional): Cervical Squamous Cell Carcinoma & Endocervical Adenocarcinoma 2/194 cases (1 %). (PPTX 77 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Verhoeft, K.R., Ngan, H.L. & Lui, V.W.Y. The cylindromatosis (CYLD) gene and head and neck tumorigenesis. Cancers Head Neck 1, 10 (2016). https://doi.org/10.1186/s41199-016-0012-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41199-016-0012-y
Keywords
- Head and Neck Cancer
- Cylindromatosis (CYLD)
- The CYLD cutaneous syndrome
- Turban Tumor Syndrome
- Brooke-Spiegler Syndrome (BSS)
- Multiple Familial Trichoepithelioma (MFT1)
- Familial Cylindromatosis (FC)
- tumorigenesis
- Deubiquitinating (DUB)
- Nuclear Factor-kB (NF-kB)
- TNF-receptor associated factor (TRAF) proteins
- and B-cell lymphoma 3 (Bcl-3)