Abstract
The study was formulated to identify the effect of Mangifera indica leaf extract in inhibiting the growth and metamorphosis of Culex quinquefasciatus larva. Bioassay-guided extraction identified the bioactive fraction, after which GC-MS characterized it. The larvicidal activity was analyzed by administrating extract in various concentrations and then subjecting the mortality rate for probit analysis. The morphological and physiological impact upon larvae was understood by histological analysis and acetylcholinesterase activity assay. The results suggested that the extract possessed a high degree of larvicidal activity, whereas the Dose50 was 225.158 ± 15.168 with a Total Chi-Square of 13.09 and p-value of 0.11. The histological studies revealed notable aberrations among the study subjects compared to the control group due to diminished abdominal tissue integrity.
It was also observed that the extract could inhibit the acetylcholinesterase activity, with an LD 50 of 0.9512 µg/ml. The observations made in these studies may be utilized to develop a potential larvicidal agent that could act upon multiple targets.
Similar content being viewed by others
Background
Mosquitoes are notorious for transmitting infectious diseases, making them a global public health concern. These bloodsucking insects transmit various pathogens, including viruses, bacteria, and parasites, while feeding on their hosts. Infectious diseases transmitted by mosquitoes include malaria, dengue fever, Zika virus, chikungunya, and yellow fever [1]. The clinical manifestations of mosquito-borne diseases can range from mild flu-like symptoms to life-threatening conditions, depending on the specific pathogen involved [2]. The symptoms may include fever, headache, joint and muscle pain, rashes, and fatigue.
In some cases, neurological complications and hemorrhagic manifestations can lead to life-threatening complications or even death. Preventing and controlling mosquito-borne diseases requires vector management strategies, community education, and the development of effective vaccines and treatments. Understanding the clinical characteristics of mosquitoes as potential vectors is crucial for prompt diagnosis, treatment, and containment of potential outbreaks [3].
There are several mosquito species within the family Culicidae, each with its distinct traits. Malaria is carried mostly by Anopheles mosquitoes, one of the most infamous species. Aedes aegypti is infamous for transmitting diseases such as dengue, yellow fever, the Zika virus, and chikungunya. The disease-carrying Aedes albopictus, popularly known as the Asian tiger mosquito, has black-and-white striped legs. On the other hand, Culex mosquitoes are known carriers of West Nile Virus and Japanese encephalitis. Mansonia and Haemagogus are also linked to the spread of different kinds of encephalitis and yellow fever.
These species flourish in various settings and temperatures, and their behavioral patterns, feeding habits, and disease transmission capacity greatly impact global health issues [4]. Numerous diseases are transmitted by Culex mosquitoes, making them vital vectors for public health. Culex mosquitoes are exceptionally well-adapted to urban areas, where they commonly spawn in stagnant water. This trait, combined with their capacity to prey on birds and humans, enables them to survive in human-populated areas, raising the danger of disease transmission within the population. To lessen the detrimental impact of Culex mosquitoes on public health, disease control initiatives usually emphasize vector management and monitoring to lower mosquito populations and monitor the development of the diseases they transmit [5]. Anti-mosquito chemicals are substances designed to repel, kill, or disturb the life cycle of mosquitoes, making them vital weapons against diseases transmitted by mosquitoes. These substances include pesticides, insect repellents, and larvicides, among others. Often, insecticides kill adult mosquitoes through touch or ingestion, lowering their number and avoiding the spread of disease.
In contrast, insect repellents prevent mosquitoes from landing and feeding on humans, offering substantial personal protection. Larvicides target mosquito larvae at breeding grounds, inhibiting their maturation into adults and restricting population expansion at the source. Developing safe and effective anti-mosquito chemicals is essential for public health since they reduce the spread of diseases such as malaria, dengue, and Zika, ultimately improving the worldwide well-being of populations. To ensure the long-term effectiveness and sustainability of these compounds for mosquito control, however, responsible use, attention to safety protocols, and consideration of environmental implications are necessary [6].
The significance of natural compounds as mosquito repellents lies in their potential to provide effective, safe, and environmentally friendly alternatives to synthetic chemicals. Numerous natural compounds derived from plants, fungi, or microorganisms have inherent insecticidal and repellent properties used by indigenous communities for mosquito control for centuries [7]. Typically, these natural compounds have fewer adverse effects on non-target organisms and ecosystems, making them environmentally sustainable alternatives. In addition, the continued use of synthetic insecticides has led to resistance in mosquito populations, making it imperative to seek out new, inventive alternatives. Natural compounds offer a vast array of chemical structures, and their use can aid in circumventing the resistance issue. In regions where mosquito-borne diseases are prevalent, they are frequently accessible and inexpensive, allowing communities to conduct their vector control. This research and innovation can lead to the discovery of potent natural compounds that not only effectively combat mosquitoes but also minimize their harmful effects on the environment and human health, thereby bolstering our arsenal against mosquito-borne diseases [8]. The developing trend and favorable response of communities to phytochemicals and their environmentally beneficial behavior offer many opportunities for studying and inventing plant-based insecticides [9].
Mangifera indica leaves oil extracts have been determined to include hydrocarbons, triterpenes, phenolics, carotenoids, saponins, vitamins, and fatty acids as their principal ingredients, and these compounds are considered responsible for the repelling action on female African malarial agent Anopheles gambiae [10]. Previous studies reported the aqueous leaf extract of Mangifera indica and titanium dioxide (TiO2) and found it efficacious towards larvae of Culex quinquefasciatus with an LC50 value of 4.34 mg/L accordingly [11]. This current study analyzes the effect of essential oil from Mangifera indica leaf extract on Culex mosquito larvicidal activity.
The biocompatible nature of the extract could substitute the chemical counterparts in controlling mosquitoes and associated clinical implications based on modern scientific research.
Materials and methods
Collection and preparation of plant extract
Fresh Mangifera indica leaves were collected in and around the Thrissur district of Kerala, India, in May 2022. The sample was transported aseptically to the laboratory and analyzed for any anomalies such as diseases and infection; if any were discovered, those pieces were segregated and not used in the experiment.
The selected leaves were thoroughly rinsed to remove any dust or particles that had adhered to them. The leaves were then dried in the shade at room temperature. The leaves were finely pulverized using an electric blender. Hydro-distillation extracts volatile or polar components from plant materials using steam as an extraction solvent. A Clevenger apparatus or basic steam distillation device is frequently used for hydro-distillation. Sample combined water is heated in the Clevenger device to remove volatile components. Two layers were obtained, one aqueous and the other oil-rich. Oil can be further separated using funnels [12].
The extract was obtained by employing a Clevenger apparatus in the following manner: powdered leaf extract was subjected to hydro distillation with the Clevenger under optimal operational settings at a temperature of 40 °C. One hundred grams of extract (100 g) was dissolved with 800 cc of distilled water. The distillation lasted 3 h, after which the essential oil was collected and dehydrated with anhydrous sodium sulfate [13].
GC-MS analysis of bioactive fraction
The bioactive fractions present in Mangifera indica leaves were separated by column chromatography in which the stationary phase was constituted by silica gel 60–120 mesh size. In contrast, the organic solvents of various polarities were used as the mobile phase. Each fraction was analyzed for its bioactivity, and the fraction with maximum bioactivity was subjected to Gas Chromatography (Agilent 6890 series) equipped with HP-5MS column mass spectrometry operated at an initial column temperature of 30 °C and heated up to 300 °C at 10 °C for 15 min. Chromatographic conditions were high-purity helium’s 1.0 ml/min flow as a carrier gas in split mode. The compounds were identified in spectra based on retention time and the integral area of peaks. The similarity of compounds matched with references listed on the NIST library search [14].
Collection, maintenance and larvicidal activity of mosquitoes
The test mosquito larvae (Culex mosquito larvae) were procured from farmland in Kolazy Panchayath, Thrissur District, Kerala, India. They were then transported to MCAR (Marian Centre for Advanced Research), where they were kept under conditions of 25 °C, 75% relative humidity, and 14:10 (L/D) of light exposure.
Sets of 30 fourth instar larvae were administered the leaf extract in concentrations ranging from 0 to 500 mg/ml, with distilled water as the control. Observations of mortality and survival rates were made 24 h following exposure. The larvae received no nourishment during the testing time. All tests were carried out three times to validate the findings. The dead larvae were counted after each test container was tightly covered with a mosquito net, kept at room temperature, and protected from sunlight. The larvae were observed for behavioral changes, i.e., wriggling speed, horizontal and vertical movements, and self-biting behaviors. The larval behavior symptoms were recorded and compared with control larvae [15].
Dose-response bioassay
Dose-response bioassay was performed by collecting larvae in beakers containing sterile, deionized water. Then, different extract concentrations were made using 100 ml of water. The extract was diluted to concentrations between 0 and 500 mg/ml. Each experiment’s negative control was distilled water. All test containers were stored at room temperature without interruption. The LC50 and LC90 values of exposed larvae were determined using probit analysis [16].
Morphology and histopathology study
The third instar larvae of test mosquitoes were treated with 500 mg/ml MILE (Mangifera indica leaf extract). The control and treated larvae samples were fixed with 10% formalin, dried with a succession of ethyl alcohol, washed with xylene, and then sectioned. Standard staining techniques were used to stain the sectioned larvae and control samples with eosin and hematoxylin for light microscope observation and photographed with a Labomed microscope equipped with a digital color camera Micaps pro-HDMI [17].
Acetylcholinesterase activity assay for larvicidal activity
To determine the extract’s ability to inhibit the acetylcholinesterase enzyme, the following method prescribed by Hematpoor et al., 2016, was used with slight modifications. An electronic multichannel pipette (Eppendorf, USA) was used to transfer the extract 10 µL of enzyme solution to each well of a 96-well microtiter plate, further mixed with 20 µL of tested compounds in solution form and 150 µL of cold phosphate buffer, which were kept in refrigerator 1–4 °C. The assay microtiter plates were incubated at 25 °C for 10 min. Inhibitors were dissolved in DMSO (Merck, USA) in several concentrations ranging from 0 to 1 µg/ml and stocks diluted to give a final concentration of 0.1% DMSO (v/v) were added into test well in separated rows.
It was followed by adding 20 µL of acetylthiocholine iodide (ACTHI) (Sigma-Aldrich, USA) (0.4 mM) and DTNB (Sigma-Aldrich, USA) (0.3 mM) to the enzyme solution to observe the reaction. A yellowish or colorless solution was observed during the reaction for 30 min at room temperature. Changes in absorbance were recorded by a microplate reader (Synergy H1 Hybrid MultiMode Microplate Reader, USA) at 412 nm. Enzyme concentrations used were within the linear range of their toxicity activity.
\(\:\text{P}\text{e}\text{r}\text{c}\text{e}\text{n}\text{t}\text{a}\text{g}\text{e}\:\text{o}\text{f}\:\text{I}\text{n}\text{h}\text{i}\text{b}\text{i}\text{t}\text{i}\text{o}\text{n}=\frac{\left[\frac{\text{A}\:\text{c}\text{o}\text{n}-\text{A}\:\text{s}\text{a}\text{m}}{\text{A}\:\text{c}\text{o}\text{n}}\right]}{100}\), where A con is the absorbance of the control, and A sam is the absorbance of the test sample.
Results
Chromatographic analysis of bioactive fraction
The bioactive fraction of Magnifera indica was separated by the employment of Gas chromatography, followed by the identification by mass spectrometry. The GC-MS analysis suggested that the bioactive fraction is enriched with various bioactive fractions (Fig. 1), out of which the major compounds were identified as n-Eicosanol, which displayed itself at the retention time of 14.55 with a molecular weight of 597.1 g/mol. In contrast, Squalene and Linoleic acid were present at the retention temperature of 20 and 15.22, respectively, wherein the former weighed about 410.7 g/mol and later weighed about 280.4 g/mol.
1-Eicosanol is a naturally occurring fatty alcohol consisting of a hydroxy function at the C-1 position of an unbranched, saturated 20-carbon chain., whereas Squalene is a colorless liquid polyunsaturated hydrocarbon found naturally in many animals and plants, including human sebum. Linoleic acid, on the other hand, is a plant metabolite with two double bonds at positions 9 and 12 and Z (cis) stereochemistry.
Effect of extract on mosquito larvicidal activity
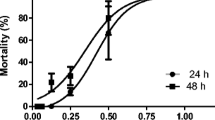
The larvicidal assay suggested that the bioactive fraction of Magnifera indica had an exceptional effect on inhibiting the persistence of Culex mosquito larvae in terms of mortality. Through the dose-response assay, it was evident that larval mortality could be initiated even in the least concentration of 50 µg/ml itself, and the mortality rate was immensely dependent upon increasing the concentration of the inhibitor (Fig. 2).
By the employment of probit analysis, it was understood that the Dose50 is 225.158 ± 15.168 with a Total Chi-Square of 13.09 and p-value of 0.11 (Table 1)Footnote 1
Morphology and histopathology study
Histological and morphological analysis revealed various information regarding extract action upon mosquito larvae. There were several behavioral changes, such as curving of the body and hook-like appearance of the anterior region exhibited by treated subjects, whereas this behavior was not seen in the control group. Similarly, the histological analysis showed significant differences between both groups. A monocellular layer (epithelium) rests on a basement membrane in the mid-gut of the third instar control. Both circular and longitudinal muscle fibers surround this membrane on the outside. The epithelium comprises columnar cells with groups of slightly regenerating cells, each with a sizeable nucleus and strongly basophilic cytoplasm.
A peritrophic membrane, which surrounds a lumen, is a detached sheath that shields the epithelium from food particles. The columnar cells appear unsaturated in position, shape, and size in the longitudinal section taken from the midgut’s anterior, middle, and posterior regions. All larvae exposed to the extract developed severe lesions, primarily affecting the mid-gut epithelium. The anterior region of the mid-cross-section gut also displayed swelling, extruded masses of cellular material, and disarray in the appearance of the columnar cells. In the posterior mid-gut, the epithelial cells appeared to have grown into the gut lumen and contained sizable cytoplasmic spaces. After 48 h, all three areas of the mid-gut disintegration appeared to be more severe (Fig. 3).
Effect of extract upon acetylcholinesterase activity
The acetylcholinesterase inhibition assay determined the extract’s ability to affect the larvae’s morphogenesis. The study suggested a clear connection between the enzyme’s activity and the extract’s presence (Fig. 4). The enzyme tends to reduce its activity with an increasing concentration of extract. At the higher concentration of extract, which is 1.5 µg/ml, the enzyme action was less than 10% compared to that of the untreated sample. Subsequently, the LD50 was determined to be 0.9512 µg/ml, whereas the regression equation of the graph was found to be y = 57.308x + 4.514.
Discussion
The conflict between man and mosquito has been noted since prehistoric times, according to the recent studies by Colón-González et al., 2021. It was comprehended that malaria suitability would increase by 1.6 months in tropical highlands in the African, Eastern Mediterranean, and Americas regions.
In contrast, Dengue suitability will increase by 4.0 months in lowlands in the Western Pacific and the Eastern Mediterranean regions, and the population at risk of both diseases may increase by up to 47 billion people by 2070 compared to 1970–1999. Therefore, finding more suitable or combined targets to control mosquitoes is essential. The current study suggests the possibility of developing a mosquito larvicide from the leaf extract of Magnifera indica that possesses morphological and physiological effects on larvae.Magnifera indica is native to South Asia, specifically present-day India, Bangladesh, and Myanmar. It has spread globally for thousands of years and thrives in tropical and subtropical regions [18. Mango (Mangifera indica) leaves, while less recognized than the fruit, have several pharmacological properties that have piqued the interest of traditional medicine systems and modern scientific research. Mango leaves, which are high in terpenoids, flavonoids, and tannins, have been shown to have antioxidant, anti-inflammatory, and antimicrobial properties [19].
The Chemometric analysis by employing gas chromatography linked with mass spectrometry suggested the presence of various bioactive compounds in the extract, such as n-Eicosanol, Squalene and Linoleic acid. Gas chromatography is a widely used method for characterizing bioactive compounds due to its ability to quantify, identify and separate the components based on their molecular weight and thereby provide a “fingerprint” of the bioactive fraction [20, 21]. It may be assumed that the larvicidal activity of the extract could be attributed to the chemical components present in the extract. A previous study reported by Perumalsamy et al., 2015, suggested that Linoleic acid and linolenic acid shall act on both AChE and octopaminergic receptors, thereby which it can inhibit the acetylcholinesterase. Similarly, n-Eicosanol was detected in the Elettaria cardamomum extract that could successfully inhibit AChE action [22].
By summing up these two observations, it may be assumed that Linolic acid and n-Eicosanol could have contributed immensely towards the acetylcholinesterase inhibition action exhibited by Mangifera indica leaf extract. The histological analysis showed similar observations with previous reports, whereas Plumeria pudica Jacq synthesized silver nanoparticles. Flower extract had resulted in histological lesions in the larvae [23], although in the current study also, the inhibitor caused a serious impact on the histological integrity of the larva. It is also noted that our observations were in terms with the observations done by Liu et al., 2020, where there were severe histological aberrations caused by Turmerone on Culex pipiens pallens Larvae, which is suggested to be the reason for the instant death of subjects. It may also be observed that the extract affected the larval gut’s tissue morphology, which would have affected the feeding habits of subjects and, therefore, inevitable mortality.
The morphological and physiological impact upon larvae was understood by histological analysis and acetylcholinesterase activity assay. The results suggested that the Mangifera indica leaf extract possessed a high degree of larvicidal activity, whereas the Dose50 was 225.158 ± 15.168 with a Total Chi-Square of 13.09 and p-value of 0.11. The GC-MS analysis suggested that the Mangifera indica leaf extract is enriched with various bioactive fractions such as n-Eicosanol, Squalene and Linoleic acid and found effective towards acetylcholinesterase inhibition.
Conclusion
Mangifera indica leaf extract could successfully inhibit the persistence and development of Culex larvae. The study suggested that the extract has multiple targets that could make this inhibitor superior to several counterparts. The extract could successfully inhibit the neural network by regulating acetylcholinesterase and, thereby, the metamorphosis of larvae into adults. In high concentrations, the extract could affect the histological integrity of larvae and, thereby, inevitably perish. This strategy shall be adopted for future larvicidal missions.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Notes
Based on probit analysis, Dose50 is 225.158 ± 15.168 with a Total Chi-Square of 13.09 and p-value of 0.11.
References
Dahmana H, Mediannikov O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens [Internet]. 2020;9:310. https://www.mdpi.com/2076-0817/9/4/310
Rahana VK, Anirudh ER, Ramesh PR, Aneesh EM, Pushpalatha E. Leaf extracts of three native medicinal plants, damages the reproductive organs of the filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). J Nat Pestic Res. 2023;6:100050.
Moola AK, Ayyadurai T, Balasubramani S, Vignesh R, Mohan PK, Sathish S, et al. Chemical composition and larvicidal activity against Aedes aegypti larvae from Hyptis suaveolens (L.) Poit essential oil. J Nat Pestic Res. 2023;3:100018.
Prabhu K, Sudharsan P, Ganesh Kumar P, Chitra B, Janani C. Impact of Piper betle L. bioactive compounds in larvicidal activity against Culex quinquefasciatus. J Nat Pestic Res. 2022;2:100013.
Guta W, Simma EA, Yewhalaw D. Species composition, blood meal sources and insecticide susceptibility status of Culex mosquitoes from Jimma area, Ethiopia. Int J Trop Insect Sci. 2021;41:533–9.
Kittichai V, Kaewthamasorn M, Samung Y, Jomtarak R, Naing KM, Tongloy T, et al. Automatic identification of medically important mosquitoes using embedded learning approach-based image-retrieval system. Sci Rep. 2023;13:10609.
da Silva MRM, Ricci-Júnior E. An approach to natural insect repellent formulations: from basic research to technological development. Acta Trop. 2020;212:105419.
da Costa KS, Galúcio JM, da Costa CHS, Santana AR, dos Santos Carvalho V, do Nascimento LD, et al. Exploring the potentiality of Natural products from essential oils as inhibitors of odorant-binding proteins: a structure- and ligand-based virtual Screening Approach to find Novel Mosquito repellents. ACS Omega. 2019;4:22475–86.
Şengül Demirak MŞ, Canpolat E. Plant-Based Bioinsecticides for Mosquito Control: Impact on Insecticide Resistance and Disease Transmission. Insects [Internet]. 2022;13:162. https://www.mdpi.com/2075-4450/13/2/162
Meza FC, Roberts JM, Sobhy IS, Okumu FO, Tripet F, Bruce TJA. Behavioural and Electrophysiological Responses of Female Anopheles gambiae Mosquitoes to Volatiles from a Mango Bait. J Chem Ecol [Internet]. 2020;46:387–96. http://link.springer.com/https://doi.org/10.1007/s10886-020-01172-8
Mahdi N, Ridha MR, Setiawan D, Praristiya MRS, Rahayu N, Atmaja BP. Bio-efficacy of Mangifera leaf extracts on mortality of Aedes aegypti and inhibition of egg hatching. Vet World [Internet]. 2022;1753–8. http://www.veterinaryworld.org/Vol.15/July-2022/20.html.
Samadi M, Abidin ZZ, Yunus R, Awang Biak DR, Yoshida H, Lok EH. Assessing the kinetic model of hydro-distillation and chemical composition of Aquilaria malaccensis leaves essential oil. Chinese J Chem Eng [Internet]. 2017;25:216–22. https://linkinghub.elsevier.com/retrieve/pii/S1004954116302208
Mukandiwa L, Eloff JN, Naidoo V. Larvicidal activity of leaf extracts and seselin from Clausena anisata (Rutaceae) against Aedes aegypti. South Afr J Bot. 2015;100:169–73.
Vadakkan K. Acute and sub-acute toxicity study of bacterial signaling inhibitor Solanum torvum root extract in Wister rats. Clin Phytoscience. 2019;5:19.
Al-Solami HM. Larvicidal activity of plant extracts by inhibition of detoxification enzymes in Culex pipiens. J King Saud Univ - Sci. 2021;33:101371.
Tabanca N, Ali Z, Bernier UR, Epsky N, Nalbantsoy A, Khan IA et al. Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum Lobelianum. 2018;16:324–32.
Al-Mehmadi RM, Al-Khalaf AA. Larvicidal and histological effects of Melia azedarach extract on Culex quinquefasciatus say larvae (Diptera: Culicidae). J King Saud Univ - Sci. 2010;22:77–85.
Alaiya MA, Odeniyi MA. Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: a review. Futur J Pharm Sci. 2023;9:29.
Kaurav M, Kanoujia J, Gupta M, Goyal P, Pant S, Rai S, et al. In-depth analysis of the chemical composition, pharmacological effects, pharmacokinetics, and patent history of mangiferin. Phytomedicine Plus. 2023;3:100445.
Vadakkan K, Cheruvathur MK, Chulliparambil AS, Francis F, Abimannue AP. Proteolytic enzyme arbitrated antagonization of helminthiasis by Cinnamomum cappara leaf extract in Pheretima Posthuma. Clin Phytoscience. 2021;7:23.
Vadakkan K, Hemapriya J, Selvaraj V. Quorum quenching intervened in vivo attenuation and immunological clearance enhancement by Solanum torvum root extract against Pseudomonas aeruginosa instigated pneumonia in Sprague Dawley rats. Clin Phytoscience [Internet]. 2019;5:24. https://clinphytoscience.springeropen.com/articles/https://doi.org/10.1186/s40816-019-0120-4
Chowdhury S, Kumar S. Alpha-terpinyl acetate: a natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. J Funct Foods. 2020;68:103892.
Suriyakala G, Sathiyaraj S, Gandhi AD, Vadakkan K, Mahadeva Rao US, Babujanarthanam R. Plumeria pudica Jacq. flower extract - mediated silver nanoparticles: Characterization and evaluation of biomedical applications. Inorg Chem Commun [Internet]. 2021;126:108470. https://linkinghub.elsevier.com/retrieve/pii/S1387700321000290
Acknowledgements
The authors thank the Marian Centre for Advanced Research (MCAR), the Research division of St. Mary’s College (Autonomous), Thrissur. Kerala, India, and DST-FIST for providing us with research infrastructure.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or Non-profit sectors.
Author information
Authors and Affiliations
Contributions
The idea was conceived by KV and SSA, wherein KV, SSA, MRM, BKD and VMP did the design of experiments, conduction of experiments and manuscript preparation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not Applicable.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vadakkan, K., Aravoor, S.S., Mundanttu, M.R. et al. Acetylcholinesterase inhibition mediated the larvicidal activity of Mangifera indica extract against Culex quinquefasciatus. Clin Phytosci 10, 12 (2024). https://doi.org/10.1186/s40816-024-00379-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-024-00379-6