Abstract
Background
Malaria remains a significant cause of morbidity and mortality globally and continues to disproportionately afflict the African population. We aimed to evaluate the effect of home management of malaria intervention on health outcomes.
Methods
In our systematic review and meta-analysis, six databases (Pubmed, Cochrane CENTRAL, EMBASE, CAB Abstracts and Global Health, CINAHL Complete, and BIOSIS) were searched for studies of home management of malaria from inception until November 15, 2023. We included before–after studies, observational studies, and randomised controlled trials of home management intervention delivered in community settings. The primary outcomes were malaria mortality and all-cause mortality. The risk of bias in individual observational studies was assessed using the ROBINS-I tool, whilst randomised controlled trials were judged using a revised Cochrane risk of bias tool and cluster-randomised controlled trials were evaluated using an adapted Cochrane risk of bias tool for cluster-randomised trials. We computed risk ratios with accompanying 95% confidence intervals for health-related outcomes reported in the studies and subsequently pooled the results by using a random-effects model (DerSimonian–Laird method).
Results
We identified 1203 citations through database and hand searches, from which 56 articles from 47 studies encompassing 234,002 participants were included in the systematic review. All studies were conducted in people living in sub-Saharan Africa and were rated to have a low or moderate risk of bias. Pooled analyses showed that mortality rates due to malaria (RR = 0.40, 95% CI = 0.29–0.54, P = 0.00001, I2 = 0%) and all-cause mortality rates (RR = 0.62, 95% CI = 0.53–0.72, P = 0.00001, I2 = 0%) were significantly lower among participants receiving home management intervention compared to the control group. However, in children under 5 years of age, there was no significant difference in mortality rates before and after implementation of home management of malaria. In terms of secondary outcomes, home management of malaria was associated with a reduction in the risk of febrile episodes (RR = 1.27, 95% CI = 1.09–1.47, P = 0.002, I2 = 97%) and higher effective rates of antimalarial treatments (RR = 2.72, 95% CI = 1.90–3.88, P < 0.00001, I2 = 96%) compared to standard care. Home malaria management combined with intermittent preventive treatment showed a significantly lower incidence risk of malaria than home management intervention that exclusively provided treatment to individuals with febrile illness suggestive of malaria. The risks for adverse events were found to be similar for home management intervention using different antimalarial drugs. Cost-effectiveness findings depicted that home malaria management merited special preferential scale-up.
Conclusions
Home management of malaria intervention was associated with significant reductions in malaria mortality and all-cause mortality. The intervention could help decrease health and economic burden attributable to malaria. Further clinical studies are warranted to enable more meaningful interpretations with regard to wide-scale implementation of the intervention, settings of differing transmission intensity, and new antimalarial drugs.
Similar content being viewed by others
Background
Malaria is a devastating infectious disease that is a major cause of morbidity and mortality [1], with sub-Saharan Africa shouldering the heaviest burden [2]. Estimates show that there were 241 million malaria cases and 627,000 malaria-related deaths across the globe in 2020, an increase between 7 and 12% compared to 2015 [3], of which, the African region accounts for 95% of the world's malaria cases and 96% of mortality [2]. As such, prompt and effective treatment of malaria is critical in preventing progression to severe disease or complications and reducing morbidity and mortality. The increasing resistance to chloroquine has led to the use of artemisinin-based combination therapy as the first-line treatment against confirmed or suspected Plasmodium falciparum malaria [4, 5]. Alongside a functioning continuum of care that encompasses recognition of severe episodes at household and primary care levels to prompt comprehensive management with effective diagnostics and medicines, the risk of death or permanent disability could be remarkably reduced [6].
Home-based management of malaria has been promoted as a strategy to increase early diagnosis of malaria, physical access to antimalarial drugs, and use of malaria preventive treatment [7]. It is recommended under the Roll Back Malaria Initiative to reduce the burden of malaria by delivering effective antimalarial treatment to individuals with suspected malaria so that they can receive appropriate care in the comfort of their own homes [8, 9]. It encompasses components, such as establishment of a suitable platform that empowers caregivers to recognise malarial illness early and respond accordingly, community-based training programmes that equip caregivers with adequate knowledge and capacity to respond to malarial illness, and creation of an environment that facilitates the provision of antimalarial drugs as close as possible to the patients’ homes [8, 10]. As such, community health workers and community drug distributors are instrumental in implementing the home management intervention [10]. However, home management of malaria is associated with several potential disadvantages, such as unnecessary overtreatment, emergence of drug resistance, and higher costs [11]. In recent years, there have been home-based management of malaria programmes that integrate diagnosis and treatment based on test results, leading to a better community access to prompt and effective management of uncomplicated malaria, particularly in rural areas with high levels of transmission [12, 13].
Home-based malaria management intervention plays a vital role to ensure the reach of public health services, particularly for populations living in rural, remote, or hard-to-reach areas, where there is limited access to healthcare infrastructures. It allows proactive case detection and treatment via the scaling up of integrated community delivery platforms and supports a broader continuum of care for impoverished groups who often face disadvantage, discrimination and exclusion in health outcomes. Such intervention that is tailored to the local context accelerates the progress along the path to control and ultimately eliminate malaria. In line with a global effort to eradicate malaria, all suspected malaria cases should receive timely diagnostic confirmation through parasite detection methods, such as antigen-detection rapid diagnostic tests and microscopy examination of blood films. Home-based management of malaria enhances the access to prompt diagnostic testing in remote sites and contributes to effective treatment of uncomplicated malaria or other medical conditions. It alleviates the burden of preventable and treatable deaths and diminishes the risk of onward transmission of malaria in the communities [14].
Home-based management of malaria (HMM) is a promising strategy to improve public access to prompt and effective management of uncomplicated malaria for reducing the disease burden. It encompasses the provision of diagnostic tests and pharmacological therapies to patients close to their homes [12, 15]. Community case management of malaria (CCM) is designed to reach a larger fraction of the population by bringing primary health care into the communities. It involves the training of community health workers to diagnose and treat uncomplicated malaria cases within their communities [16]. On the other hand, home-based management of malaria (PECADOM) is targeted for inhabitants of remote or rural areas with difficulties in accessing health care where community health workers visit all households in their communities weekly during malaria transmission season to identify fever cases and offer case management [17]. Community Health Workers (CHWs) are trusted members of local communities who are trained to play a bridging role between patients and clinical services, transferring and interpreting health information and ensuring that patients are connected to health care [18, 19].
The impact of home management intervention on malaria morbidity and mortality has been inconclusive, with existing research depicting mixed findings [8]. To date, there is only one systematic review of home-based treatment of malaria which is limited to the use of chloroquine alone and it was found that the evidence base on home management of malaria intervention were sparse and showed inconsistent results [11]. An updated systematic review is necessary to expand the former literature searches in view of the drug resistance to chloroquine and new standard of care with artemisinin-based combination therapy for malaria [20, 21]. Therefore, the aim of the current review was to provide a comprehensive overview on the effect of home-based management of malaria and its impact on health outcomes.
Methods
This systematic review with meta-analysis was conducted by following the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) guidelines, 2020 [22].
Search strategy and selection criteria
Six online bibliographic databases were searched from inception up to November 15, 2023: Pubmed, Cochrane CENTRAL, EMBASE, CAB Abstracts and Global Health, CINAHL Complete, and BIOSIS for studies that investigated the effect of home management of malaria. No restrictions on language, age, geography, document type, or publication status were applied. In searching each database, we used a combination of English text and Medical Subject Heading terms, including “home management” and “malaria” and “artemisinin or chloroquine”. A complete search strategy is depicted in the appendix. Bibliographies of screened and selected studies, as well as review articles were manually reviewed to identify any additional relevant studies.
Titles and abstracts of retrieved articles were first screened by one author, and all potentially relevant full texts were screened and evaluated by two authors independently. In the case of any discrepancies, a third author was consulted. Studies were considered eligible for inclusion if they: (1) were before–after studies, observational studies, or randomised controlled trials; (2) pertained to the use of any antimalarial drugs, including artemisinin-based or quinine-based treatments; and (3) involved the delivery of home-based malaria prevention or treatment. Studies were excluded if they were: (1) retrospective studies, case series, case reports, cross-sectional studies, qualitative exploratory research, commentaries, or editorials; (2) studies that did not report separate data for home-based management of malaria; and (3) studies that did not provide clinical or health-related outcomes. Two reviewers independently extracted data using a standardised data abstraction form. Information extracted included study design, study period, participant characteristics, antimalarial drugs for treatment and prevention of malaria, details of home delivery of malaria care, and main study findings. Any differences were discussed and resolved through consensus.
Outcome measures
Primary outcomes of interest were malaria mortality and all-cause mortality. Secondary outcomes included number of febrile episodes treated with antimalarial drugs, proportion of effective antimalarial treatments (i.e. any antimalarial therapy that consisted of chloroquine plus sulfadoxine–pyrimethamine, quinine, or an artemisinin were deemed to be effective), incidence of clinical malaria episodes, risk of severe malaria, rates of anaemia, parasitaemia, splenomegaly, early treatment failure, late treatment failure, late parasitological failure, sensitivity and specificity for diagnosis of malaria, adherence to antimalarial medications, and adverse drug events.
Risk of bias assessment
Potential risk of bias was assessed for observational studies based on ROBINS-I tool [23], while randomised controlled trials were judged with the use of a revised Cochrane risk of bias tool (RoB 2.0) [24] and cluster-randomised controlled trials were evaluated using an adapted Cochrane risk of bias tool for cluster-randomised trials (RoB 2.0 CRT) [25].
Data analysis
Data from each of the studies were extracted and summarised by two authors independently. A standardised data abstraction form in Microsoft Word was used to outline the principal components of each individual study which included study design, study period, sample size, participant characteristics, antimalarial drugs for treatment and prevention of malaria, details of home delivery of malaria care, relevant study outcomes, and main findings. Any discrepancies and disagreements during data extraction were discussed and reviewed among two authors (KP and BC), and a third author (SL) was consulted if consensus was not reached. When comparable quantitative data were reported across multiple studies, we noted the sample sizes, proportions, and frequency statistics to facilitate subsequent pooling of estimates using random-effects models (DerSimonian–Laird method) in order to generate risk ratios (RR) for the dichotomous outcomes. Heterogeneity between studies was evaluated using I2 statistics and funnel plots were assessed visually for publication bias. All analyses were performed using RevMan version 5.3 (Cochrane Collaboration).
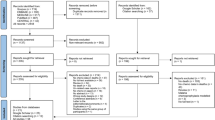
Results
The literature search yielded 1203 records. After screening of the title and abstract, 377 articles underwent full-text evaluation, of which 56 articles [15, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80] representing 47 studies were included in the systematic review (qualitative synthesis) and 40 for quantitative analysis (Fig. 1). Of these, 10 studies were pre–post in design [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], 17 were prospective observational studies [15, 41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59], 9 were randomised controlled trials [60,61,62,63,64,65,66,67,68], and 11 were cluster-randomised controlled trials [69,70,71,72,73,74,75,76,77,78,79,80]. All studies were conducted in sub-Saharan African countries and were published between 1987 and 2022. They were invariably carried out in remote rural communities (n = 40) [15, 26, 27, 29, 31,32,33,34, 36,37,38, 41, 42, 45, 47, 49, 52,53,54,55,56,57,58,59,60,61, 64, 66,67,68,69,70,71,72,73,74,75, 77, 78, 80], whilst four in peri-urban areas [44, 51, 62, 65], one each in urban areas [76], urban and peri-urban zones [63], and mixed urban and rural areas [50]. The age ranges of participants included in the studies were variable, however, most studies (76.6%) recruited children aged 15 years and younger. The studies had sample sizes ranging from 156 to 34,358 [45, 64], making up a total of 234,002 participants. Antimalarial monotherapy as well as combinations deployed in the home management of malaria were artemether–lumefantrine, chloroquine, chloroquine–sulfadoxine–pyrimethamine, quinine, artesunate–amodiaquine, artesunate–chlorproguanil–dapsone, artesunate–sulfadoxine–pyrimethamine, dihydroartemisinin–piperaquine, artesunate monotherapy, amodiaquine monotherapy, sulfadoxine–pyrimethamine, halofantrine, or Argemone mexicana decoction for treating uncomplicated malaria, rectal artesunate for treating severe malaria, and sulfadoxine–pyrimethamine–amodiaquine, sulfadoxine–pyrimethamine–piperaquine, dihydroartemisinin–piperaquine, or pyrimethamine–dapsone as a preventive therapy for malaria. Ten studies reported to utilise rapid tests for malaria diagnosis. Details of individual studies and main findings are summarised in Additional file 1: Table S1.
Efficacy of home management of malaria
Pooled results randomised controlled studies comparing home management of malaria with standard care demonstrated a reduction in the risk of febrile episodes (RR = 1.27, 95% CI = 1.09–1.47, P = 0.002, I2 = 97%) and higher effective rates of antimalarial treatments (RR = 2.72, 95% CI = 1.90–3.88, P < 0.00001, I2 = 96%) in the intervention group (Fig. 2). Among the communities who were provided home management intervention, incidence rates of malaria cases were similar compared with the control group (Table 1A). Combining home-based management with intermittent preventive malaria treatment was associated with a considerably lower incidence risk of malaria than home management intervention that exclusively provided treatment to individuals with febrile illness suggestive of malaria (Table 1B). In a comparison of mortality rates among children under the age of 5 years before and after implementation of home management of malaria, no important difference was noted (Table 2). Contrariwise, the risks of severe anaemia was significantly decreased (RR = 0.63, 95% CI = 0.45–0.88, P = 0.007, I2 = 72%) after the implementation of home-based management of malaria (Fig. 3). Mortality rates due to malaria (RR = 0.40, 95% CI = 0.29–0.54, P = 0.00001, I2 = 0%) and all-cause mortality rates (RR = 0.62, 95% CI = 0.53–0.72, P = 0.00001, I2 = 0%) were similarly reduced among participants receiving home management intervention compared to control group (Fig. 4). However, other outcome including treatment failures, or clinical failure did not differ between groups (Fig. 5). Regarding the diagnostic accuracy of home management of malaria, the relevant studies reported mean sensitivity of 82.9% and specificity of 69.2% (Additional file 1: Table S2). Delivering the home management of malaria intervention also resulted in an average of 94.9% of participants received effective treatment and 90.9% of participants had good adherence to antimalarial regimen (Additional file 1: Table S3).
Cost of home management of malaria
Five performed an economic evaluation of the home management intervention [37, 45, 51, 58, 76]. The mean cost per home visit was $14.74, which was substantially greater than the cost of follow-up by telephone calls ($0.77) [51]. The cost of delivering home management intervention was $33.83 per child per year, which was associated with lower health care expenditures per participant per year than in the control group [76]. In another study, it was estimated that the annual scale-up costs of home malaria management were $6.73 million for 20% and $11.78 million for 35% utilisation. The cost per case appropriately diagnosed and treated in home-based management was $4.22 as compared to $6.12 for health facility-based management of uncomplicated malaria [45]. Moreover, the total implementation cost of a 4-month home management programme was $12,066, with an average cost of $0.06 per child [37]. The scale-up of home-based management of malaria required $163,424.61 yearly, which was equivalent to $0.80 per person at risk (Additional file 1: Table S4) [58].
Safety of home management of malaria
17 studies documented data for adverse events [27, 30, 31, 44, 53, 55, 60, 61, 63, 65, 66, 68,69,70, 75, 77, 79]. There were no significant differences between the groups with respect to adverse event rates [60, 61, 63, 65, 69]. Most studies (64.7%) reported no serious adverse event [27, 30, 31, 55, 60, 61, 63, 66, 70, 75, 77, 79]. Two studies, respectively, identified 1.2% and 4.1% of reported adverse events were serious [44, 53]. Another study depicted severe adverse events only occurring in 3 of 178 participants (1.7%) of home malaria management, of whom two received quinine and one received artemether–lumefantrine [60]. Likewise, during a 3-month follow-up, 3 of 294 participants (1.0%) of home-based management experienced adverse events, namely two deaths in Argemone mexicana group and one miscarriage in artemisinin combination therapy group [68]. In a study comparing home malaria management with standard care, one death was recorded in each group [76]. The frequency of adverse events attributable to home malaria management only group was higher than those in combination of home malaria management and preventive therapy group (Additional file 1: Table S5) [77].
Our pooled analysis did not find any discernible differences in the risk of adverse drug events in home management of malaria interventions by use of artesunate–amodiaquine compared with other antimalarial drug combinations (Additional file 1: Fig. S1). Additionally, home-based intermittent preventive treatment had a similar risk of deterioration to severe malaria in comparison with home-based management of malaria-attributable febrile illness (Additional file 1: Fig. S2). When evaluating home management interventions with Argemone mexicana decoction versus artesunate–amodiaquine, we did not detect any differences on the risks of severe malaria in children younger than 5 years of age, parasitaemia, and serious adverse events. Nonetheless, provision of Argemone mexicana decoction at home increased the need for second-line antimalarial treatment (RR = 3.12, 95% CI = 1.21–8.01, P = 0.02, I2 = 25%) (Additional file 1: Fig. S3).
Risk of bias
All observational studies had a moderate risk of bias (Additional file 1: Fig. S4) while most randomised controlled trials had some concerns of bias relating to participation in assignment or adhering to intervention and completeness of outcome evaluation and reported result (Additional file 1: Fig. S5). Most cluster-randomised controlled trials were assessed as low risk of bias (Additional file 1: Fig. S6). Examination of the funnel plots for all the meta-analyses indicated that there was no evidence of publication bias.
Discussion
Home management of malaria is a widely implemented and long-running strategy delivered in malaria-endemic regions across sub-Saharan Africa, where antimalarial drugs are distributed by trained community health workers or community drug distributors, complemented with more recent integration of rapid diagnostic testing through the evolution of community-based programme and point-of-care technologies to minimise overtreatment or antimalarial drug resistance [13, 16]. Nevertheless, the impact of home management intervention on malaria disease burden and patient outcomes remains less understood [8]. Our systematic review and meta-analysis provides the most comprehensive evidence synthesis of the scientific literature on home-based management of malaria for children and adults in communities. It included 47 studies from 17 countries in sub-Saharan Africa, comprising over 230 thousand participants in studies conducted between 1981 and 2020. Considering all health-related outcomes at different time periods that the underlying studies have assessed, our main findings showed that home management of malaria significantly reduced malaria mortality, all-cause mortality, and risks of severe anaemia among patients presenting with symptoms of uncomplicated malaria. The strategy was also associated with better access to treatments for febrile illnesses, effective antimalarial treatments, and good adherence to medications. In terms of home-based preventive treatment, we only detected a significantly lower incidence risk of malaria, but a modest or null effect on severe malaria. The small number of studies on preventive malaria treatment precludes us from quantifying its impact on other clinical outcomes.
Our review found that the number of studies performed in rural populations far exceeds the number of studies in urban populations. Therefore, the collated evidence might not be directly and appropriately translatable to urban communities, including slum households in light of different intensity of malaria transmission [81], geographic proximity and access to health facilities [82], barriers of affordability and symptom recognition, availability of subsidised artemisinin combinations, and communication strategies for urban context [7]. Notably, over 70% of malaria cases in rural areas and 50% of malaria cases in urban areas of Africa are self-treated and formal medical care from health providers is only sought when initial treatment fails [12]. Such health care seeking behaviour underlines the importance of extending the siloed approach of home management intervention beyond rural settings so as to address the plight of populations at risk of malaria living in all types of environment. The scarcity of research in urban and peri-urban settings included in this review mirrors a lack of robust, reliable data to prove the health benefits of home malaria management strategy in urban neighbourhoods [44, 50, 51, 62, 63, 65, 76]. Notwithstanding, the findings generally support the feasibility of providing home management of malaria intervention at the level of households in urban environments.
Several studies (21.3%) utilised rapid diagnostic techniques for the detection of malaria parasites. Timely and accurate techniques for diagnosis are central to effective disease management [83]. Our study showed that rapid diagnostic tests used in the home management intervention had high sensitivity (82.9%), but moderate specificity (69.2%). False positives can arise, thus contributing to inappropriate prescribing or overtreatment with antimalarial agents, with possible undertreatment of alternative causes of febrile illness [84]. Conventional rapid diagnostic tests are unable to detect low density infections under 200 parasites/µL, particularly in non-falciparum infections and Plasmodium falciparum parasites with histidine-rich protein 2 (pfhrp2) and pfhrp3 gene deletions that have been reported to exist in Africa, Asia, and South America [85, 86]. As rapid, point-of-care diagnosis technologies has become a fundamental tool to support home-based malaria management, there is a clear need for reliable, easy-to-use, inexpensive tests that can be conducted at home settings, for instance, CRISPR (clustered regularly interspaced short palindromic repeat)-based malaria diagnostic assay [85] and haemozoin-based malaria diagnostic device [87] to provide robust clinical sensitivity and specificity that help inform decision-making in real time.
Home malaria management tackles barriers to accessing health care services and optimal health for deprived places and socioeconomically disadvantaged, underserved communities. We anticipate that the clinical burden attributable to malaria infections would be alleviated if the intervention is implemented with adequate intensity and over a sufficiently long period or in routine home visits and continuous monitoring of patients infected with malaria via phone calls or physical visits by care providers. The intensity of home management intervention will depend on the number of households in the target population being reached, the frequency of household visitations, diagnostic algorithms being performed, education on malaria preventative measures for patients and their relatives, as well as cascades for seeking medical care at primary health facilities and emergency services. In addition, only five studies in the review evaluated costs of delivering home management intervention [37, 45, 51, 58, 76]. Therefore, we do not have much information on the costs or opportunity costs of home malaria management as compared to other community-based interventions that could be undertaken to mitigate the burden of morbidity and mortality of the infection. The cost-effectiveness of home malaria management is likely to be influenced by factors, such as transmission intensity, environmental changes, and alternative access to quality health care [76, 88]. Exploration and understanding of implementation processes and context along with economic evaluation will provide imperative information to understand what worked, what did not work, and why, as well as inform potential for scale-up and public health policy for malaria control and elimination.
It is noteworthy that the current pooled analysis for the outcomes of malaria mortality and all-cause mortality employed data of studies in rural areas that used chloroquine, which may be now essentially obsolete. Our study also found that home malaria management that involved the use of medicinal herb (Argemone mexicana decoction) increased the need for re-treatment with artemisinin-based combination therapies. While Argemone mexicana decoction is traditionally used for malaria treatment in several African countries, its clinical effects are less understood [89]. Home malaria management had no effect on child mortality rates, likely to be due to waning of maternally transferred antibodies and lack of innate immune responses to malaria infections [90, 91]. None of the studies in our review ascertained the use of triple artemisinin-based combination therapies, such as dihydroartemisinin–piperaquine–mefloquine or artemether–lumefantrine–amodiaquine for home treatment of malaria. With the increasing treatment failures of conventional artemisinin-based combination therapy, recent studies have revealed that triple artemisinin-based combination therapies are efficacious, well tolerated, and safe for the treatment of uncomplicated falciparum malaria in regions with multidrug-resistant parasites, such as Southeast Asia and South Asia, while prolonging the useful therapeutic lifetime of existing antimalarial drugs that contain lumefantrine [92, 93]. Given that artemisinin-based combination therapies are more complex regimens that would likely compromise treatment adherence and fixed-dose combination has yet to be developed, home management of malaria is a key way to improve patients' adherence to medication regimens.
Our results provide insights on the clinical effects of home malaria management in settings with scarce health care resources. The key strengths of this review include comprehensive database searches, assessment of risk of bias, robust analysis, and the systematic examination of numerous clinical outcomes with the use of artemisinin-based or quinine-based treatments. As a whole, the included studies were judged to have at least moderate overall risk of bias using well-established tools, such as RoB 2.0 and ROBINS-I. The observational studies appeared to provide sound evidence for non-randomised study designs. The main limitations of our research largely pertain to the constraints inherent in the individual studies and data summarised in the meta-analysis. We included only manuscripts published in English. Although we reviewed manuscripts drawn from six online scientific databases, we did not systematically search unpublished data or grey literature. To avoid compositional bias stemming from differences in study-level sociodemographic characteristics in our analysis regardless of geographical region, age, and year. However, due to a paucity of studies published in peer-reviewed journals, we could not identify any relevant research in malaria-endemic countries outside of sub-Saharan Africa, thus precluding us from estimating the health outcomes differentially by race, ethnicity, and geographical location. Moreover, we detected considerable heterogeneity of effect estimates between studies, including febrile episodes treated with antimalarial drugs, effective treatment, parasitaemia, and other clinical outcomes. This could be due several possible explanations, such as study designs, intensity of home management intervention assessed, follow-up durations, lack of standardised data collection tools, and variation between communities and countries in population values and preferences concerning acceptability of home-based approach to managing malaria. Furthermore, a number of randomised controlled trials were performed from the 1980s until mid-2000s. Owing to unavailability of reporting guidelines at the time, these studies may lack details of trial conduct and reporting. Of note, the implementation of home management of malaria intervention may also vary over space and time with different locations scaling up and scaling down at different times. The simplistic approaches that are employed to analyse the data on the large geographic scales might underestimate the true impact or might inaccurately attribute impact to the intervention where there was none. As such, more advanced statistical and modelling methods to measure the overall impact across numerous geographic sites at different times. Spatiotemporal dynamics of clinical outcomes of malaria would be a meaningful approach to identify the impact of home malaria management with artemisinin combination therapy that serves as the front-line treatment against malaria when large surveillance datasets or clinical trials are available. Additional randomised controlled trials evaluating the effects of home malaria intervention, in particular with the use of better logistical support, diagnostic tests, and combination therapies spanning a range of geographical regions are warranted to consolidate the evidence basis for shaping policy solutions to sustainably combat the continuing challenges of malaria elimination [94, 95]. As for urban settings, digital health interventions may yield some encouraging results [96]. Albeit the current evidence suggests that the role of home-based preventive antimalarial treatment is ineffective, it will be of interest to assess its combined effects with vector control and elimination strategies, including use of mobile phone technology for patient and caregiver education [97], new class of insecticide-treated bed nets [98], interventions to address human and local vector population behaviours, or novel, low-carbon house designs [99] plus an annual booster of malaria vaccination [100].
Conclusions
This updated systematic review and meta-analysis generated evidence to suggest that home malaria management with artemisinin combination therapy led to higher rates of febrile episodes treated with antimalarial drugs and higher effective rate of antimalarial treatments. Our findings also indicated that home malaria management resulted in a good adherence to antimalarial regimen among the patients. Home management with chloroquine was associated with significantly lower incidence of malaria mortality and all-cause mortality, whilst these outcomes were not available with the newer artemisinin combination therapy. A significantly lower incidence risk of malaria was noted by combining home-based management with intermittent preventive malaria treatment. The evidence also reflects that home malaria management intervention is a predominant point of health care access for rural communities. Home malaria management intervention demonstrated a favourable cost-effectiveness profile that supported feasibility for scaling-up in typically high-transmission settings. Reliable diagnostic technologies should be incorporated into home malaria management to allow accurate diagnosis and treatment, thereby improving clinical care. Future randomised controlled trials are needed to build a stronger evidence base and capacity in home-based management of malaria that values diversity in its component designs, participant characteristics, countries, and regions. Effective home treatment algorithms could be implemented in tandem with the use of new antimalarial drugs being developed so as to reduce the massive disease burden and save lives in malaria-endemic regions which are often faced by a grave paucity of resources.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional information files.
References
Greenwood B. Treatment of malaria—a continuing challenge. N Engl J Med. 2014;371(5):474–5.
Balakrishnan VS. A new strategy is required for malaria elimination in Africa. Lancet Infect Dis. 2022;22(2):170–1.
Malaria in 2022: a year of opportunity. Lancet. 2022;399(10335):1573.
Nguyen TD, Olliaro P, Dondorp AM, Baird JK, Lam HM, Farrar J, et al. Optimum population-level use of artemisinin combination therapies: a modelling study. Lancet Glob Health. 2015;3(12):e758–66.
White NJ, Day NPJ, Ashley EA, Smithuis FM, Nosten FH. Have we really failed to roll back malaria? Lancet. 2022;399(10327):799–800.
Hetzel MW, Awor P, Tshefu A, Omoluabi E, Burri C, Signorell A, et al. Pre-referral rectal artesunate: no cure for unhealthy systems. Lancet Infect Dis. 2023;23(6):e213-e217.
Källander K, Nsungwa-Sabiiti J. Home-based management of malaria in the era of urbanisation. Lancet. 2009;373(9675):1582–4.
Nwaneri DU, Sadoh AE, Ibadin MO. Impact of home-based management on malaria outcome in under-fives presenting in a tertiary health institution in Nigeria. Malar J. 2017;16(1):187.
The L. Rolling back malaria—the next 10 years. Lancet. 2008;372(9645):1193.
Roll Back Malaria Department of the World Health Organization. The roll back malaria strategy for improving access to treatment through home management of malaria Geneva: World Health Organization; 2005. https://apps.who.int/iris/handle/10665/69057.
Hopkins H, Talisuna A, Whitty CJ, Staedke SG. Impact of home-based management of malaria on health outcomes in Africa: a systematic review of the evidence. Malar J. 2007;6:134.
Dongmo Kenfack E, Tendongfor N, Nsagha DS. Home-based intervention for the prevention and treatment of malaria among children younger than 5 years in the West Region of Cameroon: protocol for a randomized controlled trial. JMIR Res Protoc. 2021;10(3): e19633.
Pagnoni F. Home management of malaria. Lancet. 2009;374(9686):288–9.
Global technical strategy for malaria 2016–2030, 2021 update. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
Tiono AB, Kaboré Y, Traoré A, Convelbo N, Pagnoni F, Sirima SB. Implementation of Home based management of malaria in children reduces the work load for peripheral health facilities in a rural district of Burkina Faso. Malar J. 2008;7:201.
Otambo WO, Ochwedo KO, Omondi CJ, Lee MC, Wang C, Atieli H, et al. Community case management of malaria in Western Kenya: performance of community health volunteers in active malaria case surveillance. Malar J. 2023;22(1):83.
Gaye S, Kibler J, Ndiaye JL, Diouf MB, Linn A, Gueye AB, et al. Proactive community case management in Senegal 2014–2016: a case study in maximizing the impact of community case management of malaria. Malar J. 2020;19(1):166.
Bagenda F, Wesuta AC, Stone G, Ntaro M, Patel P, Kenney J, et al. Contribution of community health workers to the treatment of common illnesses among under 5-year-olds in rural Uganda. Malar J. 2022;21(1):296.
Peretz PJ, Islam N, Matiz LA. Community Health Workers and COVID-19—addressing social determinants of health in times of crisis and beyond. N Engl J Med. 2020;383(19): e108.
Karunajeewa HA, Mueller I, Senn M, Lin E, Law I, Gomorrai PS, et al. A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med. 2008;359(24):2545–57.
White NJ. Emergence of artemisinin-resistant Plasmodium falciparum in East Africa. N Engl J Med. 2021;385(13):1231–2.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
Minozzi S, Dwan K, Borrelli F, Filippini G. Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB2) improved with the use of implementation instruction. J Clin Epidemiol. 2022;141:99–105.
Eldridge S, Campbell MK, Campbell MJ, Drahota AK, Giraudeau B, Reeves BC, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2) Additional considerations for cluster-randomized trials (RoB 2 CRT). 2021. Cochrane. [Available from: https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials]
Adeoti O, Spiegelman D, Afonne C, Falade CO, Jegede AS, Oshiname FO, et al. The fidelity of implementation of recommended care for children with malaria by community health workers in Nigeria. Implement Sci. 2020;15(1):13.
Ahorlu CK, Koram KA, Seakey AK, Weiss MG. Effectiveness of combined intermittent preventive treatment for children and timely home treatment for malaria control. Malar J. 2009;8:292.
Ahorlu CK, Koram KA, Seake-Kwawu A, Weiss MG. Two-year evaluation of Intermittent Preventive Treatment for Children (IPTc) combined with timely home treatment for malaria control in Ghana. Malar J. 2011;10:127.
Ajayi IO, Browne EN, Bateganya F, Yar D, Happi C, Falade CO, et al. Effectiveness of artemisinin-based combination therapy used in the context of home management of malaria: a report from three study sites in sub-Saharan Africa. Malar J. 2008;7:190.
Ajayi IO, Browne EN, Garshong B, Bateganya F, Yusuf B, Agyei-Baffour P, et al. Feasibility and acceptability of artemisinin-based combination therapy for the home management of malaria in four African sites. Malar J. 2008;7:6.
Chinbuah AM, Gyapong JO, Pagnoni F, Wellington EK, Gyapong M. Feasibility and acceptability of the use of artemether-lumefantrine in the home management of uncomplicated malaria in children 6–59 months old in Ghana. Trop Med Int Health. 2006;11(7):1003–16.
Elmardi KA, Malik EM, Abdelgadir T, Ali SH, Elsyed AH, Mudather MA, et al. Feasibility and acceptability of home-based management of malaria strategy adapted to Sudan’s conditions using artemisinin-based combination therapy and rapid diagnostic test. Malar J. 2009;8:39.
Francis F, Ishengoma DS, Mmbando BP, Rutta ASM, Malecela MN, Mayala B, et al. Deployment and use of mobile phone technology for real-time reporting of fever cases and malaria treatment failure in areas of declining malaria transmission in Muheza district north-eastern Tanzania. Malar J. 2017;16(1):308.
Greenwood BM, Greenwood AM, Bradley AK, Snow RW, Byass P, Hayes RJ, et al. Comparison of two strategies for control of malaria within a primary health care programme in the Gambia. Lancet. 1988;1(8595):1121–7.
Menon A, Snow RW, Byass P, Greenwood BM, Hayes RJ, N’Jie AB. Sustained protection against mortality and morbidity from malaria in rural Gambian children by chemoprophylaxis given by village health workers. Trans R Soc Trop Med Hyg. 1990;84(6):768–72.
Nsungwa-Sabiiti J, Peterson S, Pariyo G, Ogwal-Okeng J, Petzold MG, Tomson G. Home-based management of fever and malaria treatment practices in Uganda. Trans R Soc Trop Med Hyg. 2007;101(12):1199-207.
Pagnoni F, Convelbo N, Tiendrebeogo J, Cousens S, Esposito F. A community-based programme to provide prompt and adequate treatment of presumptive malaria in children. Trans R Soc Trop Med Hyg. 1997;91(5):512–7.
Spencer HC, Kaseje DC, Collins WE, Shehata MG, Turner A, Stanfill PS, et al. Community-based malaria control in Saradidi, Kenya: description of the programme and impact on parasitaemia rates and antimalarial antibodies. Ann Trop Med Parasitol. 1987;81 Suppl 1:13–23.
Spencer HC, Kaseje DC, Mosley WH, Sempebwa EK, Huong AY, Roberts JM. Impact on mortality and fertility of a community-based malaria control programme in Saradidi, Kenya. Ann Trop Med Parasitol. 1987;81 Suppl 1:36–45.
Spencer HC. Community-based health development: the Saradidi, Kenya, experience continued. Ann Trop Med Parasitol. 1987;81 Suppl 1:1–147.
Ahorlu CS, Ndong IC, Okyere D, Mensah BA, Chu CE, Enos JY, et al. The Effect of Mass Testing, Treatment and Tracking on the Prevalence of Febrile Illness in Children under 15 in Ghana. Pathogens. 2022;11(10).
Ajayi IO, Falade CO, Bamgboye EA, Oduola AM, Kale OO. Assessment of a treatment guideline to improve home management of malaria in children in rural south-west Nigeria. Malar J. 2008;7:24.
Ajayi IO, Falade CO, Kale OO. An assessment of accuracy of mothers' presumptive diagnosis of fever at home in southwest Nigeria: evidence for switch to parasite-based diagnostic test. East Afr J Public Health. 2009;6(3):229-34.
Assi SB, Aba YT, Yavo JC, Nguessan AF, Tchiekoi NB, San KM, et al. Safety of a fixed-dose combination of artesunate and amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in real-life conditions of use in Côte d’Ivoire. Malar J. 2017;16(1):8.
Chanda P, Hamainza B, Moonga HB, Chalwe V, Banda P, Pagnoni F. Relative costs and effectiveness of treating uncomplicated malaria in two rural districts in Zambia: implications for nationwide scale-up of home-based management. Malar J. 2011;10:159.
Chanda P, Hamainza B, Moonga HB, Chalwe V, Pagnoni F. Community case management of malaria using ACT and RDT in two districts in Zambia: achieving high adherence to test results using community health workers. Malar J. 2011;10:158.
Delacollette C, Van der Stuyft P, Molima K. Using community health workers for malaria control: experience in Zaire. Bull World Health Organ. 1996;74(4):423–30.
Hetzel MW, Okitawutshu J, Tshefu A, Omoluabi E, Awor P, Signorell A, et al. Effectiveness of rectal artesunate as pre-referral treatment for severe malaria in children under 5 years of age: a multi-country observational study. BMC Med. 2022;20(1):343.
Lengeler C, Burri C, Awor P, Athieno P, Kimera J, Tumukunde G, et al. Community access to rectal artesunate for malaria (CARAMAL): A large-scale observational implementation study in the Democratic Republic of the Congo, Nigeria and Uganda. PLOS Global Public Health. 2022;2(9):e0000464.
Kalyango JN, Rutebemberwa E, Karamagi C, Mworozi E, Ssali S, Alfven T, et al. High adherence to antimalarials and antibiotics under integrated community case management of illness in children less than five years in eastern Uganda. PLoS One. 2013;8(3):e60481.
Kukula VA, Dodoo AA, Akpakli J, Narh-Bana SA, Clerk C, Adjei A, et al. Feasibility and cost of using mobile phones for capturing drug safety information in peri-urban settlement in Ghana: a prospective cohort study of patients with uncomplicated malaria. Malar J. 2015;14:411.
Mulebeke R, Wanzira H, Bukenya F, Eganyu T, Collborn K, Elliot R, et al. Implementing population-based mass drug administration for malaria: experience from a high transmission setting in North Eastern Uganda. Malar J. 2019;18(1):271.
Ngasala BE, Malmberg M, Carlsson AM, Ferreira PE, Petzold MG, Blessborn D, et al. Effectiveness of artemether-lumefantrine provided by community health workers in under-five children with uncomplicated malaria in rural Tanzania: an open label prospective study. Malar J. 2011;10:64.
Orimadegun AE, Amodu OK, Olumese PE, Omotade OO. Early home treatment of childhood fevers with ineffective antimalarials is deleterious in the outcome of severe malaria. Malar J. 2008;7(1):143.
Ratsimbasoa A, Ravony H, Vonimpaisomihanta JA, Raherinjafy R, Jahevitra M, Rapelanoro R, et al. Compliance, safety, and effectiveness of fixed-dose artesunate-amodiaquine for presumptive treatment of non-severe malaria in the context of home management of malaria in Madagascar. Am J Trop Med Hyg. 2012;86(2):203–10.
Ruizendaal E, Schallig H, Scott S, Traore-Coulibaly M, Bradley J, Lompo P, et al. Evaluation of Malaria Screening during Pregnancy with Rapid Diagnostic Tests Performed by Community Health Workers in Burkina Faso. Am J Trop Med Hyg. 2017;97(4):1190–7.
Sirima SB, Konaté A, Tiono AB, Convelbo N, Cousens S, Pagnoni F. Early treatment of childhood fevers with pre-packaged antimalarial drugs in the home reduces severe malaria morbidity in Burkina Faso. Trop Med Int Health. 2003;8(2):133–9.
Thiam S, Thwing J, Diallo I, Fall FB, Diouf MB, Perry R, et al. Scale-up of home-based management of malaria based on rapid diagnostic tests and artemisinin-based combination therapy in a resource-poor country: results in Senegal. Malar J. 2012;11:334.
Ye Y, Hoshen M, Kyobutungi C, Sauerborn R. Can weekly home visits and treatment by non-medical personnel reduce malaria-related mortality among children under age 5 years? J Trop Pediatr. 2007;53(4):292–3.
Achan J, Tibenderana JK, Kyabayinze D, Wabwire Mangen F, Kamya MR, Dorsey G, et al. Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial. BMJ. 2009;339: b2763.
Graz B, Willcox ML, Diakite C, Falquet J, Dackuo F, Sidibe O, et al. Argemone mexicana decoction versus artesunate-amodiaquine for the management of malaria in Mali: policy and public-health implications. Trans R Soc Trop Med Hyg. 2010;104(1):33–41.
Nahum A, Erhart A, Ahounou D, Bonou D, Van Overmeir C, Menten J, et al. Extended high efficacy of the combination sulphadoxine-pyrimethamine with artesunate in children with uncomplicated falciparum malaria on the Benin coast, West Africa. Malar J. 2009;8:37.
Niba PTN, Nji AM, Ali IM, Akam LF, Dongmo CH, Chedjou JPK, et al. Effectiveness and safety of artesunate-amodiaquine versus artemether-lumefantrine for home-based treatment of uncomplicated Plasmodium falciparum malaria among children 6–120 months in Yaoundé, Cameroon: a randomized trial. BMC Infect Dis. 2022;22(1):166.
Ouédraogo A, Tiono AB, Diarra A, Nébié IO, Konaté AT, Sirima SB. The effects of a pre-season treatment with effective antimalarials on subsequent malaria morbidity in under five-year-old children living in high and seasonal malaria transmission area of Burkina Faso. Trop Med Int Health. 2010;15(11):1315–21.
Owusu-Agyei S, Asante KP, Owusu R, Adjuik M, Amenga-Etego S, Dosoo DK, et al. An open label, randomised trial of artesunate+amodiaquine, artesunate+chlorproguanil-dapsone and artemether-lumefantrine for the treatment of uncomplicated malaria. PLoS ONE. 2008;3(6):e2530.
Sesay S, Milligan P, Touray E, Sowe M, Webb EL, Greenwood BM, et al. A trial of intermittent preventive treatment and home-based management of malaria in a rural area of The Gambia. Malar J. 2011;10:2.
Tinto H, Diallo S, Zongo I, Guiraud I, Valea I, Kazienga A, et al. Effectiveness of artesunate-amodiaquine vs. artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in Nanoro, Burkina Faso: a non-inferiority randomised trial. Trop Med Int Health. 2014;19(4):469-475.
Willcox ML, Graz B, Diakite C, Falquet J, Dackouo F, Sidibe O, et al. Is parasite clearance clinically important after malaria treatment in a high transmission area? A 3-month follow-up of home-based management with herbal medicine or ACT. Trans R Soc Trop Med Hyg. 2011;105(1):23–31.
Chinbuah MA, Kager PA, Abbey M, Gyapong M, Awini E, Nonvignon J, et al. Impact of community management of fever (using antimalarials with or without antibiotics) on childhood mortality: a cluster-randomized controlled trial in Ghana. Am J Trop Med Hyg. 2012;87(5 Suppl):11–20.
Cisse B, Cairns M, Faye E, O ND, Faye B, Cames C, et al. Randomized trial of piperaquine with sulfadoxine-pyrimethamine or dihydroartemisinin for malaria intermittent preventive treatment in children. PLoS One. 2009;4(9):e7164.
Eriksen J, Mujinja P, Warsame M, Nsimba S, Kouyaté B, Gustafsson LL, et al. Effectiveness of a community intervention on malaria in rural Tanzania - a randomised controlled trial. Afr Health Sci. 2010;10(4):332–40.
Kangwana BP, Kedenge SV, Noor AM, Alegana VA, Nyandigisi AJ, Pandit J, et al. The impact of retail-sector delivery of artemether-lumefantrine on malaria treatment of children under five in Kenya: a cluster randomized controlled trial. PLoS Med. 2011;8(5):e1000437.
Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomised trial. Lancet. 2000;356(9229):550–5.
Kouyaté B, Somé F, Jahn A, Coulibaly B, Eriksen J, Sauerborn R, et al. Process and effects of a community intervention on malaria in rural Burkina Faso: randomized controlled trial. Malar J. 2008;7:50.
Mubi M, Janson A, Warsame M, Mårtensson A, Källander K, Petzold MG, et al. Malaria rapid testing by community health workers is effective and safe for targeting malaria treatment: randomised cross-over trial in Tanzania. PLoS ONE. 2011;6(7): e19753.
Staedke SG, Mwebaza N, Kamya MR, Clark TD, Dorsey G, Rosenthal PJ, et al. Home management of malaria with artemether-lumefantrine compared with standard care in urban Ugandan children: a randomised controlled trial. Lancet. 2009;373(9675):1623–31.
Tagbor H, Cairns M, Nakwa E, Browne E, Sarkodie B, Counihan H, et al. The clinical impact of combining intermittent preventive treatment with home management of malaria in children aged below 5 years: cluster randomised trial. Trop Med Int Health. 2011;16(3):280–9.
Tine RC, Faye B, Ndour CT, Ndiaye JL, Ndiaye M, Bassene C, et al. Impact of combining intermittent preventive treatment with home management of malaria in children less than 10 years in a rural area of Senegal: a cluster randomized trial. Malar J. 2011;10:358.
Tine RC, Ndour CT, Faye B, Cairns M, Sylla K, Ndiaye M, et al. Feasibility, safety and effectiveness of combining home based malaria management and seasonal malaria chemoprevention in children less than 10 years in Senegal: a cluster-randomised trial. Trans R Soc Trop Med Hyg. 2014;108(1):13–21.
Yeboah-Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Med. 2010;7(9):e1000340.
Wilson ML, Krogstad DJ, Arinaitwe E, Arevalo-Herrera M, Chery L, Ferreira MU, et al. Urban malaria: understanding its epidemiology, ecology, and transmission across seven diverse ICEMR Network Sites. Am J Trop Med Hyg. 2015;93(3 Suppl):110–23.
McBride K, Moucheraud C. Rural-urban differences: using finer geographic classifications to reevaluate distance and choice of health services in Malawi. Health Syst Reform. 2022;8(1): e2051229.
Ochola LB, Vounatsou P, Smith T, Mabaso MLH, Newton C. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis. 2006;6(9):582–8.
Murungi M, Fulton T, Reyes R, Matte M, Ntaro M, Mulogo E, et al. Improving the specificity of Plasmodium falciparum malaria diagnosis in high-transmission settings with a two-step rapid diagnostic test and microscopy algorithm. J Clin Microbiol. 2017;55(5):1540–9.
Cunningham CH, Hennelly CM, Lin JT, Ubalee R, Boyce RM, Mulogo EM, et al. A novel CRISPR-based malaria diagnostic capable of Plasmodium detection, species differentiation, and drug-resistance genotyping. eBioMedicine. 2021;68.
Thomson R, Beshir KB, Cunningham J, Baiden F, Bharmal J, Bruxvoort KJ, et al. pfhrp2 and pfhrp3 gene deletions that affect malaria rapid diagnostic tests for Plasmodium falciparum: analysis of archived blood samples from 3 African countries. J Infect Dis. 2019;220(9):1444–52.
Kumar R, Verma AK, Shrivas S, Thota P, Singh MP, Rajasubramaniam S, et al. First successful field evaluation of new, one-minute haemozoin-based malaria diagnostic device. eClinicalMedicine. 2020;22.
Lubell Y, Mills AJ, Whitty CJ, Staedke SG. An economic evaluation of home management of malaria in Uganda: an interactive Markov model. PLoS ONE. 2010;5(8): e12439.
Tekassa T, Hasen G, Merga H, Cavin AL, Graz B, Suleman S. Knowledge of malaria and its uncomplicated treatment with Argemone mexicana L. in selected districts of Jimma Zone, Oromia Regional State, Ethiopia: a community-based cross sectional survey. Infect Drug Resist. 2022;15:3087–95.
Barua P, Beeson JG, Maleta K, Ashorn P, Rogerson SJ. The impact of early life exposure to Plasmodium falciparum on the development of naturally acquired immunity to malaria in young Malawian children. Malar J. 2019;18(1):11.
Menendez C, Saute F, Greenwood B, Schellenberg D, Snow R, Alonso PL. Avoiding another lost decade in reducing malaria burden in African infants and young children. Lancet Glob Health. 2022;10(10):e1385–6.
van der Pluijm RW, Tripura R, Hoglund RM, Pyae Phyo A, Lek D, ul Islam A, et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet. 2020;395(10233):1345–60.
Peto TJ, Tripura R, Callery JJ, Lek D, Nghia HDT, Nguon C, et al. Triple therapy with artemether-lumefantrine plus amodiaquine versus artemether-lumefantrine alone for artemisinin-resistant, uncomplicated falciparum malaria: an open-label, randomised, multicentre trial. Lancet Infect Dis. 2022;22(6):867–78.
Wambani J, Okoth P. Impact of malaria diagnostic technologies on the disease burden in the sub-Saharan Africa. J Trop Med. 2022;2022:7324281.
Adhikari B, Tripura R, Peto TJ, Callery JJ, von Seidlein L, Dysoley L, et al. Village malaria workers for the community-based management of vivax malaria. Lancet Reg Health Southeast Asia. 2023;9: 100128.
Fan BE, Chen DTY, Ponnudurai K, Winkler S. Artificial intelligence for malaria: Bridging the gap for real world usage. Lancet Reg Health Southeast Asia. 2022;5:100073.
Poespoprodjo JR, Douglas NM, Ansong D, Kho S, Anstey NM. Malaria. Lancet. 2023;402(10419):2328–45.
Messenger LA, Matowo NS, Cross CL, Jumanne M, Portwood NM, Martin J, et al. Effects of next-generation, dual-active-ingredient, long-lasting insecticidal net deployment on insecticide resistance in malaria vectors in Tanzania: an analysis of a 3-year, cluster-randomised controlled trial. Lancet Planet Health. 2023;7(8):e673-e83.
Mshamu S, Halifa S, Meta J, Mmbando A, Lindsay SW, Okumu F, et al. Studying the health benefits of improved housing in rural Tanzania: challenges and progress. Trans R Soc Trop Med Hyg. 2023;117(10):678–81.
Datoo MS, Natama HM, Somé A, Bellamy D, Traoré O, Rouamba T, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22(12):1728–36.
Acknowledgements
The authors would like to extend their heartfelt and utmost gratitude to Chulalongkorn University, Thailand for sponsoring the article processing charge of this publication.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
KP carried out conception and design, literature search, data analysis, and data interpretation and was a major contributor in writing the original draft of the manuscript. SL was responsible for data curation, formal analysis, supervision, critical revision for important intellectual content, and manuscript editing. BC performed project administration, validation, review, and editing of the manuscript. All authors read and approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Ethics approval and consent to participate were not required because this study retrieved and synthesised data from already published studies. No human participants, human data, human tissue, animals, or client-owned animals were involved.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Pooled effect estimates of home management of malaria with artesunate–amodiaquine compared to home management of malaria with other antimalarial drug combinations on risk of experiencing adverse drug events. Figure S2. Pooled effect estimates of home-based intermittent preventive treatment compared with home-based management of malaria on risk of developing severe malaria. Figure S3. Pooled effect estimates of home management of malaria with Argemone mexicana decoction compared to home management of malaria with artesunate–amodiaquine on clinical outcomes. Figure S4. Assessment of risk of bias in observational studies based on ROBINS-I tool. Figure S5. Assessment of risk of bias in individually randomised controlled trials based on RoB 2.0 tool. Figure S6. Assessment of risk of bias in cluster-randomised controlled trials based on RoB 2.0 CRT tool. Table S1. Summary of included studies. Table S2. Sensitivity and specificity of malaria diagnosis. Table S3. Antimalarial treatment following implementation of home-based interventions in communities. Table S4. Cost-effectiveness findings from relevant individual studies. Supplementary Table S5. Safety findings from individual studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kua, K.P., Lee, S.W.H. & Chongmelaxme, B. The impact of home-based management of malaria on clinical outcomes in sub-Saharan African populations: a systematic review and meta-analysis. Trop Med Health 52, 7 (2024). https://doi.org/10.1186/s41182-023-00572-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-023-00572-2