Abstract
Background
The purpose of the study was to extract carotenoids from thermophilic bacteria which show efficient antioxidant and protein oxidation inhibition properties, characterize and identify those isolates, extract the carotenoids in different solvents, quantify the carotenoids and perform concentration-dependent and solvent-dependent quantitative assays validated and analysed by appropriate statistical tests.
Methods
Three pigment-forming thermophilic strains were isolated from water sample of Paniphala hot spring, India, and tentatively identified by 16S ribosomal DNA (rDNA) homology. Different concentrations of the carotenoid extracts (100, 80, 40 and 20 μg) in three solvents, methanol, DMSO and water, were used to determine the antioxidant activity through five methods: the DPPH (1,1-diphenyl-2-picrylhydrazyl) assay, the ABTS (2,2-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid)) assay, the hydrogen peroxide assay, TOC (total antioxidant capacity) assay and inhibition of protein oxidation assay. Statistical analysis of mean, standard deviation, ANOVA and Pearson correlation coefficient was performed in Microsoft Excel statistical package.
Results
The isolates were tentatively identified as Meiothermus sp. strain RP, Meiothermus sp. strain TP and Thermus strain YY. Meiothermus sp. formed red coloured pigment, whereas Thermus sp. formed yellow coloured pigment. All of the extracts showed positive results in DPPH assay, ABTS assay and hydrogen peroxide radical scavenging assay with best results obtained when the extracts were dissolved in water. Total antioxidant capacity assay was also high in all the extracts. Protein oxidation inhibition activity was only seen in extracts of strain YY. One-way ANOVA (analysis of variance) clearly showed that choice of solvent influenced the antioxidant capacity of all of the extracts.
Conclusions
Newer and efficient antioxidative compounds are constantly being searched for, and the carotenoid extracts of RP, TP and YY have been shown to catalyze various types of antioxidative reactions, including protein oxidation inhibition by YY. Thus, all these extracts have huge potential to be industrially and pharmaceutically useful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Antioxidants, as the name suggests, have the capacity to prevent or delay the oxidation of other chemicals even when present at low concentrations with respect to the substrate to be oxidized. Depending on physical and chemicals properties, both oxidants and antioxidants can be of various forms. The reactive oxygen species include O2 − ,1O2, HO·, and HOCl, and the reactive nitrogen species include NO·and NO2 ·. Biological systems comprise variety of antioxidants which may have several modes of action and can be broadly classified into four groups: (1) enzymes like catalase and superoxide dismutase, (2) large molecules like proteins, e.g. albumin and ferritin, (3) small molecules like ascorbic acid and carotenoids, (4) some hormones like estrogen and melatonin. Carotenoids in particular are very proficient in quenching singlet oxygen. Two possible mechanisms come into play when antioxidants deactivate free radicals: hydrogen atom transfer (HAT) and single electron transfer (SET). In HAT, the antioxidant quenches free radicals by hydrogen donation (X· + AH→ XH + A· where X represents the substrate and A the antioxidant) while in SET, an electron is transferred to reduce any compound (X· + AH → X− + AH·+) where X represents the substrate and A the antioxidant) [1,2,3]. Carotenoids, a type of pigment, by virtue of their electron rich polyene chain being susceptible to attack by oxidizing free radicals and electrophilic reagents, act as excellent antioxidant and pro-oxidants. The mode of action is not constant, the variable factors including the nature of carotenoid (number of conjugated double bonds, substituents like oxygen), solvent polarity, nature of free radicals, presence of other molecules having antioxidant properties, carotenoid aggregation and orientation in the biological membrane. Normally, carotenoids deactivate free radicals by electron transfer (SET) and form carotenoid radical cations [4]. Many known carotenoids like astaxanthin, actinioerythrol, lycopene, zeaxanthin and β-carotene and many other carotenoid extracts, whose exact composition is yet to be determined, have been reported to have excellent antioxidant properties [5]. Many assays have been devised for determination of antioxidant properties like DPPH method, ABTS method, hydrogen peroxide scavenging assay, phosphomolybdenum method, ferricthiocyanate method and ferric-reducing antioxidant power (FRAP) assay. In the DPPH assay, delocalisation of the electrons of the molecule 1,1-diphenyl-2-picrylhydrazyl not only makes it a stable free radical but also provides it a deep violet colour absorbing maximally at 517 nm in methanol/ethanol solution. Any antioxidant capable of donating a hydrogen atom reduces DPPH accompanied by colour loss, which can then be used to determine the scavenging property of a molecule [6]. Similarly, hydrogen peroxide assay also makes use of its absorbance at 230 nm and then its decrease in absorbance on addition of any suitable antioxidant [7]. In ABTS assay, the blue green colour of ABTS (2,2-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid)) radicals is reduced as quenching of free radicals occur in a mechanism similar to the DPPH assay [8]. The total antioxidant capacity assay of the antioxidants can be performed with the help of the phosphomolybdenum method by phosphomolybdenum complex formation. At acidic pH, Mo (VI) is reduced to Mo (V) in the presence of any suitable antioxidant, forming green phosphomolybdenum complex [9, 10]. One of the most common methods of detection of inhibition of protein oxidation is that of detection of protein carbonyl content spectrophotometrically by the DNPH (2,4-dinitrophenylhydrazine) method as protein carbonyl content increases with oxidative stress and decreases in the presence of suitable antioxidant [11]. This study focuses on the isolation, characterization and identification of three pigmented bacterial strains of Deinococcus–Thermus phylum from Paniphala hot spring, India, and analysis of the antioxidant properties of the carotenoid extracts of the three bacteria.
Methods
Water sample collection and isolation of pigment-forming isolates
Paniphala hot spring located near Barabani in Burdwan, India, (23° 45’ 33” N, 86° 58’ 54” E) was used as the source of water sample which was collected and plated on R2A agar (Hi-MEDIA) plates for isolation of pigmented thermophilic isolates and incubated at 60 ± 1 °C for several days in the dark.
Colony and cell morphology, biochemical properties and determination of growth conditions
After obtaining pigmented isolates, their colony and cell morphology were recorded according to Smibert and Krieg [12]. Cell morphology was observed after gram staining using binocular phase contrast Microscope, Leica DM1000. For determining growth temperature range, the strains were incubated at 0, 10, 37, 45, 55, 60, 65, 70 and 80 °C. The growth pH ranges were obtained by inoculating in media adjusted to pH 2, pH 4, pH 6, pH 7, pH 10 and pH 13. Various concentrations of sodium chloride (0.1, 0.2, 0.4, 0.5, 1, 2 and 5%) were used to determine the salt tolerance of the isolates. For analysis of carbon utilization pattern, 50 μl of culture suspension was inoculated in each of the thirty five test simple carbohydrate wells of kit available from HIMEDIA (KB009 HiCarbohydrateTM Kit), India, and incubated at 60 °C to see the colour change. Biochemical tests for catalase activity, oxidase, nitrate reduction, casein hydrolysis and deoxyribonuclease (DNase) were performed as per standard protocols [12]. All the three strains were tested for their degradability of complex substrates including starch, tannic acid, chitin, cellulose, xylan, gelatin, pectin, oil and alginic acid by inoculating in media consisting of 0.15% yeast extract, 0.1% carbon source, 0.03% dipotassium phosphate and 0.0024% magnesium sulphate and observing the growth at 60 ± 1 °C.
Isolation of genomic DNA, sequencing of 16S rDNA amplicons and phylogenetic analysis of the isolates
MB505 HiPurATM Bacterial and Yeast Genomic DNA Miniprep Purification Spin kit (HIMEDIA) was used to extract genomic DNA of the isolates following instructions in the kit manual. Universal primers 27F (5′ AGAGTTTGATCMTGGCTCAG 3′) and 1492R (5′ TACGGYTACCTTGTTACGACTT 3′) were used to amplify the 16S rDNA genes in thermocycler (Eppendorf- AG, 22331 Hamburg) following standard protocol [13] which were then sequenced using Sanger technology. BLAST algorithm in NCBI (National Centre for biotechnology information) was used for determining the sequence similarity of the 16S rDNA sequences of the isolates with other 16S rDNA sequences in the reference database of GenBank [14]. MEGA 6 software was used for phylogenetic analysis and construction of phylogenetic tree of the three isolates using the neighbour-joining method [15].
Extraction of pigments and their solubility in various solvents
The isolates were inoculated in R2A broth and incubated for 24–48 h at 60 ± 1 °C. Then, the fresh cultures were used as inoculum for mass culture in 700 ml R2A broth and incubated for 14–15 days at 60 °C so that intense pigment formation occurs. Since the pigments are not diffusible in nature and not present in the supernatant, the cell masses for pigment extraction were obtained by repeated centrifugation at 8000 rpm for 5 min. Cells were washed thrice with normal saline. The washed cell pellets were used for testing extractability of their pigments in a number of solvents including ethanol, methanol, dimethylformamide (DMF), acetone, chloroform, hexane, cyclohexane, di-ethyl ether, 1,4 dioxane, propanol, toluene, xylene, butanol, water, dimethyl sulfoxide (DMSO), benzene and isobutanol (all of HPLC grade) by resuspending the cell pellet in the solvent, vortexing for 10 min and then centrifugation at 15,000×g for 30 min [16]. All of the centrifugation steps were carried out at a temperature of 25 ± 1 °C and under dim light conditions. Further, works with the pigments were also done under dim light conditions to maintain the stability of the pigments. The solvent extracts were observed under UV-visible spectrum in a UV-VIS spectrophotometer (Varian made) for detection of carotenoids. Next, the carotenoids extracted in DMSO were lyophilized to solid form and observed for their solubility in the above solvents.
Antioxidant assays
The carotenoid content from the methanolic extracts were quantified in micrograms, obtaining the extinction coefficients of the carotenoids from literature, whose absorption spectra and maxima showed similarity to the carotenoid extract of RP, TP and YY following the Beer Lambert’s law. [A = α Cm l, where A = absorbance, α = extinction coefficient, Cm = molar concentration and l = path length which is 1 cm in each case; all the parameters being considered at or near the absorption maxima in each case (475 nm for RP and TP assuming the pigment as deoxyflexixanthin and 449 nm for YY assuming the pigment as zeaxanthin-related carotenoid)] [13]. The extinction coefficients taken were 150 mM−1 cm−1 at 475 nm in methanol for RP and TP and 144,300 M−1 cm−1 at 449 nm in methanol for YY [17, 18]. For the antioxidant assays, the solid extracts were dissolved in water, methanol and DMSO in concentrations of 20, 40, 80, and 100 μg and assayed for antioxidant activity. All experiments were conducted in triplicates.
DPPH assay
For the DPPH assay, 2 ml of the sample solutions with concentrations of 20, 40, 80 and 100 μg was mixed with 0.5 mM DPPH methanolic solution (1 ml) and 2 ml of 0.1 M acetate buffer pH 5.5 and incubated for 6 h. Sets were kept as control where the carotenoid extracts were not added to the mixture. The absorbance of the mixture was observed at 517 nm. The percentage scavenging activity was calculated as [(Actrl−As)/Actrl] ×100 where Actrl denotes absorbance of the control solution where no extract is added and As denotes absorbance of the solution where the extract is added [6].
ABTS radical assay
7 mM of ABTS solution was reacted with 2.45 mM ammonium persulfate and kept in dark at room temperature for 16 h for production of ABTS radical cation. Five hundred microlitres of different concentrations (20, 40, 80 and 100 μg) of the extracts were added to 300 μl of ABTS radical solution while making the final volume up to 1 ml with distilled water. The absorbance of the mixtures was read at 745 nm. The percentage inhibition activity was calculated in a manner, similar to that of DPPH assay mentioned in the “DPPH assay”section. [19].
Hydrogen peroxide radical scavenging assay
One hundred microlitres of different concentrations of the extracts (20, 40, 80 and 100 μg) are added to 100 μl of 40 mM hydrogen peroxide solution in phosphate buffer (pH 7.4) and kept at room temperature for 30 min. The absorbances were taken at 230 nm against a blank solution of phosphate buffer. The percentage of hydrogen peroxide scavenging was calculated in a manner, similar to that of DPPH assay mentioned in the “DPPH assay”section. [7].
Total antioxidant capacity (TOC) by phosphomolybdenum method
One millilitre of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, 4 mM ammonium molybdate) was added with 100 μl of sample solution (20, 40, 80 and 100 μg) in closed cap tubes and incubated at 95 °C for 6 h. The absorbance was measured at 695 nm against blank control, and the total antioxidant capacity was determined against the antioxidant capacity of ascorbic acid. Ascorbic acid standard curve was prepared by taking concentrations of (20, 40, 80g and 100 μg) of ascorbic acid in the respective solvents (methanol, water and DMSO). The total antioxidant activity is determined as number of gram equivalents of ascorbic acid) [20].
Protein oxidative damage inhibition assay
The protein oxidation inhibition assay was done spectrophotometrically by the modified DNPH (2,4-dinitrophenyl hydrazine) method [11]. Fifty microlitres of each of bovine serum albumin (BSA), 50 μl of ferrous chloride/ citric acid (4/40 mM), 50 μl of hydrogen peroxide (4 mM) and 50 μl of sample solutions (with concentrations of 20, 40, 80 and 100 μg) were mixed and incubated in water bath at 37 °C for 60 min. Five hundred microlitres of 2,4 DNPH (10 mM in 2 N HCl) was added to the reaction mixtures and incubated for 60 min at room temperature with regular vortexing after every 15 min. After precipitation of proteins by 20% TCA (Trichloroacetic acid), the precipitate was washed with 1 ml of Ethanol: Ethylacetate (1:1) thrice and dissolved in 600 μl of 6 M guanidine (in phosphate buffer) whose pH adjustment was done by TFA (Trifluoroacetic acid) to pH 2.3. The solutions were incubated at 37 °C for 20 min and centrifuged once to remove impurities. The percentage inhibition in carbonyl formation by the carotenoid extracts were determined by measuring the absorbance at 370 nm and then calculated in a manner, similar to that of DPPH assay mentioned in the “DPPH assay” section.
Statistical analysis
All the experiments were conducted in triplicates, and mean and standard deviation of the data were obtained. One-way ANOVA was conducted to determine whether the choice of solvent had any significant effect on the antioxidant assays. The Pearson correlation coefficient was used to determine the relation between carotenoid concentration and activity. All the analyses were done by statistical software package of Microsoft Excel.
Results
Isolation of pigment forming isolates, determination of growth conditions, colony and cell morphology and biochemical properties
Three pigmented isolates were obtained, of which two formed red pigments and one formed yellow pigment. The red pigment forming isolates were named as RP and TP, and the yellow pigment forming isolate was named as YY. The isolates formed smooth, convex, circular colonies with entire margin. Gram staining of the isolates revealed gram negative non-motile rods of the strains. Length of RP and TP varies between 1 and 3 μm, and the diameter varies between 0.5 and 0.8 μm, while the length of YY varies between 0.5 and 2 μm and the width varies between 0.5 and 0.8 μm in diameter (Additional file 1: Figure S1). All the three strains are obligate thermophiles and do not show growth below 55 °C. Strains RP and TP show growth up to 70 °C showing optimal growth at 60 °C, while strain YY grow up to 80 °C, showing optimal growth at 65 °C (Additional file 1: Figure S2). Strains RP and TP cannot grow below pH 6; they show growth up to pH 10, i.e. alkaline range showing optimal growth at pH 7. Strain YY can grow within a wide range of pH 2–pH 13, showing optimal growth at pH 6 (Additional file 1: Figure S3). All three strains can tolerate up to 1% NaCl. Strain RP and TP show optimal growth at 0% NaCl. While 0.1% NaCl affects growth of RP, it does not affect growth of TP and growth declines at 0.2% NaCl concentration. Optimal growth of YY occurs at 0.4% NaCl (Additional file 1: Figure S4). Results of carbon utilization pattern and biochemical tests are noted in Additional file 1: Table S1 and Table S2, respectively.
Isolation of genomic DNA, sequencing of 16S rDNA amplicons and phylogenetic analysis of the isolates
The genomic DNA (Additional file 1: Figure S5) and the 16S rDNA amplicons were seen as sharp bands on agarose gel (Additional file 1: Figure S6). Sequencing revealed 1438, 1303 and 1312 nucleotide long contiguous of 16S rRNA genes of strains RP, TP and YY, respectively. While strain RP showed 98% sequence similarity with Meiothermus taiwanensis strain WR-30, strain TP showed 98% sequence similarity to Meiothermus cateniformans strain LY1 and strain YY showed 99% sequence similarity to Thermus scotoductus SA-01. The sequences were submitted to GenBank, NCBI. Meiothermus sp. RP, Meiothermus sp. TP and Thermus sp. YY were assigned accession numbers KP053251, KP053252 and KT239023, respectively. Evolutionary relationship tree was constructed for phylogenetic analysis between the strains and other related strains (Fig. 1).
In MEGA6, neighbour-joining method was used to determine the evolutionary relationships of Meiothermus sp. strain RP, Meiothermus strain TP and Thermus sp. YY with each other and other related taxa. Next to the branches, the percentages of replication in which the taxa clustered in the bootstrap test (1000 replicates) are there. P-distance method was used to compute the evolutionary distances whose units are number of base differences per site
Extraction of pigments and their solubility in various solvents
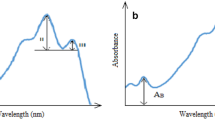
The carotenoids of the strains were extracted most efficiently in methanol, DMF and DMSO, and results of the solvent extractability of the carotenoids and solubility of the extracted carotenoids in various solvents are tabulated in Table 1. Visible spectrum of carotenoid extracts revealed typical three-finger structure of carotenoids (Fig. 2). The absorption maxima of the methanolic carotenoid extracts of strains RP, TP and YY in the visible range were observed at 478.1, 480 and 449.1 nm, respectively.
Antioxidant assays
From literature, the carotenoids were tentatively identified from their absorption spectra and pigments of related microorganisms. The carotenoid extracts of RP and TP were most probably carotenoid glycoside esters deoxyflexixanthin and/ or 1′-β-glucopyranosyl-3,4,3′,4′-tetradehydro-1′, 2′-dihydro-β, ψ-caroten-2-one, and the carotenoid extract of YY showed similarity to different forms of zeaxanthin such as zeaxanthin monoglucoside, thermozeaxanthin and thermobiszeaxanthin. Since the extinction coefficient of 1′-β-glucopyranosyl-3,4,3′, 4′-tetradehydro-1′, 2′-dihydro-β, ψ-caroten-2-one is not available, the concentration of the carotenoids were carried out using the extinction coefficients of deoxyflexixanthin in methanol for RP and TP and zeaxanthin in methanol for YY.
DPPH assay, ABTS radical assay and hydrogen peroxide radical scavenging assay
All of the extracts showed positive result in DPPH radical scavenging assay, ABTS radical scavenging and hydrogen peroxide radical scavenging assay, and the percentage inhibition property increased with increasing concentrations of extract (Fig. 3). Among all the samples, carotenoids dissolved in water showed greater scavenging activity than methanol or DMSO. Scavenging activity of 100 μg of water dissolved extracts of RP and TP showed similar DPPH scavenging activity of 80.38 ±0.64 and 80.38 ± 0.53%, respectively, while that of YY showed slightly lower activity (73.16 ± 0.85%). The ABTS radical scavenging activity was 74.3 ± 0.2% in case of RP, 74.2 ± 1.2% in case of TP and 95.5 ± 1% in case of YY. Water-dissolved YY extract showed a high amount of hydrogen peroxide scavenging activity (87.4 ± 1.15%). Details of the result are given in Additional file 1: Table S3, S4 and S5.
Determination of percent scavenging activity of different concentrations of carotenoid extracts of RP, TP and YY dissolved in methanol (M), water (W) and DMSO (D) by DPPH assay (DH), ABTS radical assay (AB) and H2O2 scavenging assay (HO). Coloured lines indicate different concentrations: blue—100 μg, red—80 μg, green—40 μg and violet—20 μg. The abbreviations are in the sequence of ‘method-organism-solvent’
Total antioxidant capacity by phosphomolybdenum method
The total antioxidant capacity assay was seen to be much higher in all the extracts particularly that of RP and TP, which increased with increasing concentrations of extract. One hundred microgrammes of water-dissolved extracts of RP, TP and YY showed total antioxidant capacities of 92.26 ± 0.94, 92.4 ± 1.2 and 78.71 ± 1, respectively (Fig. 4). Details of the result are given in Additional file 1: Table S6.
The antioxidant capacity (TOC) of different concentrations of the carotenoid extracts of strains RP, TP and YY dissolved in methanol (M), water (W) and DMSO (D). Coloured lines indicate different concentrations: blue—100 μg, red—80 μg, green—40 μg and violet—20 μg. The abbreviations are in the sequence of ‘organism-solvent’
Protein oxidative damage inhibition assay
Only extracts of YY showed inhibition of protein oxidative damage, and methanol-dissolved extracts showed the highest inhibition of 71.86 ± 0.611% and inhibition increased with decreasing concentrations (Fig. 5). Details of the result are given in Additional file 1: Table S7.
Statistical analysis
The Pearson correlation coefficient (r) value of 0.96 ± 0.04 indicates high degree of correlation between concentration of carotenoids and antioxidant activity in each case. One-way ANOVA (Analysis of variance) revealed that in most of the assays, the choice of solvent had a significant effect on the scavenging activity of all three carotenoid extracts (at 5% level of significance). The exception includes determining total antioxidant capacity of YY, where the choice of solvent had no significant effect. The details are given in Additional file 1: Table S8.
Discussion
Carotenoids like astaxanthin, actinioerythrol, lycopene [5] and thermozeaxanthin [21] have been found to have excellent antioxidant capacities. The major carotenoids found in the Deinococcus−Thermus phylum include carotenoid (di) glucoside-branched fatty acid (di) esters like thermozeaxanthin, thermobiszeaxanthin, all trans-zeaxanthin, zeaxanthin monoglucoside, and β-cryptoxanthin glucoside ester. Genus Meiothermus was reported to have 1′-β-glucopyranosyl-3,4,3′, 4′-tetradehydro-1′, 2′-dihydro-β, ψ-caroten-2-one glucose acetylated at position 6 and a series of carotenoid glycoside esters [17, 18]. The absorption spectra of the carotenoid extracts of Meiothermus sp. strains RP and TP show most similarity to the carotenoids deoxyflexixanthin and 1′-β-glucopyranosyl-3,4,3′, 4′-tetradehydro-1′, 2′-dihydro-β, ψ-caroten-2-one, both of which are carotenoid glycoside esters, so tentatively, the carotenoid extracts of strains RP and TP may be assigned as belonging to the family of carotenoid glycosides related to those two carotenoids. Similarly, the absorption spectra and maxima of strain YY show most similarity to the group of zeaxanthin and zeaxanthin derivates, so most probably, it belongs to zeaxanthin family [22]. Though a little work on the antioxidant property of Thermus filiformis carotenoid extract has been done [21], studies on the antioxidant properties of carotenoids belonging to this phylum are limited and analysis of antioxidant properties of strains related to Meiothermus sp. and Thermus scotoductus has not been done yet. The antioxidant property of carotenoids of T. filiformis was determined using the ABTS radical assay and hydrogen peroxide scavenging activity. The trolox equivalent antioxidant capacity (TEAC) and IC50 values (obtained from ABTS radical assay and hydrogen peroxide scavenging activity, respectively) were 2.87 and 2.41 μg ml−1, respectively, in DMSO. Similar to strain YY, the scavenging activity of the carotenoid extracts of T. filiformis had a positive correlation with the carotenoid concentration (R 2 > 0.95). From the scavenging activities of RP, TP and YY, the IC50 values of H2O2 scavenging can be presumed to be between 450 and 200 μg ml−1 (final concentration) in DMSO for RP and TP and <100 μg ml−1 (final concentration) in DMSO for YY, though the methods followed for the H2O2 scavenging activity determination were different from the one followed for the T. filiformis extract [21]. Lycopene extracts (final concentration 330 μg ml−1) from fruits and vegetables have shown up to 44.65% DPPH scavenging activity in distilled water, which is much less than that shown by extracts of RP (60.03 ± 0.15%), TP (60.26 ± 1.2) and YY (47.9 ± 1.7) with a final concentration of 4 μg ml−1 [23], and ethanolic extracts containing astaxanthin (25 μg ml−1) have shown maximum 57.6% scavenging activity [24]. Based on the study conducted on the carotenoid extracts of Meiothermus sp. strain RP, Meiothermus sp. strain TP and Thermus sp. strain YY, it can be undoubtedly stated that the carotenoid extracts have high antioxidative capacity and positive results in different methods firmly establish the fact. The statistically significant solvent-dependent variation of the scavenging activity may be attributed to the fact that solvent polarity plays a major factor in antioxidant capacity [4]. Besides solvent polarity, the methods used and the carotenoid composition of the carotenoid extracts might play a role in the differences of values across the strains. The methods involving DPPH and ABTS are more or less similar, i.e. quenching of free radicals, but DPPH is already a stable molecule, and the antioxidant reaction reaches a steady state more slowly; ABTS radical on the other hand has to be generated, and the reaction is rather quick [25]. The method of hydrogen peroxide scavenging is completely different, as the superoxide radical is broken down [8]. In the TOC method, a change in the oxidation state of molybdenum occurs [9], and the protein oxidation inhibition method is a method for detecting changes in carbonyl content [11]. These may be the probable reason why RP, TP and YY show difference in activities across different assays. Again, the zeaxanthin and thermobiszeaxanthin esters have carbon number ranges of 59–82, whereas deoxyflexixanthin has a carbon number of 40 and 1′-β-glucopyranosyl-3,4,3′, 4′-tetradehydro-1′, 2′-dihydro-β, ψ-caroten-2-one has a carbon number of 46. Though all of them are glycoside esters, the molecular mass are widely different and so are the differences in activity among the three strain extracts [18, 22].
There are some minor biochemical differences between the two Meiothermus strains RP and TP. While RP can utilize glycerol and sucrose, which TP is unable to utilize, unlike RP, TP can utilize melezitose as sole source of carbon. Most of their biochemical characteristics are similar to M. cateniformans and/or M. taiwanensis. The only dissimilarity lies in the positive utilization of inositol, which both M. cateniformans and M. taiwanensis are unable to do, while M. ruber is capable of utilizing. All the strains are catalase and oxidase positive. While most of the Meiothermus strains have a growth temperature range starting at a minimum temperature of 35–40 °C, both strains RP and TP are unable to grow below 55 °C [26, 27]. Features, like utilization of xylanase, cellulase, chitinase, pectinase and alginate lyase, present in strain RP and TP cannot be compared due to lack of information about other strains. The biochemical characteristics of Thermus sp. strain YY are more similar to T. scotoductus and T. filiformis than T. aquaticus. Like T. filiformis, the strain can utilize lactose, glucose, melibiose, sucrose, trehalose and starch. The strain is oxidase negative and can utilize gelatin and glycerol unlike other strains of Thermus. Though all the strain of Thermus showed optimal growth at pH 7.5, strain YY shows optimal growth at pH 6. Features, like utilization of cellulase, chitinase and alginate lyase, present in strain YY cannot be compared due to lack of information about other strains [28, 29].
Conclusions
Oxidative stress seems to contribute to many human physiological problems and diseases like cardiac arrests, neurodegenerative diseases, cancer, diabetes mellitus, rheumatoid arthritis, gastrointestinal disease, renal disorder, pulmonary disorders, eye diseases and pregnancy problems. Free radicals generated from oxidative stress lead to cellular stress, degeneration, aging and death, damaging cell membranes, proteins, RNA, DNA, etc. Diet sources provide us with antioxidants, but an externally supplied good antioxidant complex supplement is more effective as the different antioxidants act on different types of harmful oxidative reactions and radicals at various sites, thus providing an all-round protection to the resultant ill effects and resultant disorders and diseases. In food industry, antioxidants increase shelf life of foods like oil, fats and meat. They are also widely used in pharmaceuticals and cosmetics and prevent degradation of plastics, rubber, gasoline, lubrication oil, etc. [30, 31]. Newer and efficient antioxidative compounds are constantly being searched for, and the carotenoid extracts of RP, TP and YY have been shown to catalyze various types of antioxidative reactions, including protein oxidation inhibition by YY. Thus, all these extracts have huge potential to be industrially and pharmaceutically useful, and the carotenoid composition of the strains RP, TP and YY might be further determined for finding out the exact mechanism behind their antioxidative properties.
References
Badrinath AV, Rao KM, Chetty CMS, Ramkanth S, Rajan TVS, Gnanaprakash K. A review on in-vitro antioxidant methods: comparisons, correlations and considerations. Int J PharmTech Res. 2010;2:1276–85.
Proir RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–302.
Apak R, Güçlü K, Demirata B, Özyürek M, Çelik SE, Bektaşoğlu B, Berker KI, Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–547.
El-Agamey A, McGarvey. Carotenoid radicals. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids: Natural Functions; 2008. p. 119-150.
Britton G. Functions of intact carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids: Natural Functions; 2008. p. 189-211
Kurechi T, Kikugawa K, Kato T. Studies on the antioxidants. XIII. Hydrogen donating capability of antioxidants to 2, 2-diphenyl-1-picrylhydrazyl. Chem Pharm Bull. 1980;28:2089–93.
Ruch RJ, Cheng S, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–8.
Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans. Antioxidant activities of carotenes and xanthophylls. FEBS Letters. 1996; 384: 240-242
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of Vitamin E1. Anal Biochem. 1999;269:337–41.
Alam MN, Bristi NH, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–52.
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–8.
Smibert RM, Krieg NR. Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. Methods for general and molecular bacteriology. Washington DC: ASM Press; 1994. p. 607–54.
Innis MA, Gelfand DH. Optimization of PCRs. In: Innis MA, Gelfand DH, Sininsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. p. 3–12.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25.
Meckel RA, Kester AS. The extractability of carotenoid pigments from non-photosynthetic bacteria with solvents and detergents: implications for the location and binding of pigments. J Gen Microbiol. 1980;120:111–6.
Britton G. UV visible spectroscopy. In: Britton G, Liaaen-jensen S, Pfander H, editors. Carotenoids: spectroscopy, vol. 1B. Switzerland: Birkhauser Verlag; 1995. p. 13–62.
Takaichi S, Maoka T, Yamada M, Matsuura K, HAikawa Y, Hanada S. Absence of carotenes and presence of a tertiary methoxy group in a carotenoid from a thermophilic filamentous photosynthetic bacterium Roseiflexus castenholzii. Plant Cell Physiol. 2001;42:1355–62.
Selvam NT, Vengatakrishnan V, Murugesan S, Kumar SD. Free radical scavenging activity of methanolic extract of Grewia tiliaefolia bark in in-vitro model systems. Res J Pharma, Biol Chem Sci. 2010;1:502–9.
Aliyu AB, Ibrahim MA, Musa AM, Musa AO, Kiplimo JJ, Oyewale AO. Free radical scavenging and total antioxidant capacity of root extracts of Anchomanes difformis Engl. (Araceae). Acta Pol Pharm. 2013;70:115–21.
Mandelli F, Miranda VS, Rodrigues E, Mercadante AZ. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J Microbiol Biotechnol. 2012;28:1781–90.
Tian B, Yuejin H. Carotenoid biosynthesis in extremophilic Deinococcus–Thermus bacteria. Trends Microbiol. 2010;18:512–20.
Shahzad T, Ahmad I, Choudhry S, Saeed MK, Khan MN. DPPH free radical scavenging activity of tomato, cherry and watermelon: lycopene extraction, purification and quantification. Int J Pharm Pharm Sci. 2014;6:223–8.
Zhang X, Zhang X, Fu L, Zhu H, Zhang B. Effect of extraction and drying methods on antioxidant activity of astaxanthin from Haematoccus pluvialis. Food Bioprod Process. 2016;99:197–203.
Shalaby EA, Shanab SMM. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Ind J Geo-Marine Sci. 2013;42:556–64.
Zhang X, Zhang W, Wei B, Xu X, Zhu X, Wu M. Meiothermus cateniformans sp. nov., a slightly thermophilic species from north-eastern China. Int J Syst Evol Microbiol. 2010;60:840–4.
Chen M, Lin G, Lin Y, Tsay S. Meiothermus taiwanensis sp. nov., a novel filamentous, thermophilic species isolated in Taiwan. Int J Syst Evol Microbiol. 2002;52:1647–54.
Hudson JA, Morgan HW, Daniel RM. Thermus filiformis sp. nov., a filamentous caldoactive bacterium. Int J Syst Bacteriol. 1987;37:431–6.
Kristjánsson JK, Hjörleifsdóttir S, Marteinsson VT, Alfredsson GA. Thermus scotoductus sp. nov., a pigment - producing thermophilic bacterium from hot tap water in Iceland and including Thermus sp. X-1. Syst Appl Microbiol. 1994;17:44–50.
Hamid AA, Aiyelaagbe OO, Usman LA, Ameen OM, Lawal A. Antioxidants: its medicinal and pharmacological applications. Afr J Pure Appl Chem. 2010;4:142–51.
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84.
Acknowledgements
We thankfully acknowledge the Honourable Vice Chancellor, Burdwan University, for providing necessary infrastructural facilities.
Funding
This work was supported by grant of DST-INSPIRE (Innovation in Scientific Pursuit for Inspired Research, Department of Science and Technology) (IF 140017), Ministry of Science and Technology, Govt. of India. The funding body played significant role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The 16S rDNA sequences supporting the manuscript are available in GenBank, NCBI. Meiothermus sp. RP, Meiothermus sp. TP and Thermus sp. YY were assigned accession numbers KP053251 (https://www.ncbi.nlm.nih.gov/nuccore/967510706/), KP053252 (https://www.ncbi.nlm.nih.gov/nuccore/KP053252) and KT239023 (https://www.ncbi.nlm.nih.gov/nuccore/KT239023), respectively. Other datasets supporting the manuscripts are included in the supporting information word file Additional file 1: Figures S1–S6 and Tables S1–S8.
Authors’ contributions
TM, SKM and SB made substantial contributions to acquisition of data and analysis and interpretation of data, have been involved in drafting the manuscript and revising it critically for important intellectual content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TM, SKM and SB have participated sufficiently in the work to take public responsibility for appropriate portions of the content. All authors read and approved the final manuscript.
Authors’ information
TM is topper at university level in both graduation and post graduation (gold-medallist) and ranked 88 and 13 at two national level tests GATE and CSIR-UGC NET (LS), respectively. Currently, TM has 15 publications (https://www.researchgate.net/profile/Trinetra_Mukherjee2) and SKM has 74 publications (https://www.researchgate.net/profile/Subhra_Mukhopadhyay3/publications?pubType=article). SKM is associate professor of the Department of Microbiology, The University of Burdwan. TM, SB and SKM are working on the bioprospecting of thermophiles from hot springs.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Supporting material. Figure S1. Gram negative rods of a) strain RP b) strain TP c) strain YY, as observed under oil immersion lens. Figure S2. Growth of strains RP, TP and YY at different temperatures. Figure S3. Growth of strains RP, TP and YY at different pH. Figure S4. Salt tolerance of strains RP, TP and YY. Figure S5. Agarose gel electrophoresis of genomic DNA of strains YY, RP and TP, present in lanes a, c and d, respectively. Figure S6. Agarose gel electrophoresis of 1.5 kb 16S rDNA amplicons of strains YY, RP and TP. Table S1. Carbon utilization pattern of the three strains. Table S2. Results of biochemical tests. Table S3. DPPH scavenging activity of different concentrations of carotenoid extracts of strains RP, TP and YY dissolved in water, methanol and DMSO. Table S4. ABTS radical scavenging activity of different concentrations of carotenoid extracts of strains RP, TP and YY dissolved in water, methanol and DMSO. Table S5. H2O2 scavenging activity of different concentrations of carotenoid extracts of strains RP, TP and YY dissolved in water, methanol and DMSO. Table S6. Total antioxidant capacity (TOC) of different concentrations of carotenoid extracts of strains RP, TP and YY dissolved in water, methanol and DMSO. Table S7. Percent inhibition activity of protein oxidation by different concentrations of carotenoid extracts of strain YY dissolved in water, methanol and DMSO. Table S8. Analysis of variance (ANOVA) of the antioxidant properties of the carotenoid extracts showing the effects of solvent for DPPH assay, ABTS assay, hydrogen peroxide assay, total antioxidant capacity assay (TOC) and protein oxidation inhibition (POI) assay. (DOC 1250 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mukherjee, T., Bose, S. & Mukhopadhyay, S.K. Antioxidant properties of the carotenoid extracts of three Deinococcus–Thermus phylum bacteria, Meiothermus sp. strains RP and TP and Thermus sp. strain YY from Paniphala hot spring, India. Nutrire 42, 7 (2017). https://doi.org/10.1186/s41110-017-0032-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-017-0032-3