Abstract
Background
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease with all-cause mortality increasing globally. Dietary magnesium (Mg), an anti-inflammatory nutrient, has been proven to be associated with the all-cause mortality. The association of dietary Mg intake and all-cause mortality in RA patients remains unknown. The aim of this study was to assess the association between dietary Mg intake and all-cause mortality in RA patients.
Methods
RA patients were extracted from the NHANES 1999–2018, and followed for survival through December 31, 2019. Dietary Mg intake data were obtained from 24-h dietary recall interview. The association between dietary Mg intake and RA patients’ all-cause mortality was explored based on weighted univariate and multivariate Cox proportional hazard models and described as absolute risk difference (ARD), hazard ratios (HRs) and 95% confidence intervals (CIs). This association was further explored in subgroup analyses based on different age, gender and body mass index (BMI).
Results
Totally 2,952 patients were included. Until 31 December 2019, a total of 825 deaths were documented. RA patients with higher dietary Mg intake had a 11.12% reduction of all-cause mortality (ARD=-11.12%; HR = 0.74, 95%CI: 0.56–0.99) in the fully adjusted model, especially in female (HR = 0.68, 95%CI: 0.47–0.98), aged < 65 years (HR = 0.59, 95%CI: 0.37–0.94) and BMI ≤ 30 kg/m2 (HR = 0.62, 95%CI: 0.42–0.91).
Conclusion
RA patients who consumed adequate dietary Mg from diet as well as supplements may had a lower risk of all-cause mortality.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA), an autoimmune disease featured by systemic inflammation and invasive destruction of multiple joints, greatly affects the quality of patients’ life [1,2,3]. RA affects between 0.5 and 1% population worldwide, and the risk of female were two to three times more than men [4]. Related studies reported an approximately 50-60% increase in mortality in RA patients compared with healthy people [5, 6]. Active prevention and treatment are essential to improving the outcomes of RA patients.
Inflammation may be an important factor leading to premature death in RA patients [7]. It has been shown that approximately a quarter of the all-cause mortality in RA patients was mediated by inflammation mediates [8]. Several inflammation cytokines, such as tumor necrosis factor-α, interleukin-1 and 6, were significantly increased in RA patients [9,10,11]. These inflammatory cytokines result in the progression of RA synovitis, which leads to joint destruction and ultimately disability and premature death [12, 13]. Currently, several studies have shown the potential interaction between dietary nutrients and immune diseases [14, 15]. Several dietary patterns and supplements, such as the Mediterranean diet (MD), dietary total antioxidant capacity, vitamin D and probiotics, have proven to be associated with arthritis or osteoarthritis [16, 17]. Magnesium (Mg), a biologically active mineral, acts as a cofactor in hundreds of enzymatic reactions in the human body. Previous observations studies reported that Mg intake was inversely related to inflammatory diseases including hypertension, type 2 diabetes mellitus and cardiovascular disease (CVD) [18]. Mendes PMV et al. [19] reported the all-cause mortality risk was reduced by 6% for every 100 mg/d increase of dietary Mg intake. In addition, several studies have suggested that insufficient dietary Mg intake increase the poor prognosis risk in various cancers [20,21,22]. Less in known, however, the relationship of Mg intake and all-cause mortality in RA patients.
Herein, we evaluated the relationship of dietary Mg intake and RA patients’ all-cause mortality based on the National Health and Nutrition Examination Survey (NHANES) database. This study was aimed to lay a theoretical foundation for the good prognosis of RA patients from the perspective of improving the diet.
Methods
Study design and RA patients
In this retrospective cohort study, data of RA patients were extracted from the NHANES 1999–2018. NHANES is a survey conducted by National Center for Health Statistics (NCHS), a part of the Centers for Disease Control and Prevention (CDC) and is responsible for assessing the health and nutritional status for the U.S. civilian. The NHANES protocols are approved by the NCHS Ethics Review Board of the US CDC and all participants provided written informed consent. According to the Ethics Review Board of Second Affiliated Hospital of Nanchang University, cross-sectional studies have been exempted from the ethical review.
The included criteria were: (1) patients diagnosed as RA; (2) patients aged ≥ 18 years old; (3) patients with complete dietary Mg intake information. The excluded criteria were: (1) missing important covariates [white blood cell (WBC), BMI, smoking and RA medication use]; (2) missing survival data.
Outcome and follow-up
From baseline through 31 December 2019, the vital status and cause of death information were followed by the National Center for Health Statistics (obtained at: NCHS Data Linkage - Mortality Data - Public-Use Files (cdc.gov). Vital status was ascertained by probabilistic matching of subjects to the National Death Index based on social security number, name, sex and date of birth. Details of the linkage methods have been reported previously (obtained at: 2011 Linked Mortality Files Matching Metholodogy (cdc.gov). The follow-up time of the study was calculated from the NHANES 1999–2018 examination data until the last known data alive or censored through 31 December 2019 [23].
Mg intake assessment
Dietary Mg intake data were obtained through 24-h dietary recall interview. This interview was carried out via face-to-face at the Mobile Examination Center (MEC). The U.S. Department of Agriculture (USDA) Automated Multiple-Pass Method was utilized to collect dietary recall recorded during the physical health examination and participants were required to recall all of the food consumed in the 24-h prior to the interview, including information on the time of intake, amount and type food, and detailed food descriptions [24]. Then, each reported food item was linked to the USDA’s Food and Nutrient Database for Dietary Studies (FNDDS) to assign 8-digit food codes. The USDA’S food codes were also used to sort the foods into the What We Eat in America (WWEIA) food categories and subcategories. The dietary Mg intake was grouped into two groups in present study according to whether meeting the recommended nutrient intake (RNI). The RNI of dietary Mg was 400 and 420 mg/d for male aged 18–30 years and 31 years and above, respectively; 310 and 320 mg/d for female aged 18–30 years and 31 years and above, respectively [25].
Potential covariates
Demographic variables included age, gender, race and marital status. Smoking status was defined as never smokers, former smokers (smoking at least 100 cigarettes in life) and current smokers (smoking less than 100 cigarettes in life) [26]. Mg intake were calculated as the total of dietary Mg intake and Mg-supplement intake (supplements deficiency were counted as 0). Physical activity was presented as the metabolic equivalent task (MET) and calculated by the following formula: recommended MET × exercise time for corresponding activities (min/day) × the number of exercise days per week (day) [27].
The medical history data adopted in this study were determined on the basis of the medical condition questionnaires. Diabetes was defined as hemoglobin A1C (HbA1c) ≥ 6.5% or fasting glucose ≥ 126 mg/dL or 2 h oral glucose tolerance test (OGTT) blood glucose ≥ 200 mg/dL or diagnosed as diabetes by doctors [28]. Hypertension was defined as systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 80 mmHg or taking blood pressure medication or diagnosed as hypertension by doctors [29].Subjects with total cholesterol (TC) ≥ 200 mg/dL (5.2 mmol/L), or triglyceride (TG) ≥ 150 mg/dL (1.7 mmol/L), or low density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL (3.4 mmol/L), or high density lipoprotein cholesterol (HDL-C) ≤ 40 mg/dL (1.0 mmol/L), or diagnosed as hypercholesterolemia by doctors or receiving cholesterol-lowering therapy and lipid-lowering drugs were defined as dyslipidemia [30]. Cardiovascular disease (CVD) was assessed by the question of “Ever told you had angina or heart failure or heart attack or coronary heart disease or stroke or congestive heart failure "[30]. Cancer was assessed by “Ever told you had any cancer or malignancy”. The mean bone mineral density (BMD) of white females aged 20–29 years was used as the reference value. Osteoporosis was defined as participants with any BMD score of 2.5 standard deviations or more below the norm. Participants with all BMD values of 1.0 standard deviations or more above the norm were considered normal BMD, and other subjects were considered low bone mass [31]. Drugs that affect the absorption of Mg were identified based on participants’ self-reported use the tetracycline and loop diuretics.
Statistical analysis
All statistical tests were performed by SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). Continuous data were expressed as mean and standard error (S.E.), and the weighted t-test was used for comparison between all-cause deaths and survivor groups. Categorical data were described by case number and percentage [n (%)], and weighted χ2 test was utilized to compare the differences between all-cause deaths and survivor groups. Missing data imputation was conducted using multivariate imputation by chained equations (MICE). Sensitivity analyses were performed to compare whether the results were different before and after imputation (Supplementary Table S1). The weighted univariable and multivariate Cox proportional hazard models were used to explore the association between dietary Mg intake and RA patients’ all-cause mortality (Supplementary Table S2), described as hazard ratios (HRs), absolute risk difference (ARD) and 95% confidence intervals (CIs). Model I was a crude model. Model II was a fully adjusted model and accounted for age, race, PIR, osteoporosis, fracture, drug influence magnesium absorption, CVD, energy and calcium. The association was further explored through different subpopulations stratified by age, gender and BMI. Two-sided P-value < 0.05 was regarded as statistically significant.
Results
Characteristics of RA patients
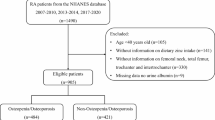
Figure 1 shows the flow chart of population screening. A total of 2,952 RA patient were screened. Among them, 324 patients missing complete dietary Mg intake information, 384 missing WBC measurement, 56 patients missing BMI data, 2 patients missing smoking status, and 5 patients missing survival data were excluded. Finally, 2,181 patients were included, with the mean age of 57.52 (0.40) years. Of which, 825 (37.83%) were all-cause deaths. The rate of dietary Mg intake above the RNI was significant higher in the survival group than in all-cause mortality group (29.48% vs. 18.60%). Characteristics of studied RA patients were presented in Table 1. Differences were found in age, race, PIR, marital status, physical activity, the history of osteoporosis, fracture, hypertension cancer CVD, cancer, diabetes and CKD, the use of antirheumatics and drug influence Mg absorption and the intake of energy, calcium and Mg between two groups (P < 0.05).
Dietary mg intake and all-cause mortality
The relationship of dietary Mg intake and RA patients’ all-cause mortality was shown in Table 2. After adjusted age, race, PIR, osteoporosis, fracture, drug influence mg absorption, CVD, energy and calcium in model II, compared with the unreached RNI of dietary Mg group, RA patients who reaching the RNI of dietary Mg had a 11.12% reduction of all-cause mortality (ARD=-11.12%; HR = 0.74, 95%CI: 0.56–0.99). After excluding the patients missing dietary Mg intake and survival information, we observed the association of dietary Mg intake and all-cause mortality in RA patients remain robust (Supplementary Table S3).
Dietary mg intake and all-cause mortality stratified by age, gender and BMI
Table 3 shown the relationship of dietary Mg intake and all-cause mortality among RA patients stratified by age, gender and BMI. After adjusted for all covariates, patients who reached the RNI of Mg was also associated with the lower all-cause mortality and this relationship was pronounced in patients with female (HR = 0.68, 95%CI: 0.47–0.98), aged < 65 years (HR = 0.59, 95%CI: 0.37–0.94) and BMI ≤ 30 kg/m2 (HR = 0.62, 95%CI: 0.42–0.91) (all P < 0.05), respectively.
Discussion
The association between dietary Mg intake and the all-cause mortality in RA patients was explored in this retrospective cohort study. We observed that there was a closely association of dietary Mg intake and all-cause mortality in RA patients. Keeping a higher dietary Mg intake may be a beneficial measure to improve the prognosis of RA patients.
The known pathogenesis of RA includes inflammatory cell infiltration, synovial hyperplasia, and bone destruction. A study quantifying causes of mortality of RA suggested inflammation mediated about a quarter of the excess relative RA mortality risk [8]. Therefore, reducing inflammation by all available means would be of great benefit in reducing the high mortality rate in RA population. In recent years, diet and nutrients, as potential environmental factors that affect the development and course of diseases, have attracted great attention from scholars. Nutrients regulate inflammatory states in the body, so it has emerged in nutrition science to classify the pro-inflammatory or anti-inflammatory properties of certain foods [32]. Similarly, this phenomenon applies to people with RA. More and more studies have pointed out Mg as common anti-inflammatory and antioxidant dietary nutrition has a great benefit to the alleviation of RA. Mg has a strong anti-inflammatory effect, and higher Mg intake has been proven to be related to lower inflammatory markers levels [33, 34]. Mg deficiency was related to an increased risk of many clinical diseases and mortality [35,36,37,38]. Short-term low Mg diet was related to elevated levels of inflammatory factors, which was consistent with findings proposed by Brenner et al. [38]. A prospective cohort study suggested that there was a negative relationship between high intake of antioxidant vitamins A, E, Mg and selenium and lower all-cause mortality [39]. A systematic review of Bagheri et al. [14] pointed out dietary Mg intake was related to a lower all-cause and cancer mortality, but not with CVD mortality. Moreover, no significant association was observed between supplemental and total Mg intake and the risk of all-cause, CVD and cancer mortality. In present study, dietary Mg intake was calculated as the total amount of dietary and supplement intake. The relationship between the source of dietary Mg intake and the all-cause mortality risk warrants attention in future studies.
Previous studies also shown the all-cause mortality risk was higher in female RA patients compared with male, which is consistent with our findings. RA has the characteristics of sexual dimorphism in clinical manifestations [40]. Women were 3-folds more likely to develop RA than men. Gender may show significant differences in autoimmune dysfunction and vaccination response [41, 42]. Because of differences in body composition and structure, women may be more susceptible than men to inflammation or immune burden during the process of sex differentiation [41]. The production of gonadal-specific hormones in women during special periods such as childbearing age, pregnancy, and lactation may affect the incidence or course of RA. One study has shown that disease activity tends to improve spontaneously in 75% of pregnant women, with episodes occurring in up to 90% of cases after delivery [43]. Mg plays a vital part in regulating endothelial function and is associated with circulatory markers of systemic inflammation and endothelial dysfunction in women [44, 45]. All these results indicate that gender should be carefully considered in the clinical treatment of RA population.
In the age stratification analyzed by subgroups in this study, we found the all-cause mortality risk in RA patients also showed significant differences in the factor of age and the association between all-cause mortality and dietary Mg intake was pronounced in RA patients who aged < 65 years. It is well known CVD is the most serious complication of RA and the important cause of death in RA patients [46]. An previous study shown that the incidence of CVD events in RA patients younger than 50 were 2.6-folds higher compared to the general population, while in old age the incidence was only 1.3 times [41]. With the deepening of clinical research on RA, scholars have found that increasingly serious pathological injury can aggravate the degree of destruction of articular cartilage and bone, increase the risk of osteoporosis fracture, and affect the quality of prognosis. A cohort study also showed that RA patients have a significantly higher risk of a first fracture before aged ≤ 50 years than patients aged > 50 years, taking into account reasons such as glucocorticoid use, smoking, and alcohol [41]. The pathogenesis of RA patients in the two age groups is different, so clinical medication should also be different. Joint deformation is more obvious in the middle-aged and elderly group, and the elderly group should pay more attention to the degree of organ involvement and other complications in addition to joint damage.
Moreover, we also found RA patients with low BMI had a higher all-cause mortality risk compared to overweight and obese patients. Studies generally believe that low BMI is a risk factor for osteoporosis [47]. Lean individuals with dietary Mg intake of < 200 mg/d versus > 200 mg/d are also at higher risk for hypertension, which may increase another key pathophysiological mediator associated with Mg deficiency.
Therefore, we provided reference for the management of RA based on the relationship of dietary Mg intake and the all-cause mortality. For clinicians and policymakers, as well as RA patients, it was essential to be aware of the benefits of dietary Mg for health management of RA. In addition, it was a beneficial move to add the Mg-enriched foods to dietary diet such as some nuts, legumes, and fiber-rich whole grains. Our study also has some limitations. First, despite the 24-h dietary recall interview was the valid method to obtain the dietary intake data, participants’ memory bias may bring a difficult to acquiring the accurate evaluation. Moreover, the weighted multifactor Cox regression model included as many covariates related to RA population as possible, but the confounding effects of unconsidered or unknown factors still could not be ruled out. Finally, information such as disease history in this study obtained through questionnaires may exist the recall bias, which may affect the results of the study.
Conclusion
Higher dietary Mg intake may be an efficient measurement to improve the prognosis of RA patients, especially in RA patients with female, aged < 65 years and BMI ≤ 30 kg/m2. Further large-scale prospective cohort study is needed to explore this beneficial effect of dietary Mg intake and health outcome of RA patients.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here NHANES, NHANES Questionnaires, Datasets, and Related Documentation (cdc.gov).
References
Woodworth TG, den Broeder AA. Treating to target in established rheumatoid arthritis: challenges and opportunities in an era of novel targeted therapies and biosimilars. Best Pract Res Clin Rheumatol. 2015;29(4–5):543–9. https://doi.org/10.1016/j.berh.2015.10.001.
Sparks JA. Rheumatoid Arthritis. Ann Intern Med. 2019;170(1):Itc1-itc16. https://doi.org/10.7326/aitc201901010.
Lin YJ, Anzaghe M, Schülke S. Update on the Pathomechanism, diagnosis, and Treatment options for Rheumatoid Arthritis. Cells. 2020;9(4). https://doi.org/10.3390/cells9040880.
Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(Suppl 3):S2. https://doi.org/10.1186/ar4174.
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, Buchbinder R, Woolf A, March L. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22. https://doi.org/10.1136/annrheumdis-2013-204627.
Løgstrup BB, Ellingsen T, Pedersen AB, Darvalics B, Olesen KKW, Bøtker HE, Maeng M. Cardiovascular risk and mortality in rheumatoid arthritis compared with diabetes mellitus and the general population. Rheumatology (Oxford). 2021;60(3):1400–9. https://doi.org/10.1093/rheumatology/keaa374.
Cheng CF, Liao HJ, Wu CS. Tissue microenvironment dictates inflammation and disease activity in rheumatoid arthritis. J Formos Med Assoc. 2022;121(6):1027–33. https://doi.org/10.1016/j.jfma.2022.01.026.
Videm V, Houge IS, Liff MH, Hoff M. Inflammation mediates approximately one quarter of excess relative all-cause mortality in persons with rheumatoid arthritis: the Trøndelag Health Study. Sci Rep. 2022;12(1):18599. https://doi.org/10.1038/s41598-022-21977-9.
Brandt B, Rashidiani S, Bán Á, Rauch TA. DNA methylation-governed gene expression in Autoimmune Arthritis. Int J Mol Sci. 2019;20(22). https://doi.org/10.3390/ijms20225646.
Moran-Moguel MC, Petarra-Del Rio S, Mayorquin-Galvan EE, Zavala-Cerna MG. Rheumatoid arthritis and miRNAs: a critical review through a functional view. J Immunol Res. 2018;2018. https://doi.org/10.1155/2018/2474529.
Bhaskaram P. Micronutrient malnutrition, infection, and immunity: an overview. Nutr Rev. 2002;60(5 Pt 2):S40–45. https://doi.org/10.1301/00296640260130722.
Childs CE, Calder PC, Miles EA. Diet and Immune function. Nutrients. 2019;11(8). https://doi.org/10.3390/nu11081933.
Arablou T, Aryaeian N, Djalali M, Shahram F, Rasouli L. Association between dietary intake of some antioxidant micronutrients with some inflammatory and antioxidant markers in active rheumatoid arthritis patients. Int J Vitam Nutr Res. 2019;89(5–6):238–45. https://doi.org/10.1024/0300-9831/a000255.
Bagheri A, Naghshi S, Sadeghi O, Larijani B, Esmaillzadeh A. Total, dietary, and Supplemental Magnesium Intakes and Risk of All-Cause, Cardiovascular, and Cancer Mortality: a systematic review and dose-response Meta-analysis of prospective cohort studies. Adv Nutr. 2021;12(4):1196–210. https://doi.org/10.1093/advances/nmab001.
Taşdemir Mecit BB, Orhan S. Magnesemia in COVID-19 ICU patients: the relationship between serum magnesium level and mortality. Magnes Res. 2022;35(3):80–7. https://doi.org/10.1684/mrh.2022.0504.
Amirkhizi F, Hamedi-Shahraki S, Rahimlou M. Dietary total antioxidant capacity is associated with lower disease severity and inflammatory and oxidative stress biomarkers in patients with knee osteoarthritis. J Health Popul Nutr. 2023;42(1):104. https://doi.org/10.1186/s41043-023-00450-x.
Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, Di Franco M. Dietary habits and Nutrition in Rheumatoid Arthritis: can Diet Influence Disease Development and Clinical manifestations? Nutrients. 2020;12(5). https://doi.org/10.3390/nu12051456.
Dana N, Karimi R, Mansourian M, Javanmard SH, Laher I, Vaseghi G. Magnesium intake and lung cancer risk: a systematic review and meta-analysis. Int J Vitam Nutr Res. 2021;91(5–6):539–46. https://doi.org/10.1024/0300-9831/a000598.
Mendes PMV, Bezerra DLC, Dos Santos LR, de Oliveira Santos R, de Sousa Melo SR, Morais JBS, Severo JS, Vieira SC. Do Nascimento Marreiro D. Magnesium in breast Cancer: what is its influence on the progression of this disease? Biol Trace Elem Res. 2018;184(2):334–9. https://doi.org/10.1007/s12011-017-1207-8.
Tian P, Xiong J, Wu W, Shi S, Chen A, Chen K, Chen W, Wu A, Liao Y. Impact of the malnutrition on mortality in rheumatoid arthritis patients: a cohort study from NHANES 1999–2014. Front Nutr. 2022;9(993061). https://doi.org/10.3389/fnut.2022.993061.
Li B, Chen L, Hu X, Tan T, Yang J, Bao W, Rong S. Association of serum uric acid with all-cause and Cardiovascular Mortality in Diabetes. Diabetes Care. 2023;46(2):425–33. https://doi.org/10.2337/dc22-1339.
Ostojic SM, Korovljev D, Stajer V. Food Sci Nutr. 2021;9(10):5746–54. https://doi.org/10.1002/fsn3.2543. Dietary intake of creatine and risk of medical conditions in U.S. older men and women: Data from the 2017–2018 National Health and Nutrition Examination Survey.
Hong C, Zhu H, Zhou X, Zhai X, Li S, Ma W, Liu K, Shirai K, Sheerah HA, Cao J. Association of Blood Urea Nitrogen with Cardiovascular diseases and all-cause mortality in USA adults: results from NHANES 1999–2006. Nutrients. 2023;15(2). https://doi.org/10.3390/nu15020461.
Dietary NHANES. Data 2021.
Tang M, Liu M, Zhang Y, Xie R. Association of family income to poverty ratio and vibration-controlled transient elastography quantified degree of hepatic steatosis in U.S. adolescents. Front Endocrinol (Lausanne). 2023;14(1160625). https://doi.org/10.3389/fendo.2023.1160625.
Kenwood BM, Zhu W, Zhang L, Bhandari D, Blount BC. Cigarette smoking is associated with acrylamide exposure among the U.S. population: NHANES 2011–2016. Environ Res. 2022;209(112774). https://doi.org/10.1016/j.envres.2022.112774.
Mendes MA, da Silva I, Ramires V, Reichert F, Martins R, Ferreira R, Tomasi E. Metabolic equivalent of task (METs) thresholds as an indicator of physical activity intensity. PLoS ONE. 2018;13(7):e0200701. https://doi.org/10.1371/journal.pone.0200701.
Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, Zhu K, Zhang X, Liu Y, Lin X, Chen L, Shan Z, Liu L, Pan A, Liu G. Associations of serum carotenoids with risk of Cardiovascular Mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. 2022;45(6):1453–61. https://doi.org/10.2337/dc21-2371.
Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160–4. https://doi.org/10.1016/j.tcm.2019.05.003.
Liang JH, Pu YQ, Liu ML, Hu LX, Bao WW, Zhang YS, Kakaer A, Zhao Y, Chen YC, Pu XY, Huang SY, Jiang N, Huang S, Dong GH, Chen YJ. Joint effect of whole blood metals exposure with dyslipidemia in representative US adults in NHANES 2011–2020. Environ Sci Pollut Res Int. 2023;30(42):96604–16. https://doi.org/10.1007/s11356-023-28903-0.
Looker AC, Orwoll ES, Johnston CC Jr., Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Min Res. 1997;12(11):1761–8. https://doi.org/10.1359/jbmr.1997.12.11.1761.
Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T. Strengthening the Immune System and reducing inflammation and oxidative stress through Diet and Nutrition: considerations during the COVID-19 Crisis. Nutrients. 2020;12(6). https://doi.org/10.3390/nu12061562.
Barbagallo M, Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes Res. 2009;22(4):235–46. https://doi.org/10.1684/mrh.2009.0187.
Cubała WJ, Landowski J, Dziadziuszko M, Chrzanowska A, Wielgomas B, Magnesium. C-reactive protein, and cortisol in drug-naïve patients with short illness-duration, first episode major depressive disorder: possible immunomodulatory role for magnesium. Magnes Res. 2016;29(4):169–74. https://doi.org/10.1684/mrh.2016.0413.
Shi Z, Abou-Samra AB. Association of low serum magnesium with diabetes and hypertension: findings from Qatar Biobank study. Diabetes Res Clin Pract. 2019;158(107903). https://doi.org/10.1016/j.diabres.2019.107903.
Kostov K. Effects of Magnesium Deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. 2019;20(6). https://doi.org/10.3390/ijms20061351.
Faa G, Saba L, Fanni D, Kalcev G, Carta M. Association between Hypomagnesemia, COVID-19, respiratory tract and lung disease. Open Respir Med J. 2021;15:43–5. https://doi.org/10.2174/1874306402115010043.
Groenendijk I, van Delft M, Versloot P, van Loon LJC, de Groot L. Impact of magnesium on bone health in older adults: a systematic review and meta-analysis. Bone. 2022;154(116233). https://doi.org/10.1016/j.bone.2021.116233.
Wang W, Wang X, Cao S, Duan Y, Xu C, Gan D, He W. Dietary antioxidant indices in relation to all-cause and cause-specific mortality among adults with diabetes: a prospective cohort study. Front Nutr. 2022;9(849727). https://doi.org/10.3389/fnut.2022.849727.
Kovacs WJ, Olsen NJ. Sexual dimorphism of RA manifestations: genes, hormones and behavior. Nat Rev Rheumatol. 2011;7(5):307–10. https://doi.org/10.1038/nrrheum.2010.231.
Favalli EG, Biggioggero M, Crotti C, Becciolini A, Raimondo MG, Meroni PL. Sex and management of rheumatoid arthritis. Clin Rev Allergy Immunol. 2019;56(3):333–45. https://doi.org/10.1007/s12016-018-8672-5.
Ghosh S, Klein RS. Sex drives dimorphic Immune responses to viral infections. J Immunol. 2017;198(5):1782–90. https://doi.org/10.4049/jimmunol.1601166.
Barbagallo M, Dominguez LJ, Galioto A, Pineo A, Belvedere M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res. 2010;23(3):131–7. https://doi.org/10.1684/mrh.2010.0214.
Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102(19):2353–8. https://doi.org/10.1161/01.cir.102.19.2353.
Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr. 2007;85(4):1068–74. https://doi.org/10.1093/ajcn/85.4.1068.
Dijkshoorn B, Raadsen R, Nurmohamed MT. Cardiovascular Disease Risk in Rheumatoid Arthritis Anno 2022. J Clin Med. 2022;11(10). https://doi.org/10.3390/jcm11102704.
Simkova SS, Dvorackova O, Velemínsky M. Assessment of healthy lifestyles in relation to BMI. Neuro Endocrinol Lett. 2022;43(7–8):393–9.
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
(1) Long Xiong, conceiving and designing the study; (2) Hantian Liu, collecting the data; (3) Hantian Liu, analyzing and interpreting the data; (4) Hantian Liu, Kui Zhang, writing the manuscript; (5) Long Xiong, providing critical revisions that are important for the intellectual content; (6) Hantian Liu, Kui Zhang, Long Xiong, approving the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not provided for this study on human participants because NHANES is a publicly available dataset. The patients/participants provided their written informed consent to participate in this study. According to the Ethics Review Board of Second Affiliated Hospital of Nanchang University, cross-sectional studies have been exempted from the ethical review.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
all authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, H., Zhang, K. & Xiong, L. Dietary magnesium intake and rheumatoid arthritis patients’ all-cause mortality: evidence from the NHANES database. J Health Popul Nutr 43, 112 (2024). https://doi.org/10.1186/s41043-024-00597-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-024-00597-1