Abstract
Background
The term “human microbiota” refers to populations of microorganisms that live harmoniously in co-existence with humans. They contribute significantly to the host's immunological response when confronted with a respiratory viral infection. However, little is known about the relationship between the human microbiome and COVID-19. Therefore, our objective is to perform a bibliometric analysis to explore the overall structure and hotspots of research activity on the links between microbiota and COVID-19 at the global level.
Methods
The research literature on the microbiota and COVID-19 published between 2020 and 2022 was obtained from the Scopus database. Bibliometric analysis and network visualization were performed with VOSviewer.
Results
Of the 701 publications selected, the USA contributed the most (n = 157, 22.40%), followed by China (n = 118, 16.83%) and Italy (n = 82, 11.70%). Hotspots in this field were “COVID-19 is associated with an altered upper respiratory tract microbiome,” “the effect of antibiotics on the gut microbiome,” as well as “patient nutrition and probiotic therapy in COVID-19.”
Conclusions
The links between microbiota and COVID-19 remain an urgent concern at present, and the use of probiotics or/and antibiotics during the pandemic needs to be further improved. This landscape analysis of the links between the microbiota and COVID-19 will provide a basis for future research.
Similar content being viewed by others
Introduction
Coronavirus disease (COVID-19) is caused by an infectious pathogen known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. This kind of virus affects the respiratory tract and causes various symptoms with different levels of consequences, from mild to serious or fatal clinical manifestations [2]. However, the pathogenicity of COVID-19 is not limited exclusively to the lungs. The gastrointestinal tract (GIT) is also affected by SARS-CoV-2 as a target [3, 4]. The healthy human gut microbiota consists of almost a trillion of microorganisms [5]. These microbes play a key role in the body through their metabolic, developmental, and protective action [6] that help in food digestion and impart protective systemic and pulmonary immunity. Once these protective barriers are damaged, microorganisms may translocate to other body sites like the respiratory tract and can eventually induce acute respiratory distress syndrome [7, 8].
Because the COVID-19 pandemic is still developing and is not completely under control, ongoing research is required to update current practices in light of this disease. Due to this, we thought it was important to perform a comprehensive and meticulous bibliometric review to provide an overview of research activity on the links between the microbiota and COVID-19 at the global level. Meanwhile, we identified the evolution of research hotspots and forecasted future research focuses using term clustering analysis, so supplying important data for follow-up investigations.
Methods
We searched the Scopus database for documents for this study, as it has access to a large number of papers and provides more citation-rich. More than 34,000 peer-reviewed academic journals can be found in Scopus, the world's largest and most comprehensive academic information source. First, we searched for terms associated with COVID-19 in article titles, abstracts, and author keywords [9,10,11]. The search was then restricted to publications using phrases relating to the microbiota [12,13,14]. A publication date range of January 2020 to September 2022 was chosen to restrict the investigation further. On September 30, 2022, the data were downloaded. Bibliometric analysis was performed with VOSviewer (version 1.6.18), and the results were visualized with the use of network maps. This resulted in improved subject comprehension and interpretation [15]. This article identified the research lines, the countries with the highest number of publications, the study areas, and the articles with the highest number of citations.
Results
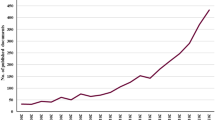
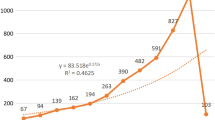
The publications on COVID-19 and microbiota analyzed in this study were 701, of which 107 were published in 2020, 249 in 2021, and 245 in 2022. Of all these publications, 334 (47.65%) were articles, 249 (35.52%) were reviews, and 118 (16.83%) were others (e.g., letters, editorials). Research on the links between COVID-19 and microbiota has been paid more and more attention. As a result, the volume of annual publications has grown over time. In addition, during the coming years, it is anticipated that the annual publication output is anticipated to expand rapidly, indicating a promising future for this area of study.
The included studies were conducted in 97 countries of the world. The top 10 publications on the links between COVID-19 and microbiota were the USA (n = 157, 22.40%), China (n = 118, 16.83%), Italy (n = 82, 11.70%), India (n = 61, 8.71%), UK (n = 42, 5.99%), Iran (n = 32, 4.56%), Australia (n = 28, 3.99%), Brazil (n = 28, 3.99%), Germany (n = 25, 3.57%), and France (n = 24, 3.42%). A map showing international cooperation between countries and regions shows that the USA and China collaborate with the majority of countries in publication (Fig. 1).
The ten most cited papers received 2,446 citations in all [16,17,18,19,20,21,22,23,24,25]. Total citations of these articles that cited the connection between microbiota and COVID-19 research ranged from 102 to 580 (Table 1).
We used VOSviewer software to analyze the terms extracted from the titles and abstracts of the 701 publications. These terms were considered significant if they appeared in a location 20 or more times (Fig. 2). Our goal was to gain a comprehensive understanding of the primary research focus of these publications. A total of 168 terms met the requirements, and they were grouped into three groups: “COVID-19 is associated with an altered upper respiratory tract microbiome (blue cluster)”; “the effect of antibiotics on the gut microbiome (green cluster)” and “patient nutrition and probiotic therapy in COVID-19 (red cluster).”
Analysis of research hotspots using cluster mapping. The VOSviewer software was used to evaluate words that appeared at least 20 times in titles and abstracts. The larger the circle of the keyword, the more frequently it appeared. The terms were classified into three clusters: “COVID-19 is associated with an altered upper respiratory tract microbiome” (blue cluster); “the effect of antibiotics on the gut microbiome” (green cluster); and “patient nutrition and probiotic therapy in COVID-19” (red cluster)
Discussion
In this bibliometric analysis study, we found 701 documents on the connection between microbiota and COVID-19 research from 2020 to 2022 in the Scopus database. This bibliometric network analysis has produced a map of global microbiota-related research on COVID-19, demonstrating a rapid increase in COVID-19 research driven by countries with many cases of the disease. According to the frequency of their publications, the USA and China were the two most productive countries in this field [26].
The results of this study indicate that the top 10 most cited references, which mainly refer to the composition of the gut microbiota, reflect the severity of COVID-19 or the role of probiotics in managing COVID-19 (such as the prevention of COVID-19). In terms of frequency, the most cited paper was “Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization,” which was published by Zuo et al. [25] in 2020 article was cited 580 times. The authors of this study found ongoing changes in the fecal microbiota throughout hospitalization compared to controls. Alterations in the fecal microbiota were associated with the severity of COVID-19. Strategies to modify the gut microbiota can reduce the severity of the disease. The fact that many hot topics were published during this time, exposing new theories and establishing new research fields [16,17,18,19,20,21,22,23,24,25] like “gut microbiota composition reflects COVID-19 severity” or the “role of probiotics in the management of COVID-19,” can also be used to explain the rise in publications on the microbiota and COVID-19. The relationship between gut microbiota and COVID-19 severity raises novel therapeutic and diagnostic ideas and guidelines to enrich the diet with probiotics and prebiotics food and supplements as one of the treatment and prevention strategies [19, 27,28,29,30,31,32].
“COVID-19 is associated with an altered upper respiratory tract microbiome” as a theme was among the main hot topics in the current study. Any change in the composition, function, or diversity of the gut microbiota (gut dysbiosis) has been shown to affect the individual immunity of many systems, including the lungs [33]. On the other hand, lung inflammation can induce intestinal dysbiosis, especially since the respiratory system has its microbiota that is affected by any infections [34, 35], which is confirmed by the reported cases of patients with respiratory infections who usually have GIT dysfunction [36]. SARS-CoV-2 was already found in many parts of GIT, including the rectum, esophagus, duodenum, stomach, and fecal samples [37,38,39]. These patients often suffer from a lack of appetite, abdominal cramps, diarrhea, nausea, and vomiting [40, 41]. Several studies have repeatedly detected SARS-CoV-2 in samples from the stool and anal swabs of patients with COVID-19, suggesting the target site of viral replication and activity [42,43,44]. These results, along with the fact that diarrhea is one of the main GIT symptoms that some patients have, point to a possible link between the GIT and respiratory tract, or mainly, between the lungs and the intestinal microbiota, which is known as the intestinal–lung axis [45, 46].
Another key topic of discussion in the current investigation was the impact of antibiotics on the gut microbiome. Dysbiosis of the gut microbiota has been associated with autoimmune diseases, infectious diseases, autoimmune diseases, and allergy disorders [47]. When pathogens are eradicated with antibiotic therapy, the typical commensal microbiota may be indiscriminately eliminated, resulting in dysbiosis of the microecosystem. Compared to healthy controls, antibiotic therapy in COVID-19 patients can significantly affect intestinal flora [48]. Antibiotic overuse may have increased during the COVID-19 pandemic due to people buying antibiotics online pharmacies rather than visiting doctors in hospitals, as previously mentioned. Since the COVID-19 epidemic, 79–96% of antibiotics have been inadvertently taken in the European region, according to a behavioral insight study by the World Health Organization (WHO) [49]. According to emerging data from interventional studies and animal models, the microbiota may be a key factor in the development of protective antibody responses to vaccination. For instance, mice that had been given antibiotics and kept germ-free exhibited lower antibody reactions to the seasonal influenza vaccine. Therefore, recognizing that the microbiota plays a crucial role in regulating immunological responses to vaccination, microbiota-targeted therapies are a promising strategy to maximize the effectiveness of the COVID-19 vaccine in addition to the treatment with COVID-19 treatment [50]. Gut dysbiosis appears to be a common clinical feature in patients with COVID-19. COVID-19 patients have a threefold increase in the diversity of fungi and pathogens. More severe symptoms were associated with patients with a high abundance of more than two Aspergillus pathogens [23, 51]. Antibiotic therapy can decrease the diversity of gut microbiota species, stimulate the development of resistance to bacterial antibiotics, and disturb the balance that usually exists, causing bacterial overgrowth such as toxigenic C. difficile. In adults, a study has shown that a combination use of meropenem, gentamicin, and vancomycin decreased the prevalence of bifidobacterium and butyrate-producing species. Furthermore, the study has revealed that the baseline composition of the intestinal microbiota was mostly restored in 1.5 months, while many other common species remained undetectable [52, 53]. As a result, antibiotic resistance has become an important public health issue worldwide [54].
The impact of patient nutrition and probiotic therapy on COVID-19 is another topic of interest. Many approaches have been studied to modulate and improve the intestinal microbiota in patients with COVID-19 by supplementation with probiotics, prebiotics, trace elements, and bacterial metabolites, revealing that supplementation could reduce the hyperinflammatory response and severity of COVID-19 [55]. Several studies explored the impact of the intestinal microbiota on respiratory infections and systemic immunity. They revealed the essential role of the commensal microbiota in enhancing antiviral responses through modulation of immune responses during normal conditions and viral infections, especially in the respiratory tract [56, 57]. Furthermore, a higher mortality rate in respiratory infections is associated with intestinal dysbiosis, possibly due to decreased secretion of regulatory immune cells (T cells) in GIT and respiratory [58]. Studies were carried out on COVID-19 patients, as patients may have unbalanced microbiota in the pharynx in addition to GIT and lungs, reinforcing the idea of a gut–lung axis where any disturbances in the mucosa of GIT may have other sites [59,60,61]. Human microbiota and probiotics can increase health benefits due to immunomodulatory effects, IgA secretion, and the activity of neutrophils and macrophages. Consequently, probiotics can protect the host against viral infections and many respiratory viruses, such as COVID-19, and prevent secondary bacterial infections [62].
However, COVID-19 can develop microbiota dysbiosis in the lungs and gut 6 months after recovery [63]. Therefore, decreased intestinal microbiomes, such as lactobacillus and bifidobacterium, and poor nutrition intake can hinder recovery [64]. Therefore, probiotics supplements have shown a significant effect in improving COVID-19 symptoms of COVID-19 such as diarrhea, headache, and cough, and support the microbiota balance in the intestine–lung axis [65, 66].
Strengths and limitations
This bibliometric study examines the links between COVID-19 and microbiota research. This study employs a well-known scientometric software tool in the construction and visualization of bibliometric networks (VOSviewer). However, there are some limitations that come with this study. To begin, our investigation is predicated mostly on quantitative analysis and somewhat marginally on qualitative analysis. Second, the retrieval is carried out primarily through the use of the Scopus database. However, it is important to note that Scopus is the most widely used scientometrics database and that visualization-based literature analysis lays the groundwork for scholars to grasp the hotspots and trends in this subject easily.
Conclusions
This study provides an overall picture of the links between microbiota and COVID-19. Hotspots in this field were “COVID-19 is associated with an altered upper respiratory tract microbiome,” “the effect of antibiotics on the gut microbiome,” as well as “patient nutrition and probiotic therapy in COVID-19.” This landscape analysis of the links between the microbiota and COVID-19 will provide a basis for future research.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available upon request from the corresponding author.
Abbreviations
- SARS‑CoV‑2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- CIT:
-
Gastrointestinal tract
References
Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022;20(5):270–84.
Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40.
Agarwal A, Chen A, Ravindran N, To C, Thuluvath PJ. Gastrointestinal and liver manifestations of COVID-19. J Clin Exp Hepatol. 2020;10(3):263–5.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32.
Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105(6):2117–22.
Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–50.
Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, Huffnagle GB. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113.
Zyoud SH, Shakhshir M, Koni A, Shahwan M, Jairoun AA, Al-Jabi SW. Olfactory and gustatory dysfunction in COVID-19: a global bibliometric and visualized analysis. Ann Otol Rhinol Laryngol. 2022;132:164–72.
Zyoud SH, Al-Jabi SW, Koni A, Shakhshir M, Shahwan M, Jairoun AA. Mapping the landscape and structure of global research on nutrition and COVID-19: visualization analysis. J Health Popul Nutr. 2022;41(1):25.
Zyoud SH, Al-Jabi SW, Shahwan MJ, Jairoun AA. Global research production pertaining to gastrointestinal involvement in COVID-19: a bibliometric and visualised study. World J Gastrointest Surg. 2022;14(5):494–505.
Zyoud SH, Al-Jabi SW, Amer R, Shakhshir M, Shahwan M, Jairoun AA, Akkawi M, Abu Taha A. Global research trends on the links between the gut microbiome and cancer: a visualization analysis. J Transl Med. 2022;20(1):83.
Zyoud SH, Shakhshir M, Abushanab AS, Al-Jabi SW, Koni A, Shahwan M, Jairoun AA, Abu Taha A. Mapping the global research landscape on nutrition and the gut microbiota: visualization and bibliometric analysis. World J Gastroenterol. 2022;28(25):2981–93.
Zyoud SH, Smale S, Waring WS, Sweileh W, Al-Jabi SW. Global research trends in the microbiome related to irritable bowel syndrome: a bibliometric and visualized study. World J Gastroenterol. 2021;27(13):1341–53.
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38.
Baud D, Dimopoulou Agri V, Gibson GR, Reid G, Giannoni E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front Public Health. 2020;8:186.
Dhar D, Mohanty A. Gut microbiota and Covid-19—possible link and implications. Virus Res. 2020;285:198018.
Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71(10):2669–78.
Infusino F, Marazzato M, Mancone M, Fedele F, Mastroianni CM, Severino P, Ceccarelli G, Santinelli L, Cavarretta E, Marullo AGM, et al. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: a scoping review. Nutrients. 2020;12(6):1718.
Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7.
Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57–69.
Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706.
Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, Chen Z, Boon SS, Chan FK, Chan PK, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70(2):276–84.
Zuo T, Zhan H, Zhang F, Liu Q, Tso EYK, Lui GCY, Chen N, Li A, Lu W, Chan FKL, et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159(4):1302-1310.e1305.
Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944-955.e948.
de Las H-P, Jambrino-Maldonado C, Rando-Cueto D, Iglesias-Sanchez PP. COVID-19 study on scientific articles in health communication: a science mapping analysis in Web of Science. Int J Environ Res Public Health. 2022;19(3):1705.
Bottari B, Castellone V, Neviani E. Probiotics and Covid-19. Int J Food Sci Nutr. 2021;72(3):293–9.
de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. Microbiota modulation of the gut-lung axis in COVID-19. Front Immunol. 2021;12:635471.
Skrajnowska D, Brumer M, Kankowska S, Matysek M, Miazio N, Bobrowska-Korczak B. Covid 19: diet composition and health. Nutrients. 2021;13(9):2980.
Sundararaman A, Ray M, Ravindra PV, Halami PM. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl Microbiol Biotechnol. 2020;104(19):8089–104.
Xavier-Santos D, Padilha M, Fabiano GA, Vinderola G, Gomes Cruz A, Sivieri K, Costa Antunes AE. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: a bibliometric analysis and systematic review. Trends Food Sci Technol. 2022;120:174–92.
Farsi Y, Tahvildari A, Arbabi M, Vazife F, Sechi LA, Shahidi Bonjar AH, Jamshidi P, Nasiri MJ, Mirsaeidi M. Diagnostic, prognostic, and therapeutic roles of gut microbiota in COVID-19: a comprehensive systematic review. Front Cell Infect Microbiol. 2022;12:804644.
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66.
Groves HT, Cuthbertson L, James P, Moffatt MF, Cox MJ, Tregoning JS. Respiratory disease following viral lung infection alters the murine gut microbiota. Front Immunol. 2018;9:182.
Wang H, Dai W, Feng X, Zhou Q, Wang H, Yang Y, Li S, Zheng Y. Microbiota composition in upper respiratory tracts of healthy children in Shenzhen, China, differed with respiratory sites and ages. Biomed Res Int. 2018;2018:6515670.
Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211(12):2397–410.
Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95.
Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–9.
Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–73.
Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, Yang Y, Liu B, Wang W, Wei C, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92(7):833–40.
Xiao F, Sun J, Xu Y, Li F, Huang X, Li H, Zhao J, Huang J, Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020;26(8):1920–2.
Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831-1833.e1833.
Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23.
He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J. Gut-lung axis: the microbial contributions and clinical implications. Crit Rev Microbiol. 2017;43(1):81–95.
Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17(5):553–64.
Gang J, Wang H, Xue X, Zhang S. Microbiota and COVID-19: long-term and complex influencing factors. Front Microbiol. 2022;13:963488.
World Health Organization (WHO). Preventing the COVID-19 pandemic from causing an antibiotic resistance catastrophe. 2020. https://www.who.int/europe/news/item/18-11-2020-preventing-the-covid-19-pandemic-from-causing-an-antibiotic-resistance-catastrophe. Accessed 24 Oct 2022.
Wang B, Zhang L, Wang Y, Dai T, Qin Z, Zhou F, Zhang L. Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7(1):143.
Brahma S, Naik A, Lordan R. Probiotics: a gut response to the COVID-19 pandemic but what does the evidence show? Clin Nutr ESPEN. 2022;51:17–27.
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:572912.
Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3(11):1255–65.
World Health Organization (WHO). WHO report on surveillance of antibiotic consumption: 2016–2018 early implementation 2019. http://apps.who.int/iris/bitstream/handle/10665/277359/9789241514880-eng.pdf?ua=1. Accessed 25 Oct 2022.
Chen J, Hall S, Vitetta L. Altered gut microbial metabolites could mediate the effects of risk factors in Covid-19. Rev Med Virol. 2021;31(5):1–13.
Bradley KC, Finsterbusch K, Schnepf D, Crotta S, Llorian M, Davidson S, Fuchs SY, Staeheli P, Wack A. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28(1):245-256.e244.
Neyt K, Lambrecht BN. The role of lung dendritic cell subsets in immunity to respiratory viruses. Immunol Rev. 2013;255(1):57–67.
Grayson MH, Camarda LE, Hussain SA, Zemple SJ, Hayward M, Lam V, Hunter DA, Santoro JL, Rohlfing M, Cheung DS, et al. Intestinal microbiota disruption reduces regulatory T cells and increases respiratory viral infection mortality through increased IFNγ production. Front Immunol. 2018;9:1587.
Budding A, Sieswerda E, Wintermans B, Bos M. An age dependent pharyngeal microbiota signature associated with SARS-CoV-2 infection. Available at SSRN 3582780 2020.
Fan J, Li X, Gao Y, Zhou J, Wang S, Huang B, Wu J, Cao Q, Chen Y, Wang Z, et al. The lung tissue microbiota features of 20 deceased patients with COVID-19. J Infect. 2020;81(3):e64–7.
Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, Chen Z, Chan PK, Ng SC, Boon SS, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70(2):276–84.
Mahooti M, Miri SM, Abdolalipour E, Ghaemi A. The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment? Microb Pathog. 2020;148:104452.
Hung YP, Lee CC, Lee JC, Tsai PJ, Ko WC. Gut dysbiosis during COVID-19 and potential effect of probiotics. Microorganisms. 2021;9(8):1605.
Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7(1):17–44.
Gutierrez-Castrellon P, Gandara-Marti T, Abreu YAAT, Nieto-Rufino CD, Lopez-Orduna E, Jimenez-Escobar I, Jimenez-Gutierrez C, Lopez-Velazquez G, Espadaler-Mazo J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes. 2022;14(1):2018899.
King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41–54.
Mak JWY, Chan FKL, Ng SC. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol Hepatol. 2020;5(7):644–5.
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
SZ designed the study, collected the data, analyzed the data, made significant contributions to the existing literature search and interpretation of the manuscript, and wrote the manuscript; MS contributed to the conceptualization and methodology of the study, participated in the interpretation of the data, contributed to the manuscript writing, and made revisions to the initial draft; AA, AK, AJ, MS, and SA participated in the interpretation of the data, contributed to the manuscript writing, and made revisions to the initial draft; all authors provided a critical review and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There was no need for ethical approval because the data for the bibliometric research were extracted directly from the database without further human intervention.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zyoud, S.H., Shakhshir, M., Abushanab, A.S. et al. Mapping the output of the global literature on the links between gut microbiota and COVID-19. J Health Popul Nutr 42, 3 (2023). https://doi.org/10.1186/s41043-023-00346-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-023-00346-w