Abstract
Background
Responsiveness to erythropoiesis-stimulating agents (ESAs) is thought to be related to prognosis in patients on hemodialysis. A multi-center, prospective cohort study was conducted to investigate the effects of hyporesponsiveness to long-acting ESAs on cardiovascular events and mortality in Japanese patients on chronic hemodialysis.
Methods
A total of 127 chronic hemodialysis patients treated with long-acting ESAs were followed-up prospectively. Responsiveness to ESA was evaluated using an erythropoietin resistance index (ERI) calculated by dividing the weekly body-weight-adjusted ESA dose by the hemoglobin concentration. The primary endpoint of this survey was defined as a combination of cardiovascular events and all-cause deaths. The association between hyporesponsiveness to ESAs evaluated by the highest quartile of the ERI and the primary endpoint was investigated.
Results
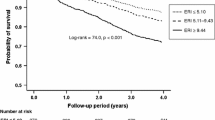
During the follow-up period (median 4.6 years), 32 patients reached the primary end point. Kaplan-Meier curve analysis showed that patients with ESA hyporesponsiveness belonging to the highest quartile of the ERI reached the primary end point more frequently than those without (P = 0.031). Cox regression analysis showed that an ERI in the highest quartile was an independent predictor of the primary end point, even after adjustment using a propensity score (hazard ratio 2.76, 95% confidence interval 1.19–6.40).
Conclusions
ESA hyporesponsiveness in hemodialysis patients treated with long-acting ESAs is related to cardiovascular events and death.
Similar content being viewed by others
Introduction
Anemia, a common complication in all stages of chronic kidney disease (CKD), is more pronounced in end-stage renal disease (ESRD) patients on chronic dialysis, and it is one of the independent risk factors for cardiovascular disease and death in these patients [1,2,3]. Since treatment of anemia with erythropoietin-stimulating agents (ESAs) reportedly reduced the need for blood transfusions and improved quality of life [4, 5], ESAs have been widely used as first-line drugs for renal anemia in ESRD patients in daily practice. However, evidence from randomized, controlled trials suggested that treatment with ESA to higher target hemoglobin levels increased the risk of mortality in subjects on chronic hemodialysis [6, 7], and higher doses of ESA and lower achieved hemoglobin levels have been found to be related to increased mortality [8, 9]. Relationships between poor prognosis and ESA hyporesponsiveness in patients on chronic dialysis have been reported in several studies [10,11,12], although there are no quantitative definitions of ESA hyporesponsiveness based on the evidence from studies examining the prognosis of patients in relation to ESA responsiveness. In addition, the definitions of ESA hyporesponsiveness were rather different among these studies. The average dose of ESA and hemoglobin levels are reportedly lower in Japan than in Western countries [13], but the relationship between ESA responsiveness and prognosis in Japanese patients on chronic dialysis has been evaluated in few studies [14, 15], and most of the ESAs used in these studies were epoetin alfa and beta. Recently, Sakaguchi et al. reported that the long-acting ESA use was associated with an increased risk of mortality compared with short-acting ESA use in Japanese patients on chronic hemodialysis [16]. As this association was reportedly relevant particularly among patients with high ERI, more careful attention should be needed on ESA hyporesponsiveness in the long-acting ESA user. However, the relationship of ESA hyporesponsiveness to poor prognosis in dialysis patients treated with long-acting ESAs, darbepoetin alfa and epoetin beta pegol, has not been sufficiently investigated. Therefore, the aim of the present study was to investigate whether ESA hyporesponsiveness is related to the risk of cardiovascular disease and death in Japanese patients on chronic hemodialysis with the long-acting ESAs, darbepoetin alfa and epoetin beta pegol.
Patients and methods
Study design and population
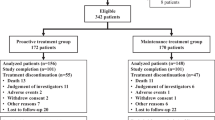
This multi-center, prospective, observational cohort study included 127 stable patients receiving maintenance hemodialysis in the dialysis unit of 3 hospitals (Fujita General Hospital, Tani Hospital, and Hohrai East Clinic). Inclusion criteria were age ≥ 20 years and receiving dialysis treatment for at least 3 months. Recruitment was performed between January and May 2015, and 203 patients were initially recruited. Of the originally enrolled patients, the following were excluded from this analysis: those who could not answer the questionnaire due to dementia or brain disease (n = 59), and those with acute/chronic inflammatory disease and active malignancy (n = 9). After additional exclusion of those not receiving ESA (n = 6), those receiving short-acting ESA (n = 1), and those who withdrew consent (n = 1), 127 participants were included in the study (Fig. 1).
Data collection
Blood samples were collected just before starting hemodialysis. Hemoglobin, serum albumin, calcium, phosphorus, intact parathyroid hormone (PTH), C-related protein (CRP), ferritin, and transferrin saturation (TSAT) were measured according to the automated standardized laboratory techniques in the clinical laboratory of each institution. Serum total calcium was adjusted for albumin using the formula proposed by Payne [17]. Dialysis dose were measured by single-pool Kt/V by the Daugirdas method [18]. Information on medications at baseline, as well as history of cardiovascular disease, was obtained from the patients’ medical records. Cardiovascular disease included myocardial infarction, angina pectoris, congestive heart failure, arrhythmias, stroke, cerebrovascular disorder, chronic arteriosclerosis obliterans, and aortic dissection.
ESA responsiveness
To evaluate the dose-response effect of therapy with ESAs, an erythropoietin resistance index (ERI) was used, calculated as the weekly weight-adjusted ESA dose divided by the hemoglobin level: weekly ESA dose (U/week)/post-dialysis body weight (kg) × hemoglobin (g/dL) [19]. The ERI was calculated only at the start of the study. All ESAs used in the present study were long-acting ESAs; darbepoetin alfa was used in 116 patients, and epoetin beta pegol was used in 11 patients. To compare with previous reports on the ERI with epoetin, darbepoetin alfa and epoetin beta pegol doses were converted in calculating the ERI based on the ratio of their equivalent peptide masses (200 units of epoetin to 1 μg darbepoetin alfa and epoetin beta pegol, as previously described) [20]. On the basis of the ERI value, subjects were divided into four quartiles; subjects in the highest quartile were defined as those with ESA hyporesponsiveness in the present study.
Prospective follow-up
The patients were prospectively followed-up until November 2019 or until the study end point was reached. The primary endpoint of this survey was defined as a combination of cardiovascular events (fatal and nonfatal myocardial infarction, angina pectoris, sudden death, congestive heart failure, arrhythmia, stroke, cerebrovascular disorder, chronic arteriosclerosis obliterans, subarachnoid hemorrhage, and aortic dissection) and all-cause death. Outcomes were surveyed every 12 months using the hospital medical records. All patients from the baseline cohort were assessed during follow-up.
Statistical analysis
The baseline characteristics of the study patients are expressed as percentages for categorical data and median and interquartile ranges for continuous variables with skewed distributions. The Kruskal-Wallis test was used to compare median values, and Tukey’s test was used to evaluate differences in proportions. Patient survival was assessed using the Kaplan-Meier method with the log-rank test. The hazard ratios (HRs) and 95% confidence intervals (CIs) of the primary end point and all-cause death according to ERI levels were estimated by a Cox proportional hazards model analysis. Due to the limited number of patients, the results of multivariate Cox proportional hazard analysis were adjusted by the propensity score in the present study. A propensity score analysis was performed using a multivariate logistic regression model, including confounding variables (age, sex, dialysis facility, diabetes, history of cardiovascular disease, dialysis duration, calcium, phosphorus, albumin, hemoglobin, CRP, intact PTH, TSAT, ferritin, use of renin-angiotensin-aldosterone system inhibitor, and Kt/V). The score was then incorporated into the multivariate analysis as a covariate. All data were analyzed using SPSS software (version 26; IBM Corporation, Chicago, IL, USA). Associations with P < 0.05 were considered significant.
Results
Table 1 summarizes the baseline characteristics according to ERI quartile of the study population. The ERI was calculated for all patients, with a median (IQR) of 5.36 (2.73–11.31). Subjects belonging to quartile four (ERI level ≥ 11.31) were defined as the high ERI group with ESA hyporesponsiveness in the present study. The median (IQR) hemoglobin concentration was 10.6 (10.1–11.1), and the median (IQR) ESA dose was 15 (10–30) μg/week. The median values for body mass index, hemoglobin concentration, and serum albumin decreased with increasing ERI levels. Lower hemoglobin levels and higher ESA dose were seen in patients with higher ERI levels (Table 1). The ERI correlated with age (r = 0.25, P = 0.004), body mass index (r = − 0.30, P = 0.001), hemoglobin (r = − 0.33, P < 0.001), serum albumin (r = − 0.26, P = 0.003), and CRP (r = 0.19, P = 0.035).
The median follow-up time was 4.6 years (IQR 4.4–4.8 years). During the follow-up period, 32 of 127 (25%) patients reached the primary end point (9 cardiovascular events and 23 all-cause deaths occurred, with only 4 cardiovascular deaths). The causes of death were sepsis (6 cases); uremia (5 cases); malignancy (4 cases); respiratory failure (2 cases); myocardial infarction (1 case); stroke (1 case); cerebrovascular disorder (1 case); subarachnoid hemorrhage (1 case); and unknown (2 cases).
In order to generate a propensity score, multivariate logistic regression analysis was performed using high ERI (≥ 11.31) as an independent variable (Supplemental table). Intact PTH had an inverse relationship to high ERI. Kaplan-Meier curve analysis showed that patients with ESA hyporesponsiveness belonging to quartile four (ERI ≥ 11.31) reached the primary end point more frequently than those without (Fig. 2). The incidence of all-cause death was also higher in the ESA hyporesponsiveness group with a higher ERI (Fig. 3). The results of univariate Cox regression analysis showed that a high ERI (≥ 11.31) was a predictor of both the primary end point and all-cause death (Table 2). A high ERI remained an independent predictor for both the primary end point and all-cause death after adjusting by the propensity score.
Discussion
The results of this prospective survey in a cohort of Japanese patients on chronic hemodialysis showed that ESA hyporesponsiveness evaluated by the ERI is an independent predictor of adverse outcomes such as cardiovascular events and deaths. Since the relationship between the ERI and poor prognosis in dialysis patients has already been reported [12, 14, 15], the present study is confirmatory. However, most studies of the ERI have been performed in patients treated with a short-acting ESA, epoetin, reporting that the ERI is related to the risk of cardiovascular events and all-cause mortality. The relationship between the ERI and poor prognosis has not been sufficiently investigated in dialysis patients treated with long-acting ESAs. The present study showed the relationship of the ERI to cardiovascular events and all-cause mortality in dialysis patients treated with long-acting ESAs, darbepoetin alfa and epoetin beta pegol, in a prospective observational study.
To compare with previous reports on the ERI with epoetin, darbepoetin alfa and epoetin beta pegol doses were converted by multiplying by 200 in calculating the ERI in the present study. Several observational studies have examined the relationship between ESA hyporesponsiveness calculated by the same equation as in this study and poor prognosis in dialysis patients [12, 14, 15]. The findings of these studies were in accord with the results of the present study, and higher ERI levels were related to the risk of mortality and cardiovascular events in patients on chronic hemodialysis. A poor prognosis was reportedly associated with ERI levels ≥ 10.0 and ≥ 9.44, respectively, in Japanese patients on dialysis [14, 15]; thus, the present result that an ERI level > 11.3 was related to the risk of cardiovascular disease and death was similar to these results, although both ESA doses and ERI levels reported in European patients on dialysis were much higher than in the present study [12].
In the present study, the ERI had a significant positive correlation with CRP and negative correlations with body mass index and serum albumin, which reflect inflammation and malnutrition in dialysis patients. Both the nutritional state and the presence of inflammation are recognized factors related to a poor prognosis in dialysis patients, so-called malnutrition-inflammation-atherosclerosis syndrome, and they were reportedly also related to ESA hyporesponsiveness [21,22,23,24,25]. Since low hemoglobin levels increase the risk of cardiovascular disease and death, ESA therapy is crucial in dialysis patients, but treatment of anemia with high-dose ESAs could have a negative effect on the prognosis of these patients. Mortality rate was reportedly higher in long-acting ESA user than in short-acting ESA user, and the increased rate of mortality was more prominent in patients with high dose ESA and high ERI [16]. Thus, management of nutritional status and chronic inflammation is needed to improve ESA hyporesponsiveness, as well as the poor prognosis, in patients who need high-dose ESAs to increase their serum hemoglobin concentrations to target levels, in particular in long-acting ESA user, before increasing the dose of ESAs.
Iron deficiency is considered one of the factors associated with both ESA responsiveness and prognosis in patients on dialysis. The Japanese nationwide registry data, including 142,339 Japanese patients on chronic dialysis, has shown a U-shaped relationship between the ERI and TSAT, with the bottom of the ERI curve around a TSAT of 30–40% [26]. However, there were no differences in ferritin and TSAT levels among the ERI levels in the present study. Significant differences were not observed in the iron states between patients who reached the primary end point and those who did not. These results might have been affected by the much lower levels of ferritin (median 34 ng/dL) in the present study with a limited number of subjects. Therefore, relationships among the iron states, responsiveness to ESAs, and prognosis in dialysis patients should be investigated across various ranges of ferritin levels in a longitudinal study with sufficient numbers of patients or in a randomized, controlled trial.
The present study has several limitations. First, single measurements of the ERI and covariates at baseline might have led to some misclassification of ESA responsiveness, and it was not possible to examine the effects of changes from the baseline category during follow-up. Also, some patients treated with long-acting ESAs at baseline might have been converted to short-acting ESAs during follow-up. Second, the sample size was small. Since the number of patients reaching the primary end point was relatively small, multivariate adjustment for covariates at baseline might be insufficient for evaluating the association between ERI levels and poor outcomes. Therefore, the results of multivariate Cox regression analysis were adjusted by the propensity score calculated from sufficient confounding variables at baseline in order to avoid this statistical inadequacy. Third, since this study was an observational study, it remains unclear whether a poor prognosis in dialysis patients is associated with a high dose of ESAs itself or factors causing ESA hyporesponsiveness, such as malnutrition, chronic inflammation, and iron states. These limitations of the present study need to be addressed in future studies.
Conclusions
The present study showed that ESA hyporesponsiveness evaluated by the ERI was associated with increased risks of cardiovascular events and all-cause death in Japanese patients on chronic hemodialysis treated with long-acting ESAs. ERIs were related to malnutrition and chronic inflammation, and they had significant and independent effects on poor prognosis in these patients. Although there are no quantitative definitions of ESA hyporesponsiveness based on the evidence from studies examining the prognosis of dialysis patients, the ERI, easily calculated in daily practice, could be a useful tool for predicting the risk of cardiovascular events and deaths in patients treated with ESAs, including both short- and long-acting. Further investigations are still necessary to confirm these findings in an observational survey of sufficient size and with better statistical methods, and to determine whether interventions for improving hyporesponsiveness to ESAs reduce the risk of cardiovascular events and deaths in patients with ESA hyporesponsiveness in a clinical trial.
Availability of data and materials
The dataset generated and/or analyzed during the current study are available from corresponding author on reasonable request.
Abbreviations
- ESA:
-
Erythropoiesis-stimulating agent
- ERI:
-
Erythropoietin resistance index
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- PTH:
-
Parathyroid hormone
- CRP:
-
C-related protein
- TSAT:
-
Transferrin saturation
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Akizawa T, Pisoni RL, Akiba T, Saito A, Fukuhara S, Asano Y, Hasegawa T, Port FK, Kurokawa K. Japanese haemodialysis anaemia management practices and outcomes (1999-2006): results from the DOPPS. Nephrol Dial Transplant. 2008;23(11):3643–53. https://doi.org/10.1093/ndt/gfn346.
Inaba M, Hayashino Y, Shoji T, Akiba T, Akizawa T, Saito A, Kurokawa K, Fukuhara S. Disappearance of association in diabetic patients on hemodialysis between anemia and mortality risk: the Japan dialysis outcomes and practice pattern study. Nephron Clin Pract. 2012;120(2):c91–c100. https://doi.org/10.1159/000335979.
Akizawa T, Saito A, Gejyo F, Suzuki M, Nishizawa Y, Tomino Y, Tsubakihara Y, Akiba T, Hirakata H, Watanabe Y, Kawanishi H, Bessho M, Udagawa Y, Aoki K, Uemura Y, Ohashi Y, JET Study Group. Low hemoglobin levels and hypo-responsiveness to erythropoiesis-stimulating agent associated with poor survival in incident Japanese hemodialysis patients. Ther Apher Dial. 2014;18(5):404–13. https://doi.org/10.1111/1744-9987.12155.
Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets and blood transfusions in hemodialysis patients without symptomatic cardiac disease receiving erythropoietin therapy. Clin J Am Soc Nephrol. 2008;3(6):1669–75. https://doi.org/10.2215/CJN.02100508.
Leaf DE, Goldfarb DS. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int. 2009;75(1):15–24. https://doi.org/10.1038/ki.2008.414.
Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–90. https://doi.org/10.1056/NEJM199808273390903.
Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369(9559):381–8. https://doi.org/10.1016/S0140-6736(07)60194-9.
Zhang Y, Thamer M, Stefanik K, Kaufman J, Cotter DJ. Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis. 2004;44(5):866–76. https://doi.org/10.1016/S0272-6386(04)01086-8.
Streja E, Kovesdy CP, Greenland S, Kopple JD, McAllister CJ, Nissenson AR, et al. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52(4):727–36. https://doi.org/10.1053/j.ajkd.2008.05.029.
Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJV, Pfeffer MA. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363(12):1146–55. https://doi.org/10.1056/NEJMoa1005109.
Kilpatrick RD, Critchlow CW, Fishbane S, Besarab A, Stehman-Breen C, Krishnan M, Bradbury BD. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(4):1077–83. https://doi.org/10.2215/CJN.04601007.
Panichi V, Rosati A, Bigazzi R, Paoletti S, Mantuano E, Beati S, Marchetti V, Bernabini G, Grazi G, Rizza GM, Migliori M, Giusti R, Lippi A, Casani A, Barsotti G, Tetta C, on behalf of the RISCAVID Study Group. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant. 2011;26(8):2641–8. https://doi.org/10.1093/ndt/gfq802.
McFarlane PA, Pisoni RL, Eichleay MA, Wald R, Port FK, Mendelssohn D. International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int. 2010;78(2):215–23. https://doi.org/10.1038/ki.2010.108.
Okazaki M, Komatsu M, Kawaguchi H, Tsuchiya K, Nitta K. Erythropoietin resistance index and the all-cause mortality of chronic hemodialysis patients. Blood Purif. 2014;37(2):106–12. https://doi.org/10.1159/000358215.
Eriguchi R, Taniguchi M, Ninomiya T, Hirakata H, Fujimi S, Tsuruya K, Kitazono T. Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: the Q-Cohort study. J Nephrol. 2015;28(2):217–25. https://doi.org/10.1007/s40620-014-0121-9.
Sakaguchi Y, Hamano T, Wada A, Masakane I. Types of erythropoietin-stimulating agents and mortality among patients undergoing hemodialysis. J Am Soc Nephrol. 2019;30(6):1037–48. https://doi.org/10.1681/ASN.2018101007.
Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T, CKD-MBD Guideline Working Group, Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247–88. https://doi.org/10.1111/1744-9987.12058.
Daugirdas JT. The post: pre dialysis plasma urea nitrogen ratio to estimate K.t/V and NPCR: validation. Int J Artif Organs. 1989;12(7):420–7.
Lopez-Gomez JM, Portoles JM, Aljama P. Factors that condition the response to erythropoietin in patients on hemodialysis and their relation to mortality. Kidney Int Suppl. 2008;111:S75–81.
Scott SD. Dose conversion from recombinant human erythropoietin to darbepoetin alfa: recommendations from clinical studies. Pharmacotherapy. 2002;22(9 Pt 2):160S–5S. https://doi.org/10.1592/phco.22.14.160S.33398.
Locatelli F, Andrulli S, Memoli B, Maffei C, Del Vecchio L, Aterini S, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21(4):991–8. https://doi.org/10.1093/ndt/gfk011.
Singh AK, Coyne DW, Shapiro W, Rizkala AR, Group DS. Predictors of the response to treatment in anemic hemodialysis patients with high serum ferritin and low transferrin saturation. Kidney Int. 2007;71(11):1163–71. https://doi.org/10.1038/sj.ki.5002223.
Bradbury BD, Critchlow CW, Weir MR, Stewart R, Krishnan M, Hakim RH. Impact of elevated C-reactive protein levels on erythropoiesis- stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transplant. 2009;24(3):919–25.
Gaweda AE, Goldsmith LJ, Brier ME, Aronoff GR. Iron, inflammation, dialysis adequacy, nutritional status, and hyperparathyroidism modify erythropoietic response. Clin J Am Soc Nephrol. 2010;5(4):576–81. https://doi.org/10.2215/CJN.04710709.
Rattanasompattikul M, Molnar MZ, Zaritsky JJ, Hatamizadeh P, Jing J, Norris KC, Kovesdy CP, Kalantar-Zadeh K. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant. 2013;28(7):1936–45. https://doi.org/10.1093/ndt/gfs368.
Hamano T, Fujii N, Hayashi T, Yamamoto H, Iseki K, Tsubakihara Y. Thresholds of iron markers for iron deficiency erythropoiesis-finding of the Japanese nationwide dialysis registry. Kidney Int Suppl. 2015;5(1):23–32.
Acknowledgements
The authors would like to thank Ayumi Kanno for her assistance in data collection.
Funding
The authors received no funding for this work.
Author information
Authors and Affiliations
Contributions
KT wrote the paper with input from all authors. All authors have approved the manuscript. Research idea and study design: KT; data acquisition: MF, HS, TI, AO, SW, MK, HK, HK, YT, JA, HS; data analysis/interpretation: KT, HS, KS; statistical analysis: KT; supervision or mentorship: JK.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol complied with the Declaration of Helsinki and was approved by the ethics committee at Fukushima Medical University (acceptance no. 2069). All patients received an explanation of the procedures and possible risks of this study and gave their written, informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table.
Factors related to high ERI (≥11.31) in CKD patients on multivariate logistic regression analysis for generating propensity score.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tanaka, K., Fujiwara, M., Saito, H. et al. Hyporesponsiveness to long-acting erythropoiesis-stimulating agent is related to the risk of cardiovascular disease and death in Japanese patients on chronic hemodialysis: observational cohort study. Ren Replace Ther 7, 13 (2021). https://doi.org/10.1186/s41100-021-00332-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-021-00332-0