Abstract
Background
Gene–diet interaction is related to the progression of diabetes and cardiovascular diseases biomarkers. We aimed to evaluate the interaction between diet quality indices and BDNF Val66Mat (rs6265) on cardiometabolic markers among diabetic patients.
Methods
This cross-sectional study was conducted on 634 patients with type 2 diabetes mellitus, which were randomly recruited from diabetic centers in Tehran. Dietary intakes were estimated by a previously validated semi-quantitative food frequency questionnaire comprising 147 items. All participants were categorized into three categories, based on healthy eating index (HEI), diet quality index (DQI), and phytochemical index (PI) scores. Polymerase chain reaction was used for genotyping the BDNF Val66Met. Interactions were tested using analysis of covariance in adjusted and crude models.
Results
Our result showed that higher DQI, HEI, and PI scores significantly decrease body mass index and waist circumference among individuals with Met/Met, Val/Met, and Val/Val genotypes (P interactions < 0.05). Moreover, the highest quartile of the DQI and PI, compared to the lowest, showed lower TG level among Met allele carriers compared to Val/Val homozygotes (P interaction = 0.004 and 0.01, respectively) and a faster reduction in IL-18 and TC level was seen among Met/Met, Val/Met who had higher HEI intake than those with Val/Val genotype.
Conclusions
BDNF Val66Met polymorphism may interact with HEI, DQI, and PI. We have revealed that Met allele acts as a protective allele for diabetic patients and may have a beneficial influence on cardio-metabolic factors through regulating dietary intake.
Similar content being viewed by others
Background
Type 2 diabetes mellitus (T2DM) as a major multi-factorial chronic health concern is rising rapidly worldwide, and cardiovascular diseases (CVDs) are known as the most common disorders and the main cause of death among patients with T2DM [1]. Conclusive evidence suggests a genetic basis for T2DM and the development of its complications [2]. One of the key target genes which might be involved in cardiometabolic functions via different mechanisms is brain-derived neurotrophic factor (BDNF), which plays a significant role in the regulation of eating behavior and energy expenditure among both animals and human via inhibition of food intake and increasing physical activity [3, 4]. It is thought that Met-allele carriers are related to higher intake of carbohydrate, protein, and total energy and contributes to the modulation of insulin, leptin, ghrelin, and pro-inflammatory cytokines levels [5]. However, some studies found no association [6]. Since eating habits play a central role in the onset and progression of chronic diseases, especially T2DM and CVDs, significant improvement in diabetic dyslipidemia, obesity, and other cardiometabolic risk factors can be achieved via diet management [7]. To determine the nutritional status of individuals, changes in food choices and consumption, and their effect on health, overall diet quality indices have been developed [8]. Healthy eating index (HEI) and diet quality index-international (DQI-I) encourage people to have a diversified, balanced, and healthy diet [9], and phytochemical index (PI), shows the amount of phytochemicals intake in a diet [10].
A few studies have evaluated the association between DQI and HEI with CVDs risk factors among diabetic patients. An inverse relationship between HEI and obesity but no relationship between DQI and body mass index (BMI) has been reported in a recent review among Chinese people [11]. Also, two different Iranian research demonstrated an inverse link between higher HEI scores with a lower risk of obesity and an increased risk of metabolic syndrome [12]. It seems that higher HEI scores which reflect a healthy dietary pattern rich in plant foods and high fiber content play an important role in lowering abdominal obesity, BMI, and waist circumference (WC) [13]. Conversely, a study on elderly Iranian people reported reduced CVDs risk factors among individuals with higher HEI scores [14]. Moreover, a cross-sectional study on diabetic women showed no association between HEI, DQI, and CVDs risk factors [15]. These contradictory results might be related to genetic variations [16]. Since numerous studies highlighted gene-based personalized dietary recommendations for improving health outcomes [17], more gene–diet interactions are needed to focus not only on the association between macro- and micronutrient intake and genetics but also on the impact of dietary patterns on genetics and metabolic health. However, there are limited studies investigating the interactions between BDNF Val66Met and dietary intake on metabolic markers. A research revealed lower prevalence of diabetes and its complication among adults with Val/Met genotype who had low energy and protein intake [18]. A recent interaction study on diabetic patients indicated higher TG and lower HDL-C levels among Val/Met carriers with higher dietary antioxidant capacity [5]. To our knowledge, there is no research investigating the interaction between HEI, DQI, PI, and BDNF polymorphisms on anthropometric and cardiometabolic risk factors. This study aimed to investigate the possible interactions between BDNF Val66Met and dietary indices on metabolic markers among T2DM patients in Iran.
Methods
Study design and subjects
This study is part of a larger investigation in which 634 T2DM patients (252 men and 382 women) aged 35–65 years were randomly recruited from diabetes centers such as the Iranian diabetes society and other health centers in Tehran [19]. This group is defined as having either fasting blood sugar (FBS) levels ≥ 126 mg/dl and consuming glucose-lowering medicines. The exclusion criteria were as follows: being under 35 or over 65 years old, insulin-using patients, pregnant, or lactating women.
General information including age, gender, job, smoking and alcohol abuse, lipid-lowering drug consumption, family history of T2DM, and other diseases was collected through interviews. Anthropometric data (body mass index (BMI) and WC) and physical activity (METs) information were taken according to standard protocols [19]. Physical activity was calculated as the metabolic equivalent of task (MET h/day) [20] by a validated and reliable physical activity questionnaire [21]. The study was conducted based on the Declaration of Helsinki and was approved by the ethics committee of Tehran University of Medical Sciences (TUMS) (no. 15060), and the written consent form was obtained from all participants.
Assessment of dietary intake and indices
The participant’s usual dietary intake during the last year was evaluated through face-to-face interviews with a trained dietitian and using a semiquantitative food frequency questionnaire (FFQ) for 147 food items. This questionnaire was validated by Esmaillzadeh et al. [22]. The subjects were asked to report the frequency of food item consumption in a day, a week, a month, or a year. The amounts listed for each food were converted to grams per day using household measures [19]. Nutritionist III software (version 7.0, N-Squared Computing) was employed to assess energy and nutrient intake.
Three dietary indices were used for evaluating diet quality including the Healthy Eating Index (HEI), assessing dietary intake according to the 2015–2020 dietary guidelines for Americans (DGA) based on a 1000 kcal/day diet. HEI-2015 score ranged from 0 to 100 and consisted of 13 components (vegetables, fruits, beans, seafood or plant-based and total protein foods can receive a score ranging from 0 to 5, whole grains, dairy, fatty acids can receive a score ranging from 0 to 5 and refined grains, saturated fats, sodium, and added sugars are moderated) (higher intakes receive lower scores) [23]. We calculated the score based on responses from the FFQs.
The Diet Quality Index-International (DQI-I) was a second dietary measurement that focuses on four main aspects of a healthy diet (variety, adequacy, moderation, and overall balance). The score for each category is calculated as the sum of the scores for each component in that category. Overall food group variety includes meat/poultry/fish/eggs/dairy/beans/grain/fruit/vegetable (0–15 points), and within-group variety for protein source includes meat/poultry/fish/dairy/beans/eggs (0–5 points), protein sources, vegetable, fruit, grain, fiber, protein, iron, calcium, vitamin C group (0–5 points), moderation foods such as saturated and total fat, cholesterol (CL), sodium and junk foods (0–30 points), macronutrient, and fatty acid (0–10 points) [24]. The total DQI-I score (ranging from 0 to 100 points) is the sum of the scores for the four categories.
Moreover, phytochemical index (PI) is known as the percentage of calorie intake derived from foods rich in phytochemicals including fruits, vegetables, whole grains, nuts, seeds, vegetable juices, soy products, and olive oil. The dietary phytochemical index (DPI) was calculated according to the modified method previously developed by McCarty [25]; [PI = (phytochemical-rich foods g/d/ total food intake g/d) × 100].
Biochemical assessment and genotyping
An overnight fasting venous blood sample was collected for each subject; the total antioxidant capacity (TAC) [26] of serum was measured by spectrophotometry. It evaluates the overall power of all antioxidants in the body [27]. Serum enzymatic activity of superoxide dismutase (SOD), known as an enzymatic antioxidant [27], was assessed by colorimetric method (Cayman Chemical Company, USA). Interleukin-18 (IL-18), pentrexin-3 (PTX3), and 8-isoprostane F2α (PGF2α) were measured using ELISA method (Shanghai Crystal Day Biotech Co., Ltd). The sensitivity of IL-18 and PTX3 ELISA kit was 28 ng/l and 0.05 ng/ml, respectively. Genomic DNA was isolated from whole blood using salting-out extraction method [28]. Polymerase chain reaction (PCR) was used for genotyping the BDNF Val66Met, followed by 8% polyacrylamide gel electrophoresis. PCR amplification of rs6265 polymorphism was performed by the following primers: forward, 5′-CACTAGCCCAGAGAGAGGAGTG-3′, Reverse, 50-TGAGCCCAGCCGCACACTAAC.
Statistical analysis
Normal distribution of data was measured using Kolmogorov–Simonov test. Logarithmic transformations were applied to variables with skewed distribution. Participants were divided into two groups: those with Val/Val and Val/Met genotypes versus those of Met/Met homozygotes (Met/Met group). The data were presented as frequency (%) for categorical variables and as mean ± SD for continuous variables. An independent T test was used to compare the quantitative variables between the two groups, and Chi-square test was used to compare the qualitative variables.
The association between diet quality indices and anthropometric or biochemical parameters was evaluated by one-way analysis of variance (ANOVA). The interactions between BDNF Val/Met polymorphism and DEI, DQI, and PI on BMI, WC, TC, HDL-C, LDL-C, LDL/HDL, TG, CRP, PTX3, IL18, TAC, SOD, PGF2α, leptin, and ghrelin were tested using analysis of covariance (ANCOVA) test in two multivariate interaction models, before and after adjustment for potential confounders including age, sexuality, smoking, alcohol consumption, and physical activity. The data were analyzed by IBM SPSS (SPSS Inc., Chicago, IL, USA, version 26), and P value < 0.05 was considered statistically significant.
Results
This cross-sectional study was conducted on 634 T2DM patients with sex distribution of 39.7% and 60.3% in men and women, respectively. The prevalence of the Val66Met genotype among study participants was as follows: The Met allele carrier group had a frequency of 44.2% (Val/Met + Met/Met), and accordingly, the major allele frequency was 55.8% (Val/Val). Anthropometric, general characteristics, nutrient intakes, and comparison of clinical parameters among participants according to BDNF Val66Met genotypes are presented in Tables 1 and 2, respectively. Leptin level was significantly higher among Met allele carriers, while the ghrelin concentration was significantly higher among Val homozygotes (P = 0.008 and P = 0.02, respectively). Although we found no significant difference regarding other clinical and general parameters across BDNF Val66Met genotypes among the groups, it was seen that Met allele carriers (Met/Met + Val/Met) had nominally significantly higher energy intakes and correspondingly higher intakes of all macronutrients (fat, protein, and carbohydrate) than those with Val/Val genotype.
Association between cardiometabolic markers and dietary indices
All participants were categorized into three tertiles, based on HEI, DQI, and DPI scores. Analysis for general and biochemical markers between the tertiles is presented in Table 3. Patients in the first quartile of HEI, DQI, and DPI were more likely to be obese. They had higher BMI levels (P = 0.01, P = 0.02, and P = 0.03, respectively). Also, WC level was higher among patients in the first quartiles of HEI and DPI (P = 0.01 and P = 0.08, respectively). Patients in the last quartile of HEI and DPI had higher levels of LDL-C (P = 0.007 and P = 0.00, respectively). Moreover, a higher level of HDL-C was seen among the patients in the last quartile of HEI (P = 0.01). Regarding HEI, greater adherence reduced the inflammatory markers. There were lower PGF2α (P = 0.03) and PTX (P = 0.02) levels among patients in the second and third quartile, respectively.
Interaction between dietary indices with BDNF Val66Met variants on cardiometabolic markers
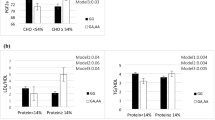
Significant interactions between BDNF Val66Met polymorphism and DQI, HEI, and PI scores on cardiometabolic markers are shown in Figs. 1, 2, and 3. Significant interactions were observed between HEI and Val66Met polymorphism for BMI and WC (P = 0.001 and P = 0.0, respectively). Our results revealed that higher scores for all diet-quality indices are significantly associated with lower BMI and WC values among all participants. It remained consistently significant even after adjusting for various potential confounders including age, weight, height, and physical activity. Also, interactions between DQI and DPI and Val66Met polymorphism for BMI and WC were significant after adjustment.
The interaction between BDNF Val66Met genotypes and quartiles of diet quality index (DQI) on: A body mass index (BMI), B waist circumference (WC), C total antioxidant capacity (TAC), D superoxide dismutase (SOD), E leptin mean, F high-density lipoprotein (HDL) and standard error. P values for the interaction obtained in two models using ANCOVA. P*: unadjusted; P**: adjusted for age, sexuality, smoking, alcohol consumption, and physical activity
The interaction between BDNF Val66Met genotypes and quartiles of healthy eating diet (HEI) on: A body mass index (BMI), B waist circumference (WC), C pentraxins (PTX), D prostaglandin F2 Alpha (PGF2) E leptin mean, F high-density lipoprotein (HDL) mean and standard error. P values for the interaction obtained in two models using ANCOVA. P*: unadjusted; P**: adjusted for age, sexuality, smoking, alcohol consumption and physical activity
The interaction between B. DNF Val66Met genotypes and quartiles of dietary phytochemical index (PI) on: A body mass index (BMI), B waist circumference (WC) C leptin mean, D high-density lipoprotein (HDL) mean and standard error. P values for the interaction obtained in two models using ANCOVA. P*: unadjusted; P**: adjusted for age, sexuality, smoking, alcohol consumption, and physical activity
The highest quartile of DQI was associated with elevated levels of SOD among individuals with Val/Met and Met/Met (P interaction = 0.01).
Discussion
This cross-sectional study aimed to assess the interactions between BDNF Val66Met polymorphism and HEI, DQI, and DPI on anthropometric and metabolic markers among diabetic patients. We found patients with lower scores of HEI, DQI, and DPI were more likely to be obese. There was a significant decreasing trend in the odds of BMI and WC across increasing quartiles of all measured dietary indices. Also, WC level was significantly lower among diabetic patients with higher scores for HEI and DPI. It is noteworthy that patients in the highest group of DQI and DPI had lower total fat intake including saturated fats and dietary cholesterol and lower TC level compared with those in the lower quartiles. In addition, higher protein intake was observed among the highest quartile of all the indices compared to the lower groups. A few studies which have investigated the association between dietary indices and anthropometric or cardiometabolic risk factors, reported controversial results. The inverse association between diet quality indices and obesity was approved by some previous studies [29, 30]. Quatromoni et al. found an inverse relationship between higher adherence to DQI and lower weight gain in an 8-year cohort study [31]. It has been demonstrated that unhealthy dietary patterns, rich in saturated fatty acids (SFAs), sugar, and red or processed meat which have low scores for HEI promote fat accumulation in different adipose tissues, particularly abdominal fat which is strongly related to the risk of metabolic disorders and atherogenesis [32]. However, the exact mechanism of diet quality scores on BMI values is not fully understood [33].
It should be considered that monitoring the overall eating patterns which reflect the intake of all food groups is more effective for finding about diet quality than investigating the effects of particular micro- or macronutrients [34]. Surprisingly, we observed a significant decrease in BMI and WC among those who had higher energy intake. However, higher scores for HEI were directly associated with higher energy and fat intake and showed a healthier dietary pattern among patients including lower intake of saturated fats and higher consumption of protein and healthier oils rich in polyunsaturated fatty acids (PUFAs) when compared to those with lower scores who consumed less total energy but more saturated fatty acids and cholesterol. Several studies have shown higher HEI scores are directly linked to healthier lifestyles and eating behaviors [35, 36]. As previous studies suggested, saturated fatty acid intake has been known as important nutrient responsible for weight gain in modern societies and is inversely associated with WC [37]. It has been suggested that the saturation level of fats in the diet contributes to the rate of fat storage as well as the amount of fat intake [10, 38]. A study that examined the effect of fatty acid composition of the diet in mice, revealed a diet rich in PUFAs prevents myocellular lipid accumulation. It showed that the highest increase in BMI was associated with a diet rich in palmitic acid (C16:0) [10]. This finding was in line with other reports indicating that there is a preference for oxidizing PUFAs due to oxidizing more rapidly than saturated fatty acids, while saturated fat seems to be stored in muscle rather than oxidizing for energy [39, 40]. It has been shown that TG composition in adipose tissue reflects habitual dietary fat intake over a short and long time [41, 42]. Our result would be another approval for this relationship. As expected, higher PUFAs and lower SFAs intake among diabetic patients in the highest quartile of HEI were also associated with a decrease in levels of LDL-C and inflammatory markers (PTX, and PGF2α) and an increase in HDL-C. Although some research among Iranians reported no significant association between HEI/AHEI and lipid profile, CRP level, and other CVDs risk factors [43, 44], several reported little association between DQI or HEI and inflammatory biomarkers [45] or showed this relationship only among men, not women [35].
To our knowledge, the association between diet quality indices and BMI or metabolic markers has been widely investigated. However, a few recent studies have highlighted gene-nutrition interaction on health outcomes [5, 42]. Thus, the current study aimed to investigate the possible interplay between dietary indices (HEI, DQI, and DPI) and BDNF Val66Met polymorphism in relation to cardiometabolic markers among patients with T2DM.
We present novel findings regarding the interaction between HEI, DQI, DPI, and BDNF Val66Met polymorphism on BMI and WC levels. Moreover, we found significant interactions between DQI and Val66Met polymorphism on SOD, HDL, and leptin, between HEI and the Val66Met polymorphism on PGF2α, HDL, and leptin levels, and between DPI and this polymorphism on HDL levels. We observed BDNF variants affected daily dietary intake such as total energy, carbohydrates, fiber, protein, and fat in diabetic patients. Generally, Met allele carrier group had significantly higher nutritional intake compared to subjects with Val/Val genotype. It might be related to their leptin level, which basically was higher among Met allele carriers. They were also more likely to increase their leptin level in higher scores of HEI and DQI. Further major finding is related to a significant decrease in cardiometabolic risk factors among diabetic patients with higher adherence to HEI and DQI. Met allele carriers were more likely to have experienced a faster reduction in IL-18 and TC levels by having higher HEI intake compared to those with Val/Val genotype. Interestingly, Met allele carriers who consumed diets higher in DQI had a significant decrease in TG level, while it showed an increase for those carrying Val/Val. Several studies have reported that the relationship between T2DM and BDNF Val66Met depends on diet [18, 46]. They found a positive relationship between BDNF and obesity or type 2 diabetes development in humans due to its role in eating behavior and energy homeostasis. Xian-Yong Ma et al. reported a significant interaction between BDNF Val66Met and PUFAs intake on elevated risk of obesity [47].
Abaj et al. showed a significant interaction between dietary insulin index and dietary insulin load scores and BDNF Val66Met on BMI level [48]. Consistent with our result, a study showed that the risk of T2DM was lower among participants with Val/Met genotype even with a diet rich in carbohydrates, while Val/Val participants with lower energy intake were more likely to develop diabetes [18]. Previous studies that first demonstrated in rats showed that BDNF modulates metabolic function by affecting appetite and controlling food intake, energy metabolism, and insulin sensitivity [49, 50]. Moreover, BDNF contributes to the regulation of energy metabolism through peripheral neurons which are involved in the maintenance of energy balance [51]. It has been shown previously that hyperphagia, obesity, and metabolic imbalances are related to BDNF deletion in both knockout mice and humans [52, 53]. So, it is not surprising that there is a lower circulating BDNF among diabetic patients and individuals with obesity or metabolic disorder compared with healthy people [54, 55]. Nakagawa et al. revealed that heterozygous BDNF knockout mice are hyperphagic and obese and weight loss due to appetite suppression has occurred in BDNF-infused diabetic mice [56]. Furthermore, Met allele carriers have been shown to have lower plasma BDNF levels compared to Val/Val homozygotes [57, 58]. On the other hand, BDNF expression can regulate by nutritional intake. A recent study on rodents reported higher BDNF expression after a high-fat diet [59]. Gyorkos et al. found that a carbohydrate-restricted diet increases BDNF levels in Met allele carriers [53]. Moreover, a recent study showed high glycemic index foods have a negative effect on cardiometabolic factors among Val/Val participants possibly due to lowering BDNF levels [48]. Studies found a protective effect among Met allele carriers even in high-energy and high-carbohydrate diets [18, 48]. The present study revealed the positive effect of high HEI and DQI on lipid profile especially among Met allele carriers when compared to Val/Val. Although the intake of SFAs and CL has been decreased and PUFAs intake has been increased among both genotypes in higher quartiles for HEI, we revealed that increased HEI did not influence on negative consequences of Val/Val genotype in terms of TG and CL levels. Similar to what has been seen among Val/Val carriers with higher DQI and DPI intake. Despite increased fiber intake and decreased cholesterol consumption among both genotypes, IL-18 and TG levels decreased only among Met carrier group. Moreover, we have seen that increased physical activity and fiber intake in higher DPI scores could decrease TAC, SOD, and PGF2α levels in Met/Met and Val/Met, while these factors have been increased among individuals with Val/Val genotype even after having less fat intake. We found an especially interesting protective impact for Met allele among diabetic patients. It is suggested that diet quality indices may regulate the association between BDNF Val66Met and cardiometabolic factors by making changes in BDNF expression.
The main limitation of this research is that we could not measure the blood sugar and serum BDNF levels of participants due to time and budget limitations. So, we missed potentially relevant interactions. Besides, although we controlled for several potential confounders, the effects of remaining confounders cannot be ignored. Thus, further investigations in different populations are needed to confirm the findings.
Conclusions
The present study revealed a protective effect of Met allele against cardiometabolic markers through modulating dietary patterns among individuals with T2DM. Participants with Met/Met and Val/Met genotypes who had higher DQI and DPI intake have experienced a significant decrease in TG level, while it has increased in homozygous patients for Val allele. Moreover, a major decrease in BMI levels among all patients with higher adherence to HEI, DQI, and DPI was seen. However, it has been shown that consuming higher PUFAs is much more effective for lowering the risk of obesity rather than lower energy and fat intake, especially among those with higher HEI.
Availability of data and materials
The data are not publicly available due to containing private information of participants. Data are available from the authors upon reasonable request. Correspondence and requests for materials should be addressed to F.K. and M.R.
Abbreviations
- CVDs:
-
Coronary vascular diseases
- HEI:
-
Healthy eating index
- DQI:
-
Diet quality index
- PI:
-
Phytochemical index
- PCR:
-
Polymerase chain reaction
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- SNPs:
-
Single-nucleotide polymorphisms
- BDNF:
-
Brain-derived neurotrophic factor
- DQI-I:
-
Diet quality index-international
- PI:
-
Phytochemical index
- DPI:
-
Dietary phytochemical index
- FBS:
-
Fasting blood sugar
- TAC:
-
Total antioxidant capacity
- SOD:
-
Superoxide dismutase
- IL-18:
-
Interleukin-18
- PTX3:
-
Pentrexin-3
- PGF2α:
-
8-Isoprostane F2α
- PUFAs:
-
Polyunsaturated fatty acids
- SFAs:
-
Saturated fatty acids
- CL:
-
Cholesterol
References
Naeini Z, Toupchian O, Vatannejad A, Sotoudeh G, Teimouri M, Ghorbani M, et al. Effects of DHA-enriched fish oil on gene expression levels of p53 and NF-κB and PPAR-γ activity in PBMCs of patients with T2DM: a randomized, double-blind, clinical trial. Nutr Metab Cardiovasc Dis. 2020;30(3):441–7.
Abaj F, Rafiee M, Koohdani F. Interaction between CETP polymorphism and dietary insulin index and load in relation to cardiovascular risk factors in diabetic adults. Sci Rep. 2021;11(1):1–10.
Xu B, Xie X. Neurotrophic factor control of satiety and body weight. Nat Rev Neurosci. 2016;17(5):282–92.
Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45.
Abaj F, Rafiee M, Koohdani F. Interaction between dietary total antioxidant capacity and BDNF Val66Met polymorphism on lipid profiles and atherogenic indices among diabetic patients. Sci Rep. 2021;11(1):1–19.
Vidović V, Maksimović N, Novaković I, Damnjanović T, Jekić B, Vidović S, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with body mass index, fasting glucose levels and lipid status in adolescents. Balk J Med Genet BJMG. 2020;23(1):77.
Sami W, Ansari T, Butt NS, Ab Hamid MR. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci. 2017;11(2):65.
Chen G-C, Koh W-P, Neelakantan N, Yuan J-M, Qin L-Q, van Dam RM. Diet quality indices and risk of type 2 diabetes mellitus: the Singapore Chinese health study. Am J Epidemiol. 2018;187(12):2651–61.
Guerrero MLP, Pérez-Rodríguez F, Hueda M. Diet quality indices for nutrition assessment: types and applications. Funct Food Improve Health Through Adequate Food. 2017;1:283–308.
Apgar JL, Shively CA, Tarka SM Jr. Digestibility of cocoa butter and corn oil and their influence on fatty acid distribution in rats. J Nutr. 1987;117(4):660–5.
Kadam I, Neupane S, Wei J, Fullington LA, Li T, An R, et al. A systematic review of diet quality index and obesity among Chinese adults. Nutrients. 2021;13(10):3555.
Saraf-Bank S, Haghighatdoost F, Esmaillzadeh A, Larijani B, Azadbakht L. Adherence to healthy eating index-2010 is inversely associated with metabolic syndrome and its features among Iranian adult women. Eur J Clin Nutr. 2017;71(3):425–30.
Asghari G, Mirmiran P, Rashidkhani B, Asghari-Jafarabadi M, Mehran M, Azizi F. The association between diet quality indices and obesity: Tehran lipid and glucose study. Arch Iran Med. 2012;15(10):599–605.
Rashidipour-Fard N, Karimi M, Saraf-Bank S, Baghaei MH, Haghighatdoost F, Azadbakht L. Healthy eating index and cardiovascular risk factors among Iranian elderly individuals. ARYA Atheroscler. 2017;13(2):56.
Daneshzad E, Larijani B, Azadbakht L. Diet quality indices and cardiovascular diseases risk factors among diabetic women. J Sci Food Agric. 2019;99(13):5926–33.
Tudor L, Konjevod M, NikolacPerkovic M, SvobStrac D, NedicErjavec G, Uzun S, et al. Genetic variants of the brain-derived neurotrophic factor and metabolic indices in veterans with posttraumatic stress disorder. Front Psychiatry. 2018;9:637.
Toro-Martín D, Arsenault BJ, Després J-P, Vohl M-C. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients. 2017;9(8):913.
Daily JW, Park S. Interaction of BDNF rs6265 variants and energy and protein intake in the risk for glucose intolerance and type 2 diabetes in middle-aged adults. Nutrition. 2017;33:187–94.
Rafiee M, Sotoudeh G, Djalali M, Alvandi E, Eshraghian M, Javadi F, et al. The interaction between apolipoprotein b insertion/deletion polymorphism and macronutrient intake on lipid profile and serum leptin and ghrelin levels in type 2 diabetes mellitus patients. Eur J Nutr. 2019;58(3):1055–65.
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80.
Klishadi R, Khosravi A, Famouri F, Sadeghi M, Shirani S. Assessment of physical activity of adolescents in Isfahan. J Shahrekord Univ Med Sci. 2001;3(2):55–66.
Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain consumption and the metabolic syndrome: a favorable association in Tehranian adults. Eur J Clin Nutr. 2005;59(3):353–62.
Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602.
Kim S, Haines PS, Siega-Riz AM, Popkin BM. The diet quality index-international (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr. 2003;133(11):3476–84.
McCarty MF. Proposal for a dietary “phytochemical index.” Med Hypotheses. 2004;63(5):813–7.
Borén J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. J Clin Investig. 1998;101(5):1084–93.
Zamani E, Sadrzadeh-Yeganeh H, Sotoudeh G, Keramat L, Eshraghian M, Rafiee M, et al. The interaction between ApoA2−265T> C polymorphism and dietary fatty acids intake on oxidative stress in patients with type 2 diabetes mellitus. Eur J Nutr. 2017;56(5):1931–8.
Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215.
Tande DL, Magel R, Strand BN. Healthy eating index and abdominal obesity. Public Health Nutr. 2010;13(2):208–14.
De Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34(5):1150–6.
Quatromoni PA, Pencina M, Cobain MR, Jacques PF, D’agostino RB. Dietary quality predicts adult weight gain: findings from the Framingham offspring study. Obesity. 2006;14(8):1383–91.
Monfort-Pires M, Folchetti LD, Previdelli AN, Siqueira-Catania A, de Barros CR, Ferreira SRG. Healthy eating index is associated with certain markers of inflammation and insulin resistance but not with lipid profile in individuals at cardiometabolic risk. Appl Physiol Nutr Metab. 2014;39(4):497–502.
Osadnik K, Osadnik T, Lonnie M, Lejawa M, Reguła R, Fronczek M, et al. Metabolically healthy obese and metabolic syndrome of the lean: the importance of diet quality. Analysis of MAGNETIC cohort. Nutr J. 2020;19(1):1–13.
Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, et al. Rationale and design of the dietary approaches to stop hypertension trial (DASH): a multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5(2):108–18.
Drewnowski A, Fiddler EC, Dauchet L, Galan P, Hercberg S. Diet quality measures and cardiovascular risk factors in France: applying the healthy eating index to the SU. VI. MAX study. J Am Coll Nutr. 2009;28(1):22–9.
McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the alternate healthy eating index. Public Health Nutr. 2006;9(1a):152–7.
Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ. 2012;345:e7666.
Chen IS, Subramaniam S, Vahouny GV, Cassidy MM, Ikeda I, Kritchevsky D. A comparison of the digestion and absorption of cocoa butter and palm kernel oil and their effects on cholesterol absorption in rats. J Nutr. 1989;119(11):1569–73.
Leyton J, Drury P, Crawford M. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br J Nutr. 1987;57(3):383–93.
DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72(4):905–11.
Hirsch J, Farquhar JW, Ahrens E Jr, Peterson ML, Stoffel W. Studies of adipose tissue in man: a microtechnic for sampling and analysis. Am J Clin Nutr. 1960;8(4):499–511.
Dayton S, Hashimoto S, Dixon W, Pearce ML. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J Lipid Res. 1966;7(1):103–11.
Khakpouri S, Safari M, Ghazizadeh H, Parizadeh SMR, Nematy M, Tayefi M, et al. The relationship between the healthy eating index and an alternate healthy eating index with the risk factors for cardiovascular disease in a population from northeastern Iran. Transl Metab Syndr Res. 2019;2(1):1–6.
Li J, Demirel A, Azuero A, McLain A, Yarar-Fisher C. The relationship between healthy eating index-2015 and cardiometabolic risk factors in people with long-standing spinal cord injury. Curr Dev Nutr. 2020;4(Supplement_2):540.
Fung TT, McCullough ML, Newby P, Manson JE, Meigs JB, Rifai N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–73.
Han J. Rare syndromes and common variants of the brain-derived neurotrophic factor gene in human obesity. Prog Mol Biol Transl Sci. 2016;140:75–95.
Ma X-Y, Qiu WQ, Smith CE, Parnell LD, Jiang Z-Y, Ordovas JM, et al. Association between BDNF rs6265 and obesity in the Boston puerto rican health study. J Obes. 2012. https://doi.org/10.1155/2012/102942.
Abaj F, Rafiee M, Koohdani F. Interactions of dietary insulin index and dietary insulin load with BDNF Val66Met polymorphism in relation to cardiometabolic markers in Iranian diabetic patients: a cross-sectional study. Br J Nutr. 2021;128:1–22.
Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–23.
Lapchak PA, Hefti F. BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. NeuroReport. 1992;3(5):405–8.
Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98.
Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15(10):1748–57.
Gyorkos A, Baker MH, Miutz LN, Lown DA, Jones MA, Houghton-Rahrig LD. Carbohydrate-restricted diet and exercise increase brain-derived neurotrophic factor and cognitive function: a randomized crossover trial. Cureus. 2019;11(9):e5604.
Chaldakov GN, Fiore M, Stankulov IS, Manni L, Hristova MG, Antonelli A, et al. Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: A role for NGF and BDNF in cardiovascular disease? Prog Brain Res. 2004;146:279–89.
Krabbe K, Nielsen A, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–8.
Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, et al. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49(3):436–44.
Eyileten C, Kaplon-Cieslicka A, Mirowska-Guzel D, Malek L, Postula M. Antidiabetic effect of brain-derived neurotrophic factor and its association with inflammation in type 2 diabetes mellitus. J Diabetes Res. 2017. https://doi.org/10.1155/2017/2823671.
Dooley LN, Ganz PA, Cole SW, Crespi CM, Bower JE. Val66Met BDNF polymorphism as a vulnerability factor for inflammation-associated depressive symptoms in women with breast cancer. J Affect Disord. 2016;197:43–50.
Liu X, Zhu Z, Kalyani M, Janik JM, Shi H. Effects of energy status and diet on Bdnf expression in the ventromedial hypothalamus of male and female rats. Physiol Behav. 2014;130:99–107.
Acknowledgements
The authors thank all the patients for participating in this study.
Funding
This work was supported by Tehran University of Medical Sciences (Grant Number 15060).
Author information
Authors and Affiliations
Contributions
ZN helped in investigation, visualization, and writing the original manuscript. FA contributed to software, formal analysis. MR was involved in reviewing, editing, and project administration. FK helped in funding acquisition, supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted based on the Declaration of Helsinki and was approved by the Ethics Committee of the Tehran University of Medical Sciences (TUMS) (no. 15060), and the written consent form was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Naeini, Z., Abaj, F., Rafiee, M. et al. Interactions of BDNF Val66met and dietary indices in relation to metabolic markers among patient with type 2 diabetes mellitus: a cross-sectional study. J Health Popul Nutr 42, 34 (2023). https://doi.org/10.1186/s41043-023-00375-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-023-00375-5