Abstract
Background
In the first and only literature review, conducted in 2009, of human insulin analog- induced lipoatrophy, there were 12 published cases, including 1 with aspart, 1 with detemir, 1 with NovoMix 30 and none with detemir plus aspart. It is perceived that insulin analog induced-lipoatrophy is increasing. We conducted a 2015 literature review of published reports of lipoatrophy induced by aspart, detemir, detemir plus aspart, and NovoMix30. We also report a new case of detemir plus aspart and glulisine induced lipoatrophy.
Methods
Our focused literature searches (limited to 1995–2014) in PubMed, Embase, and Web of Science, using a combination of insulin analog and lipoatrophy terminology, was conducted in early January 2015.
Results
From the 520 unique citations there were 33 (from 13 papers and 9 abstracts) lipoatrophy cases induced by detemir (n = 5), aspart (n = 21), detemir plus aspart (n = 4) and NovoMix 30 (n = 3), representing 30 new cases since 2009. Many of these reported cases were females (76 %), had type 1 diabetes mellitus (T1DM) (94 %) and were in young persons (61 %). A 41-year-old T1DM woman developed lipoatrophy on her upper thighs, arms and abdomen 14 months after injecting detemir plus aspart at the same sites. Later on, after a year on continuous subcutaneous insulin infusion (CSII) using aspart and then glulisine, she developed lipoatrophy at the infusion sites. When CSII insulin was switched to lispro she did not develop lipoatrophy after 10 months. Meanwhile, the original lipoatrophy sites significantly improved.
Conclusions
Our literature review uncovered 30 new published cases of aspart, detemir, aspart plus detemir and NovoMix 30-induced lipoatrophy since 2009. The largest increase in cases was in aspart- induced lipoatrophy. Recent surveys showed most rapid acting insulin analog-induced lipoatrophy were associated with CSII. In our review of the reported cases, 85.7 % cases of aspart-induced lipoatrophy were associated with CSII. As in previous reports, we showed lipoatrophy was more common in females, T1DM and young persons. Our patient may be the 5th published case of detemir plus aspart-induced lipoatrophy and possibly the first case report of glulisine induced lipoatrophy. She believed both detemir plus aspart and glulisine induced the lipoatrophy.
Similar content being viewed by others
Background
Insulin-induced lipoatrophy was a very common cutaneous complication of insulin therapy, found in 25- 55 % of patients injecting bovine and porcine insulin [1]. It became less common (<10 %) with purer animal and human insulin [1]; and uncommon (about 1 %) after human insulin analogs became available [2]. Lipoatrophy has been reported in patients using basal (glargine [3] and detemir [4]), rapid-acting (lispro [5], aspart [6] and glulisine [7]) and mixture [8] insulin analogs injections. In the first and only literature review of human insulin analog-induced lipoatrophy, done in November 2009 [9], there were 12 cases, including 1 with aspart, 1 with detemir, 1 with NovoMix 30 (Biphasic aspart – 30 % aspart 70 %, NPH insulin) and none with detemir plus aspart. Insulin analog induced lipoatrophy is perceived to be increasing in prevalence [2]. In this paper we report a literature review conducted in early January 2015 of published reports of lipoatrophy induced by aspart, detemir, detemir plus aspart, and NovoMix30 injections and report a new case of lipoatrophy induced by detemir plus aspart as well as glulisine injection. We will not cover lipoatrophy induced by lispro, lispro mixtures, and glargine in detail.
Methods
We conducted focused literature searches in early January 2015 in PubMed, Embase, and Web of Science using a combination of insulin analog and lipoatrophy terminology. We used MeSH and EMTREE controlled terms when appropriate for broad concepts, such as “insulin analog” [mesh] and lipodystrophy [mesh:noexp]. We supplemented each controlled term with a comparable set of title/abstract keywords, going so far as to include all individual insulin analogs (‘lispro’, ‘humalog’, ‘aspart’, ‘novolog’, ‘detemir’, ‘levemir’, ‘glulisine’, ‘apidra’, and ‘glargine’, ‘lantus’). We also conducted a similar search in Web of Science to primarily identify conference abstracts that were not found in PubMed or Embase. We limited all searches to articles and abstracts published between 1995 (when the first insulin analog was launched) and 2014, and deliberately designed the searches to miss, when possible, citations pertaining to HIV-related lipoatrophy. We exported citations into Endnote X6 (Thomson Reuters) and used its functionality to eliminate duplicates.
We report a new case of a female type 1 diabetes mellitus (T1DM) patient who developed lipoatrophy when injecting detemir plus aspart in the same site. Later on, when using continuous subcutaneous insulin infusion (CSII) to deliver aspart and then glulisine, she also developed lipoatrophy at the infusion sites. We obtained written informed consent from the patient for publication of this case report and the images.
We requested information on lipoatrophy associated with aspart and/or detemir injections from the manufacturer of these insulin analogs.
Results
Literature search
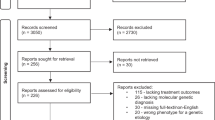
The literature search of PubMed, EMBASE and Web of Science yielded 273, 252 and 119 citations respectively, giving a combined total of 644 citations (Fig. 1). After the duplicates were eliminated, we had 520 unique citations. Two authors (MT and NE) reviewed each of the 520 citations and concurred on those identified as lipoatrophy induced by insulin analog aspart, detemir, detemir plus aspart and NovoMix 30 injections. We found 33 reported cases from 18 citations (13 papers/letters/ observations/vignette and 9 abstracts). From the references of 13 papers, pearling was done to determine whether they included other published abstracts/papers on lipoatrophy induced by aspart, detemir, detemir plus aspart, NovoMix 30 and glulisine injections. We found none.
The 33 lipoatrophy cases are induced by detemir (n = 5) [4, 10, 11], aspart (n = 21) [6, 10, 12–22], detemir plus aspart (n = 4) [11, 23, 24], and NovoMix 30 (n = 3) [8, 10] injections (Table 1). One of the detemir cases [4], one of the aspart cases [6] and one of the NovoMix30 cases [8] were previously described in the 2009 literature review [9], giving 30 new cases since then: 4 detemir cases [10, 11], 20 aspart cases [10, 12–22], 2 NovoMix 30 [10], and 4 detemir plus aspart cases [11, 23, 24]. The characteristics of the 33 cases are:
Gender
Of the 5 patients with detemir-induced lipoatrophy, the gender of 4 was stated. All were females. Of the 21 patients with aspart-induced lipoatrophy, the gender of 20 was stated. Of these, 13 were females and 7 were males. All 4 patients with detemir plus aspart-induced lipoatrophy were females. Of the 3 patients with NovoMix 30-induced lipoatrophy, 1 was female and the gender of the other 2 was not stated.
Type of diabetes
All 5 patients with detemir-induced lipoatrophy had T1DM. Twenty of the 21 patients with aspart-induced lipoatrophy had T1DM and one had type 2 diabetes mellitus (T2DM). Among the 4 adult female patients with detemir plus aspart-induced lipoatrophy, 2 had T1DM and 2 had T2DM. All 3 patients with NovoMix 30-induced lipoatrophy had T1DM.
Age
Four of the 5 patients with detemir-induced lipoatrophy were adults and 1 was an adolescent. Fourteen of the 21 patients with aspart-induced lipoatrophy were children, 3 were adults, 3 adolescents and 1 had no age mentioned. All 4 patients with detemir plus aspart- induced lipoatrophy were adults. Two patients with Novo-Mix 30-induced lipoatrophy were children and 1 an adult.
Case
We report a 41-year-old Caucasian woman with T1DM diagnosed in April 2010 who developed lipoatrophy on her upper thighs, arms and abdomen injection sites in June 2011 after being on multiple daily insulin regimen of detemir plus aspart for 14 months (Fig. 2). Her Hemoglobin A1c improved from 10.9 % at diagnosis to 6.4-7.4 % after starting these insulin analogs. She injected both insulin analogs in the same sites and could not specify which insulin analog was the cause of these indentations. In September 2011 she started CSII using Omnipod with aspart. New lipoatrophy developed at the infusion sites within a year. In November 2012 glulisine was used instead of aspart. When the infusion was above or below the lipoatrophy thigh sites, the lipoatrophy worsened. Therefore, she switched to abdominal sites and developed new lipoatrophy there. She was then evaluated by allergists at 2 different tertiary centers. One recommended steroid therapy and the other recommended observation. She chose clinical observation without steroid treatment. In February 2014 lispro was used instead of glulisine because the original lipoatrophy sites were not improving. In December 2014, 10 months after switching to lispro and using abdominal sites only, no new lipoatrophy had appeared at infusion sites. Her latest A1C in December 2014 was 7.2 %. Meanwhile the original lipoatrophy sites in the upper thighs, upper arms and abdomen improved significantly (Fig. 3).
Information from the manufacturer
In their response to our request for information on lipoatrophy induced by detemir, aspart, detemir plus aspart the manufacturer stated they did not have incidence data on lipoatrophy associated with these insulin analogs [25].
Lipoatrophy induced by other insulin analogs
In the same literature search 17 cases of lispro, 2 cases of lispro mixture, and 7 cases of glargine induced lipoatrophy were reported. No reported case of glulisine-induced lipoatrophy was found. These cases are not described in detail in this paper; but their details can be obtained from the authors. Our new case of detemir plus aspart induced lipoatrophy also developed lipoatrophy when using glulisine, making it the first published reported case of glulisine induced lipoatrophy.
Discussion
Based on our 2015 literature search of published reports, our adult female patient with T1DM may be the 5th published case of detemir plus aspart-induced lipoatrophy. Aspart was launched in 2000 and detemir in 2006. The first published report on detemir plus aspart-induced lipoatrophy in 2011 was followed by 3 more cases in 2013. Among the previously reported 4 adult female patients with detemir plus aspart-induced lipoatrophy, 2 had T1DM [11] and 2 had T2DM [23, 24]. When both detemir and aspart are injected in the same site, it is difficult to identify whether one or both of these insulin analogs induced the lipoatrophy as either can cause it. According to our patient, she injected aspart and detemir in the same sites and could not determine which insulin analog induced the lipoatrophy. When she was on CSII using aspart and then glulisine she also developed lipoatrophy at the infusion sites.
In the past, CSII was reported to treat lipoatrophy induced by human insulin [26]. Today, lipoatrophy induced by rapid-acting insulin analog is often associated with CSII. Two recent surveys reported 83.3 % [27] and 87 % [2] of patients with lipoatrophy induced by rapid-acting insulin analogs use CSII. In our report 18 of the 21 (85.7 %) cases of aspart-induced lipoatrophy were associated with CSII and only 3 cases injected aspart. The infusion may cause the lipoatrophy [22]. Together continuous exposure to insulin and continuous mechanical irritation by the infusion catheter may trigger events that lead to lipoatrophy. Some patients did not develop lipoatrophy when injecting aspart but did so when infusing aspart via CSII [17, 22]. Others develop lipoatrophy with both injection and infusion implying the delivery method does not matter. Our patient had aspart-induced lipoatrophy within a year at the infusion sites when on CSII. She also developed lipoatrophy at the infusion sites when infusing glulisine via CSII after a year. Ten months after infusing lispro via CSII, she has not developed lipoatrophy at the infusion sites. This may be because she has not infused lispro long enough (one year or longer) as lipoatrophy can develop from 4 weeks [9] to 5.5 years [24] after starting insulin. It may also be because lispro does not induce lipoatrophy in her. Although all 5 insulin analogs can induce lipoatrophy, some patients have lipoatrophy induced by one but not another [17].
Clinical presentation
Lipoatrophy is the loss of subcutaneous fat at insulin injection/infusion sites as demonstrated by biopsy [18, 28] and MRI [29]. In the past, 10 - 55 % of diabetic patients injecting impure bovine or porcine insulin developed lipoatrophy [1, 30]. With the availability of purer animal and human insulin, the prevalence of lipoatrophy decreased to 0.2-1.4 % [30, 31]. With insulin analog injections/infusions, the prevalence of lipoatrophy had been reported to be 2.5 % in a single center study [27] and 1.1 % in a multicenter survey [2].
There are no reported objective data for exact onset of insulin-induced lipoatrophy. The reported first observed onsets of insulin-induced lipoatrophy range from 4 weeks to 2 years [9], 2–3 to 23 months [26] to 6–24 months [1] after initiation of insulin therapy. In our literature search the first observed onsets of lipoatrophy range from 2 months to 5.5 years.
Lipoatrophy is more common in females in reported cases of lipoatrophy. In our review, the gender of 29 patients was identified. Of these 22 (75.8 %) were females and 7 males. Our patient is female. Why lipoatrophy is more prevalent in women remains unclear. There may be a reporting bias in this female gender predominance as this data is based on case reports and not clinical trials or MedWatch reports.
Lipoatrophy can overlap with other autoimmune diseases [21, 32]. Female T1DM patients with lipoatrophy have a higher risk of developing Hashimoto’s thyroiditis and celiac disease [32]. Autoimmune diseases affect 8 % of the population and of these 78 % are females [33]. Whether lipoatrophy and autoimmune diseases in females share a common etiology remains to be established. In females with T1DM and insulin-induced lipoatrophy, the physician should screen for other autoimmune disease(s). Our patient has Hashimoto’s thyroiditis with hypothyroidism; she does not have celiac disease or primary adrenal insufficiency.
Many of the reported cases of lipoatrophy induced by insulin analogs are in the pediatric population. Why this is remains unanswered. In our review, 20 (60.6 %) of the 33 cases were in the pediatric age group: 1 with detemir, 17 with aspart, and 2 with NovoMix 30. Like the previous 4 reported cases of lipoatrophy induced by detemir plus aspart injections, our case is an adult. Similarly, 4 of the 5 cases of lipoatrophy induced by detemir were adults. This probably reflects the age of patients using detemir plus aspart and detemir alone. Lipoatrophy more commonly occurs in T1DM partly because many cases are in the pediatric age group and possibly due to a potential immune-mediated inflammation causing lipoatrophy in patients with autoimmune type 1 diabetes. In our report, 31 of the 33 patients had T1DM and 2 had T2DM. Our patient has T1DM.
Lipohypertrophy sites from insulin injection can lead to impaired insulin absorption [34, 35]. To the best of our knowledge there are no published insulin absorption studies done in insulin analog-induced lipoatrophy sites. But, it is reasonable to expect variable insulin absorption in lipoatrophy sites with loss of adipose tissue, making glycemic control challenging. Some patients improved their glycemic control when insulin injection site is changed from the lipohypertrophy to an unaffected site [36]. One patient with lipoatrophy had improved glycemic control when the injection site was changed [11]. Another patient improved his glycemic control when given intra-peritoneal insulin suggesting that the lipoatrophy may have been causing poor insulin absorption [37]. Changing the injection or cannula site in our patient did not significantly affect her glycemic control. Clinically, insulin-induced lipoatrophy can be cosmetically distressing and disfiguring to the patient. For these reasons, the clinician should inspect the insulin injection/infusion sites regularly to identify lipodystrophy (lipohypertrophy and/or lipoatrophy), especially in patients with erratic glycemic control, so measures to manage the problem and prevent the development of further areas of lipoatrophy can be implemented.
As all these are reported cases, the number of cases of lipoatrophy induced by each insulin analog cannot be compared. However, all insulin analogs can induce lipoatrophy.
Etiology
Many possible etiologies have been considered for insulin-induced lipoatrophy. These include cresol preservative in insulin, alcohol used for sterilization of syringes and needles, injury to the fat cells, glycolytic ferments in the insulin, possible nerve injury [38], mechanical trauma from repeat injections, and cryotrauma from cold insulin [10]. Immune etiologies have also been suggested - immune reaction to insulin [39, 40] and immune complex mediated inflammation [26]. Lipoatrophic lesions occurred in individuals using animal insulins who have high levels of circulating anti-insulin antibodies and the edges of lipoatrophy lesions were characterized by deposition of immunologic proteins within dermal vessel [39]. Although this had been questioned, a strong relationship between lipoatrophy and insulin antibodies was recently reported in adults with T2DM on recombinant human insulin or insulin analogs [41]. The immune-complex mediated inflammatory response involves local macrophage release of tumor necrosis factor α causing adipocyte dedifferentiation [26, 42] in patients using both animal and recombinant human insulin. Increased numbers of degranulating mast cells that stain positively for tryptase and chymase antibodies have also been seen in skin biopsies of patients with lipoatrophic sites of insulin analogs [43]. Histology has shown small adipocyte lobules with hyperplastic capillaries, loss of adipose tissue, areas of membranous lipodystrophy usually lined by an acellular homogeneous eosinophilic material and a focal lymphoid cell infiltration abutting hypodermis blood vessels [26, 44] in patients using both animal and human insulins. Although several studies have demonstrated a possible immune basis to the etiology of lipoatrophy, there are others which do not. Jermendy et al. reported absence of inflammatory cells and no local immune mechanisms in the biopsy specimen [44]. Milan et al. suggested that adipose tissue metabolic changes play a role in lipoatrophy as they did not identify any inflammatory cells in the skin biopsy specimens of their three T1DM patients with lipoatrophy by insulin analogs [28]. This study demonstrated a decrease in fat cell volume with adipocytes losing their lipid content leading to the hypothesis that adipocytes chronically exposed to high local insulin levels could develop insulin resistance resulting in an increase in the lipolytic process causing lipoatrophy. A significant down-regulation of leptin expression along with an increase in free fatty acid was also seen which was thought to result in the recruitment of fat cell precursors [28].
Treatment
A change in insulin formulation, avoiding injections in the lipoatrophy sites (our patient noted this when the Omnipod was used near the sites), and changing the insulin needle daily have helped resolve lipoatrophy in some patients [3]. There are also case reports of adding glucocorticoid therapy such as dexamethasone or betamethasone to the insulin analog [20, 45]; but this can cause blood glucose fluctuations if the betamethasone/insulin analog solution becomes inhomogenous resulting in erratic insulin administration [20]. Administering low-dose oral glucocorticoid such as prednisone 5–10 mg daily [46, 47] has also been used to improve lipoatrophy. Corticosteroids are able to induce differentiation of adipocytes and have immune-modulating properties [45]. However, addition of glucocorticoid therapy can result in worsening glycemic control and increased insulin requirements [20]. Our patient declined the recommendation of using steroids. Yet, with time and not using the lipoatrophy sites, the original lipoatrophy began to improve (Fig. 3).
Changing the mode of insulin delivery, such as using CSII, can potentially improve lipoatrophy in patients using human insulin injections [48]. However, in our literature review, 18 of the 21 cases of lipoatrophy induced by aspart were associated with CSII which has been hypothesized to contribute to the development of lipoatrophy [22]. Why the largest increase in aspart-induced lipoatrophy occurred in those on CSII remains unclear. There may be a reporting bias in this group as this data is based on case reports and not clinical trials and Medwatch reports. Our patient developed lipoatrophy when on CSII using aspart and glulisine.
In 2 small studies topical 4 % sodium cromolyn in petrolatum solvent was partially effective therapy for early lipoatrophy areas and prevention of the development of new such areas [43, 49]. Our literature review described 2 other cases of lipoatrophy induced by aspart which improved with sodium cromolyn therapy [15, 19]. Cromolyn stabilizes mast cells that are tryptase-positive/chymase-positive and prevents the release of histamine in the presence of antigen-IgE antibody reactions [43].
Finally, 2 case reports described treatment of insulin-induced lipoatrophy. The first one is treating lipoatrophy successfully with an insulin jet –injector [50]. In an extremely refractory case Noud et al. used intraperitoneal insulin delivered by Diaport [37]. Inhaled insulin can potentially be used in patients with lipoatrophy induced by injected insulin. Lipoatrophy was not described as an adverse event in the Afreeza® product monograph [51]. We are aware that in Afreeza® trials high anti-insulin antibodies titers were documented. To the best of our knowledge no case of insulin lipoatrophy has been described in the Afreeza® clinical trials. Possible explanations for this include [a] without repeated injections of insulin no atrophy occurs despite the high insulin antibodies tiers; [b] an uncommon complication like insulin induced lipoatrophy may not appear in the limited number of patients who have used Afreeza thus far; and [c] the association of high insulin antibodies titers and injected insulin lipoatrophy does not imply a cause-effect association.
Conclusion
Our case may be the 5th case of detemir plus aspart induced lipoatrophy and possibly the first published case report of glulisine induced lipoatrophy. In prescribing information for Apidra [7] lipoatrophy was mentioned. There are 30 new published cases of aspart, detemir, detemir plus aspart and NovoMix induced lipoatrophy since 2009. These represent only a percentage of the cases of lipoatrophy induced by these insulin analogs. The ISPAD survey [2], the single center study [27] and the recent Rosenbloom recidivus [38] mentioned many cases (some due to these insulin analogs) which have not been published. To better understand insulin analog-induced lipoatrophy, more research on the prevalence of this cutaneous complication of insulin therapy, its etiology, pathogenesis and management need to be conducted. It is unrealistic to expect every case of insulin analog induced lipoatrophy to be published. But, sharing of data on reported, but not published, cases can be helpful.
References
Richardson T, Kerr D. Skin-Related Complications of Insulin Therapy. Epidemiology and Emerging Management Strategies. Am J Clin Dermatol. 2003;4:661–7.
Forsander GA, Malmodin OC, Kordonouri O, Ludvigsson J, Klingensmith G, Beaufort CD. An ISPAD survey of insulin-induced lipoatrophy [Abstract]. Pediatr Diabetes. 2013;14 Suppl 18:20.
Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: Long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28:2983.
del Olmo MI, Campos V, Abellan P, Merino-Torres JF, Pinon F. A case of lipoatrophy with insulin detemir. Diabetes Res and Clin Pract. 2008;80:e20–1.
Griffin ME, Feder A, Tambolane WV. Lipoatrophy Associated With Lispro Insulin In Insulin Pump Therapy. An old complication, a new cause? Diabetes Care. 2001;24:174.
Szypowska A, Skórka A, Pańkowska E. Lipoatrophy associated with rapid-acting insulin analogues in young patients with Type 1 diabetes mellitus. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2008;14:117–8.
Apidra Prescribing Information http://products.sanofi.us/apidra/apidra.html Accessed January 17, 2015.
Hussein SF, Siddique H, Coates P, Green. Lipoatrophy is a thing of the past, or is it? Diabet Med. 2007;24:1470–2.
Holstein A, Stege H, Kovacs P. Lipoatrophy associated with the use of insulin analogues: a new case associated with the use of insulin glargine and review of the literature. Expert Opin Drug Saf. 2010;9:225–31.
Babiker A, Datta V. Lipoatrophy with insulin analogues in type 1 diabetes. Arch Dis Child. 2011;96:101–2.
Agha A, Duffield E, Elrishi M. Detemir insulin related lipoatrophy: a case series. Pract Diabetes. 2013;30:296.
Kesavadev J, Kumar A, Ahammed S, Dinkar G, Jothydev S. Insulin Aspart Induced Lipoatrophy in a Patient on Insulin Pump [Abstract]. Diabetes. 2008;57 Suppl 1:578.
Bocca G, Westerlaken C. Good result for betamethasone added to an insulin analog, as treatment for insulin analog induced lipoatrophy. Report of a case [Abstract]. Horm Res. 2009;72:501–2.
Chang YT, Evans B, D’Arcangelo MR, Milstein MT, Gabbay RA. Lipoastrophy in a girl after switching insulin analog injection to a pump [Abstract]. Diabetes. 2010;59:A695.
Niinikoski H, Nanto-Salonen K, Ruusu P, Kinnala A, Putto-Launla A, Keskinen JTJP. Insuliinhoiden lapsille ainheuttama lipoatrofia. Duodecim. 2010;126:1328–32.
George PS, Robertson M, Grant L, Mackie ADR. Lipoatrophy associated with insulin aspart in continuous subcutaneous insulin infusion. Pract Diab Int. 2011;28:108.
Yazdanyar S, Dolmer BS, Strauss G. Three children with lipoatrophy associated with human rapid-acting insulin analogues. Eur J Pediat Dermatol. 2011;21:11–5.
Peteiro-Gonzalez D, Fernandez-Rodriguez B, Cabezas-Agricola JM, Araujo-Vilar D. Severe localized lipoatrophy related to therapy with insulin analogs in type 1a diabetes mellitus. Diab Res Clin Prac. 2011;91:e61–3.
Salma B, Plat F, Bourrel F, Sanchez M, Bernamo E. Succes d’un traitement par chromoglycate de sodium dans un cas de lipoatrophie insulinique sous pompe externe a insulin [Abstract]. Diabetes Metab. 2011;37:A97.
Swelheim HT, Westerlaken C, van Pinxteren-Nagler E, Bocca G. Lipoatrophy in a girl with type 1 diabetes mellitus: Beneficial effect of treatment with a glucocorticoid added to an insulin analog. Diabetes Care. 2012;35, e2.
Suththarantha J, Puthi VR, Walton S. Severe lipoatrophy complicating insulin analogue treatment: first reported case of lipoatrophy complicating the administration of insulin aspart via continuous subcutaneous insulin infusion (CSII) [Abstract]. Horm Res. 2012;78 Suppl 1:163–4.
Simeonovic M, Anuar A, Edge J, Makaya T. Lipoatrophy: re-emerging with analogue insulins, Is there a link with CSII? Pract Diabetes. 2014;31:164–6.
Tavare AN, Doolittle HJ, Baburaj R. Pan-insulin allergy and severe lipoatrophy complicating type 2 diabetes. Diabet Med. 2011;28:500–3.
Breznik V, Kokol R, Luzar B, Miljkovic J. Insulin-induced localized lipoatrophy. Acta Dermatovenerol APA. 2013;22:83–5.
Patel C, Koenig S. Medical Information Novo Nordisk Inc. Novemeber 12, 2014 (personal communication).
Radermecker RP, Pierard GE, Scheen AJ. Lipodystrophy Reactions to Insulin: Effects of Continuous Insulin Infusion and New Insulin Analogs. Am J Clin Dermatol. 2007;8:21–8.
Schnell K, Biester T, Tsioli C, Datz N, Danne T, Kordonouri O. Lipoatrophy in a large pediatric diabetes outpatient service [Abstract]. Pediatr Diabetes. 2013;14(Suppl18):20.
Milan G, Murano I, Costa S, Pianta A, Tiengo C, Zulato E, et al. Lipoatrophy Induced by Subcutaneous Insulin Infusion: Ultrastructural Analysis and Gene Expression Profiling. J Clin Endocrinol and Metab. 2010;95:3126–32.
Sackey AH. Injection-Site Lipoatrophy. N Engl J Med. 2009;361:19 e41.
Schernthaner G. Immunogenicity and Allergenic Potential of Animal and Human Insulins. Diabetes Care. 1993;16 Suppl 3:155–65.
Hajheydari Z, Kashi Z, Akha O, Akbarzadeh S. Frequency of lipodysdrophy induced by recombinant human insulin. Eur Rev Med Pharmacol. 2011;15:1196–201.
Salgin B, Meissner T, Beyer P, Haberland H, Borkenstein M, Fussenegger J, et al. Lipoatrophy is Associated with an Increased Risk of Hashimoto’s thyroiditis and Coeliac Disease in Female Patients with Type 1 Diabetes. Horm Res Paediatr. 2013;79:368–72.
Fairweather DL, Frisancho-Kiss S, Rose NR. Sex differences in Autoimmune Disease from a Pathological Perspective. Am J Pathol. 2008;173:600–9.
Johansson UB, Amsberg S, Hannerz L, Wredling R, Admason U, Arnqvist HJ, et al. Impaired Absorption of Insulin Aspart From Lipohypertrophic injection sites. Diabetes Care. 2005;28:2025–7.
Heinemann L. Insulin Absorption from Lipodystrophic Areas: A (Neglected) Source of Trouble for Insulin Therapy. J Diab Sci Technology. 2010;4:750–3.
Chowdhury TA, Escudier V. Poor glycemic control caused by insulin-induced lipohypertrophy. Br Med J. 2003;327:383–4.
Noud MN, Renard E, McBride C, Cotterill AM, Harris M. Benefits of intra-peritoneal insulin administration in a child with severe insulin-induced lipoatrophy [Abstract]. Pediat Diabetes. 2009;10 Suppl 11:100.
Rosenbloom AL. Insulin Injection Lipoatrophy Recidivus. Pediat Diabetes. 2014;15:73–4.
Reeves WG, Allen BR, Tatterstall RB. Insulin induced lipoatrophy: evidence for an immune pathogenesis. Br Med J. 1980;280:1500–3.
Raile K, Noelle V, Landgraf R, Schwarz HP. Insulin antibodies are associated with lipoatrophy but also with lipohypertrophy in children and adolescents with type 1diabetes. Exp Clin Endocrinol Diab. 2001;109:393–6.
Takahashi K, Hakozaki A, Narazaki M, Takebe N, Ishigaki Y. Insulin antibodies are associated with lipoatrophy in adults with type 2 diabetes mellitus [Abstract]. Diabetologia. 2014;57 Suppl 1:S396.
Atlan-Gepner C, Bongrand P, Farnarier C, Xerri L, Choux R, Gauthier JF, et al. Insulin-induced Lipoatrophy in Type 1 Diabetes: A possible tumor necrosis factor- α- mediated dedifferentiation of adiposities. Diabetes Care. 1996;19:1283–5.
Lopez X, Castells M, Ricker A, Velazquez EF, Mun E, Goldfine A. Human Insulin Analog-Induced Lipoatrophy. Diabetes Care. 2008;31(3):442–4.
Jermendy G, Nadas J, Sapi Z. "Lipoblastoma-like" lipoatrophy induced by human insulin: morphological evidence for dedifferentiation of adipocytes? Diabetologia. 2000;43:955–6.
Ramos AJS, Farias MA. Human Insulin-Induced Lipoatrophy: A successful treatment with glucocorticoid. Diabetes Care. 2006;29:926–7.
Chantelau EA, Praetor R, Praetor J, Poll LW. Relapsing insulin-induced lipoatrophy, cured by prolonged low-dose oral prednisone: a case report. Diabetol Metab Syndr. 2011;3:33–7.
Chantelau EA, Prator R, Prator J. Insulin-induced localized lipoatrophy preceded by shingles (herpes zoster): a case report. J of Med Case Rep. 2014;8:223–8.
Chantelau E, Reuter M, Schotes S, Stark AA. Severe lipoatrophy with human insulin successfully treated by CSII. Diabet Med. 1993;10:580–1.
Phua EJ, Lopez X, Ramus J, Goldfine AB. Cromoyln Sodium for Insulin-Indueced Lipoatrophy: Old Drug, New Use. Diabetes Care. 2013;36:e204–5.
Logwin S, Conget L, Jansa M, Vidal M, Nicolau C, Gomis R. Human Insulin-Induced Lipoatrophy, Successful treatment with a jet-injection device. Diabetes Care. 1996;19:255–6.
Afreeza Prescribing Information. http://products.sanofi.us/afrezza/afrezza.html. Accessed June 21 2015.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SS, NHE, MHT all wrote the manuscript. MM did the literature search. MHT and NHE reviewed each ofthe 520 citations and concurred on those identified as lipoatrophy induced by insulin analog aspart, detemir, detemir plus aspart and NovoMix 30 injections. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Saberi, S., Esfandiari, N.H., MacEachern, M.P. et al. Detemir plus aspart and glulisine induced lipoatrophy: 2015 literature review and report of a new case. Clin Diabetes Endocrinol 1, 10 (2015). https://doi.org/10.1186/s40842-015-0013-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40842-015-0013-5