Abstract
Background
There are multiple health benefits from participating in physical activity after a cancer diagnosis, but many people living with and beyond cancer (LWBC) are not meeting physical activity guidelines. App-based interventions offer a promising platform for intervention delivery. This trial aims to pilot a theory-driven, app-based intervention that promotes brisk walking among people living with and beyond cancer. The primary aim is to investigate the feasibility and acceptability of study procedures before conducting a larger randomised controlled trial (RCT).
Methods
This is an individually randomised, two-armed pilot RCT. Patients with localised or metastatic breast, prostate, or colorectal cancer, who are aged 16 years or over, will be recruited from a single hospital site in South Yorkshire in the UK. The intervention includes an app designed to encourage brisk walking (Active 10) supplemented with habit-based behavioural support in the form of two brief telephone/video calls, an information leaflet, and walking planners. The primary outcomes will be feasibility and acceptability of the study procedures. Demographic and medical characteristics will be collected at baseline, through self-report and hospital records. Secondary outcomes for the pilot (assessed at 0 and 3 months) will be accelerometer measured and self-reported physical activity, body mass index (BMI) and waist circumference, and patient-reported outcomes of quality of life, fatigue, sleep, anxiety, depression, self-efficacy, and habit strength for walking. Qualitative interviews will explore experiences of participating or reasons for declining to participate. Parameters for the intended primary outcome measure (accelerometer measured average daily minutes of brisk walking (≥ 100 steps/min)) will inform a sample size calculation for the future RCT and a preliminary economic evaluation will be conducted.
Discussion
This pilot study will inform the design of a larger RCT to investigate the efficacy and cost-effectiveness of this intervention in people LWBC.
Trial registration
ISRCTN registry, ISRCTN18063498. Registered 16 April 2021.
Similar content being viewed by others
Background
There are approximately 2.9 million people living with and beyond cancer (LWBC) in the UK, expected to rise to 4 million by 2030 [1]. People LWBC are at risk of adverse consequences of cancer and treatments, including fatigue, pain, osteoporosis, hypertension, cardiovascular disease, secondary cancers, anxiety, fear of cancer recurrence, and depression [2,3,4,5,6]. As a result, people LWBC often experience poorer quality of life and reduced survival when compared to the general population [2, 3, 7]. Developing interventions that can mitigate some of the negative effects experienced by people LWBC is therefore a public health priority [8].
A large body of evidence shows that physical activity has multiple benefits following a cancer diagnosis. Observational data suggest that people LWBC who are more active have reduced risk of cancer-specific and all-cause mortality (in the region of 25–41%), reduced risk of cancer recurrence, and experience less fatigue, pain, anxiety, depression, sleep disturbance, and better quality of life [9,10,11]. A recent review, including 679 exercise trials in people LWBC demonstrated that exercise training is safe across the cancer continuum and has beneficial outcomes on both physical and psychological functioning [12]. The evidence for the benefits of physical activity is particularly strong for breast, prostate, and colorectal cancer, three of the most commonly diagnosed cancers worldwide [12, 13]. Given the benefits, the World Cancer Research Fund recommends that people LWBC should aim for ≥ 150 min of at least moderate-intensity physical activity per week [14]. However, it is estimated that less than 30% of people LWBC are meeting these guidelines [15].
The Independent Cancer Taskforce has recommended that everyone diagnosed with cancer in the UK should receive physical activity advice as part of their routine care [16]. However, research from our group found that people LWBC often do not receive physical activity advice from oncology professionals as part of standard care, despite a desire to receive it [17,18,19]. Healthcare professionals (HCPs) report multiple barriers to delivery, including lack of knowledge of guidelines, feeling that they are not the ‘right person,’ and lack of time and resources [20]. This highlights the need for interventions that are feasible for implementation into care and accessible to a large number of patients. Many trials demonstrating the benefits of physical activity interventions after cancer are supervised by trained professionals, delivered in hospital or community settings, which can lead to high associated costs and limited accessibility [21]. In addition, the COVID-19 pandemic has put pressure on cancer services and changed models of care so it is likely that at least partial remote delivery will be required [22].
Digital interventions have potential for remote delivery of interventions, and smartphone apps are well-positioned as a platform due to their popularity and capabilities. Smartphone ownership continues to increase in all age groups; in 2021, 94% of adults aged over 55 in the UK owned a mobile phone, 83% of which were smartphones [23, 24]. Smartphone apps can track physical activity, deliver ‘in-the-moment’ behaviour change support, and, once developed, can be relatively cost-effective. A meta-analysis of 15 studies conducted by our group found that digital interventions could increase moderate-to-vigorous physical activity (MVPA) participation by approximately 40 min per week in people LWBC [25]. However, only two of these interventions were delivered via apps, and most were small pilot studies and used self-reported physical activity [25]. A subsequent review indicated that smartphone interventions may increase physical activity in people LWBC and that incorporating some element of personal contact could enhance efficacy [26]. This review also highlighted the importance of assessing cost-effectiveness [26]. In order for interventions to have a positive impact on long-term health, they need to promote behaviour change that will be maintained. One route to behaviour maintenance is establishing habits; behaviours which are cued by the contexts in which they are performed, rather than intentionally selected on each occasion they are performed [27]. Habit theory provides a basis on which to provide guidance to help people develop habits [28]. An additional consideration for app-based interventions is a need for sustainability beyond the end of research funding, so utilising/adapting publicly or commercially available apps could have potential.
The design of this study was further informed by qualitative user experience research evaluating existing, publicly available physical activity apps with 31 people diagnosed with breast, prostate, and colorectal cancer. This study identified that people LWBC reported a preference for an app-based physical activity intervention that targeted walking, had elements of tailoring to/recognition of their ability and cancer side-effects, and was endorsed by oncology HCPs and professional bodies [29]. Further qualitative interviews with 19 oncology clinical nurse specialists found that they were generally positive about physical activity apps and felt walking apps would be suitable for their patients before, during, and after treatment [30]. However, they highlighted the need for demonstrated efficacy before they would be willing to recommend them as part of cancer care [30]. Therefore, the ultimate aim of this work is to test the efficacy of an app-based walking intervention, informed by habit theory, and delivered to patients with breast, prostate, and colorectal cancer during their cancer care. The aims of the pilot study are to investigate the feasibility and acceptability of the outcome measures and procedures and obtain initial estimates of the parameters for the intended future primary outcome (device-measured physical activity).
Trial design
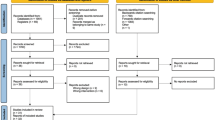
The proposed study is an individually randomised, two-arm pilot RCT comparing an app-based brisk walking intervention delivered alongside standard care, with a control (standard care) arm in people with breast, prostate, or colorectal cancer. The trial has been designed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement and its adaptation to pilot trials [31, 32]. See Fig. 1 for a flowchart of the study. The reporting of this protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (see Supplementary Material for a completed SPIRIT checklist) [33] A schedule of enrolment, interventions, and assessments based on the SPIRIT guidelines is shown in Fig. 2.
Eligibility criteria
Participants will be eligible if they have a confirmed diagnosis of breast, prostate, or colorectal cancer at a single hospital site in South Yorkshire, are aged 16 years or older, own a smartphone that uses Android or iOS (Apple) operating systems, are able to provide informed consent, have access to a computer and an email address, and are willing to complete online questionnaires. This hospital serves an area of high deprivation where cancer incidence, mortality, physical inactivity, and obesity are all high compared to the English average [34, 35]. Participants aged 16 and 17 years of age are not excluded from participation as it has been found that this group are keen to be included in cancer research and have often been overlooked in previous research [36, 37].
Exclusion criteria are as follows: having localised disease and it has been more than 6 months since completion of radical treatment (i.e. surgery to remove cancer, radiotherapy, systemic therapy with curative intent), being unable to understand spoken/written English, having an Eastern Cooperative Oncology Group (ECOG) performance status ≥ 3, a diagnosed cognitive impairment (e.g. dementia), a cognitive and/or physical impairment that prevents participation in brisk walking, a clinician-estimated life expectancy of < 6 months, or are receiving end of life care, due to have surgery to remove cancer in the next 5 months, < 6 weeks after surgery to remove cancer, report already achieving 150 min of at least moderate-intensity physical activity weekly, report previous/current use of the intervention app, or report current or recent (< 6 months) participation in a health behaviour change study.
Sample size
The target sample size for the pilot RCT is 60, with 30 participants allocated to each group. This is based on the rule of thumb that 30 or more participants are required to estimate a parameter in a feasibility study [38, 39]. A total of 90 participants will be recruited to account for potential loss to follow-up (assuming equal drop-out in both groups).
Recruitment and setting
Research nurses/members of the research team with contractual arrangements at the hospital site will search lists of current breast, prostate, and colorectal cancer patients and examine medical notes to identify potentially eligible participants. At least one clinician with responsibility for the patient’s care will review the list of potentially eligible participants and confirm whether a patient can be approached about the study.
Potentially eligible participants will be sent a letter informing them about the study. Participants who indicate interest will answer a telephone-based eligibility screening questionnaire. If eligible, participants will be sent an email with a link to the online participant information sheet and consent form (administered via REDCap electronic data capture tools hosted at University College London (UCL)) [40, 41]. The consent form (see Supplementary Material) includes asking for optional additional consent to access Hospital Episode Statistics (HES) and the National Cancer Registration and Analysis Service (NCRAS) registries to understand the willingness to consent to long-term follow-up of medical records. In this pilot, we will not access this data, but we ask participants to consent, as we would in a larger trial, in order to understand willingness to consent to this.
Randomisation
After completion of baseline assessments, participants will be individually randomised using minimisation with a 1:1 allocation ratio. Randomisation will be undertaken centrally using MinimPy (an open source, minimisation programme for allocation of participants to groups in randomised trials) [42]. Randomisation will be stratified by cancer type (breast, prostate, or colorectal) and disease status (advanced/metastatic disease vs. not). After the first participant has been randomly allocated, each subsequent participant will be allocated to the trial arm with the lowest imbalance score, with the addition of a 20% random element to reduce predictability of outcomes. The imbalance score is calculated based on hypothetical allocation of the next participant to each arm [43].
A member of the research team not involved with recruitment or data collection will use MinimPy to generate the allocation sequence as each person is recruited into the study. Intervention participants will be informed about their allocation arm by letter, with an appointment time for their intervention telephone/video call. Control participants will be informed via telephone or email.
Feasibility outcomes
The feasibility and acceptability outcomes are described in detail in Table 1 and include the recruitment and retention rates as well as app usage and engagement. These outcomes will be used to assess whether a future definitive trial could continue as per the current protocol, or if revisions are required before moving to the larger trial. The results of a power calculation will be considered alongside the recruitment and retention rates in order to estimate the number of participants that would need to be invited to provide the required sample size, to assess if this is feasible. In addition, if study enrolment is less than 30% or the 3-month retention rate is less than 65% we will consider if the trial procedures need modifying to make them more acceptable. Adaptations will be made to the assessment measures if the results indicate that these were not acceptable to participants or that participants were unable to complete them online. If more than 50% of the intervention participants do not download or use the app, or we are not able to deliver the behaviour change techniques (BCTs) to them as planned then this will trigger discussion about acceptability of the intervention. In lieu of clear published guidelines to base these values on these are pragmatic decisions that will trigger discussion about adaptation of the protocol. As part of the economic evaluation, a value of information analysis will be conducted. Value of information analysis helps ascertain the likely value of obtaining further information and therefore may provide useful information in the context of proceeding to a full RCT. While the listed criteria will be the primary criteria considered we will also examine the results from all data collected and any issue relating to successful trial delivery will inform decisions about progressing to a larger trial.
Intervention
The intervention is described according to the template for intervention description and replication (TIDiER) checklist which is provided as Supplementary Material [44]. The intervention was designed with input from people affected by breast, prostate, and colorectal cancer throughout, including our background empirical research and several Patient and Public Involvement (PPI) activities [25, 29, 30].
The intervention involves the Active 10 app along with additional behavioural support (outlined in the subsequent sections).
The Active 10 app was developed by Public Health England and will be maintained by its successor bodies. It was selected for the current study because it contained a number of the features that people LWBC and clinicians highlighted as important and is developed and maintained by a UK health agency, sponsored by the UK Government [29, 30, 45]. Screenshots of the Active 10 app are presented in Fig. 3. The app encourages users to walk briskly for 10 min (known as one ‘Active 10’) and users can set a goal to complete between 1 and 3 Active 10s per day, with the ultimate aim of reaching 30 min of at least moderate-intensity physical activity per day. Each minute of brisk walking counts towards the Active 10 goals, to reflect the recent change in the UK physical activity guidelines removing the guidance that bouts of at least 10 min were required [46]. The app distinguishes between total walking and brisk walking. Brisk walking confers greater health benefit than slower paced walking and is captured by Active 10 when participants walk at a rate of approximately 100 steps per minute or more [47].
Participants will be mailed a pack that contains a leaflet recommending the use of/describing how to download the Active 10 app as well as information about the importance of physical activity after cancer. The pack will also contain walking planners designed to support action planning and self-monitoring of walking plans. These materials will be accompanied by a letter from the participant’s clinical care team endorsing physical activity and using Active 10. Participants will also receive additional behaviour change support from the research team via two telephone/video calls, one shortly after randomisation and a second after 4 weeks. During the initial call researchers will discuss the recommended physical activity guidelines for people LWBC, the associated benefits of meeting these guidelines and of increasing physical activity by any amount; work through the concept of habit formation and using the walking planner; help with setting daily walking goals; help with developing a plan/habit for opening the app; and help with downloading the app for participants who have not already done so. During the second call, researchers will check if participants are using the app and increasing their brisk walking; remind them of their goals; and recap any of the information from the first call. These calls are intended to closely replicate conversations that a health care professional could have with a patient as part of routine care, should this intervention be implemented on a larger scale.
Theoretical basis of the intervention
Active 10 is a publicly available app and was not developed specifically for this study. Therefore, the app content was independently coded according to the Behaviour Change Technique Taxonomy (BCTTv1) (PL, NM), any discrepancies were discussed before agreement was reached on the techniques used [48]. Table 2 outlines the intervention components across the five elements of the intervention and the relevant coded BCTs [48]. The central feature of the Active 10 app is that it allows participants to monitor their activity. Self-monitoring of physical activity using a variety of technologies has been shown to successfully promote increases in physical activity in the general population and among people LWBC [49, 50]. The intervention content provided in addition to the Active 10 app was informed by habit theory and includes BCTs that have shown efficacy in promoting physical activity in inactive adults, have been associated with improved adherence to physical activity interventions in people LWBC, and that were practical to use in the context of providing brief written materials and behavioural support [28, 51,52,53,54].
Habit theory posits that habitual behaviours are performed when an impulse to act is automatically triggered in a particular situation by virtue of a mental association having formed between that situation and behaviour through repetition [27]. Habits predict behaviour particularly on days when people’s intentions are lower than usual and therefore support maintenance of behaviour, shielding it from temporary lapses in motivation [55]. In order to form a habit, a behaviour needs to be performed consistently in the same situation (termed context-dependent-repetition) [28]. It is possible to succinctly deliver this advice to participants, and interventions using this technique have shown positive changes in behaviour [56]. Distinction has been made between the habit of initiating a behaviour (i.e. an instigation habit) and that of performing it (i.e. an execution habit), and it is the instigation habit that predicts behaviour maintenance [57,58,59]. The advice given in the intervention leaflet and phone calls therefore recommend participants focus on forming instigation habits for doing physical activity, while gradually increasing the amount or intensity of activity they do on each occasion.
Control
Participants randomised to the control group will receive only the study assessments and continue with their standard care. This is so that in the definitive trial the impact of the intervention over and above standard care can be evaluated.
Measures
Measurement timepoints for the pilot are baseline (T0) and 3 months (T1; operationalised as 12–16 weeks from randomisation). When a participant provides consent they will be sent URL links to online questionnaires via email (with the option to complete these by phone with a researcher if they experience difficulties), and mailed weighing scales (Seca 803 if they weigh less than 150kg and Seca 813 if they weigh over 150kg), a tape measure (Seca 201), and an activPAL4micro accelerometer (PAL Technologies Ltd., Glasgow, UK).
Sociodemographic & disease characteristics (T0)
Details of each participant’s cancer diagnosis, treatment(s), and other health conditions (including previous cancer diagnoses) will be recorded from their hospital medical notes.
In the online questionnaires, participants will report their age, gender, employment, education, marital status, living arrangements, and ethnicity. Participants will be asked to self-report details of their cancer diagnosis, treatment, and other comorbid health conditions. This is to capture any further health information that is not included in the hospital medical records (e.g. information that would otherwise be included in GP records, or records from other hospitals). Participants’ postcodes will be used to determine socioeconomic position (Index of Multiple Deprivation) [60].
Physical activity (T0 and T1)
Physical activity will be measured using thigh-worn activPAL4micro accelerometers that participants will be asked to wear continuously for seven days (PAL Technologies Ltd., Glasgow, UK). The activPAL protocol, which follows published recommendations for using the device and expert advice from the Trial Steering Committee, includes waterproofing the device using specially designed nitrile sleeves and waterproof dressings, asking participants to wear it continuously, and including the data for analysis if 3 days of data are available [61]. Participants will be mailed instructions on how to wear the device, a log-sheet to record when the device was worn and bedtimes and waketimes, and a freepost envelope to return it. The primary outcome of the future full RCT will be activPAL-assessed average daily brisk walking (> 100 steps/min). The activPAL has shown excellent reliability and validity in measuring step number and cadence (steps/minute) and has been used in other studies with people LWBC and clinical populations [62, 63]. Other physical activity outcomes that will be explored are total daily steps, minutes of light physical activity, standing time and sitting time.
Participants will also complete the Godin Leisure-Time Exercise Questionnaire (GLTEQ) [64], which has demonstrated favourable validity and reliability against objective measures of physical activity and is widely used in oncology research [64, 65]. The questionnaire will be adapted to add a question about duration of activities to allow calculation of minutes of MVPA, in addition to the leisure score index, a practice that is common in oncology research [65].
Anthropometric outcomes (T0 and T1)
Participants’ height, weight (without outer clothing/shoes on) and waist circumference (at umbilicus) will be measured by participants in their own homes using the study weighing scales and measuring tapes provided. Written instructions will be included to help participants complete these measurements accurately. Studies suggest that self-reported weight is sufficiently reliable and accurate where objective measurement is not feasible [66, 67]. BMI will be calculated using the standard formula of weight (kg)/height (m)2. Self-measured waist circumference is also appropriate for large-scale studies where objective measurement is not feasible [68].
Well-being (T0 and T1)
Health status will be measured using the five-level EuroQol-5D questionnaire (EQ-5D-5L), which has established reliability and validity from a review of 12 studies of cancer patients [69]. This will be used to generate quality-adjusted life years (QALYs) to facilitate the cost-effectiveness analysis.
Cancer-specific quality of life will be measured using the Functional Assessment of Cancer Therapy-General (FACT-G) scale [70]. The FACT-G is a 28-item questionnaire that has excellent test-retest reliability (r = .92) and has been validated against the Functional Living Index-Cancer (FLIC) (r = .79) [70, 71].
Fatigue will be measured using the 13-item fatigue subscale of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) (previously Functional Assessment of Cancer Therapy-Fatigue (FACT-F)) questionnaire [72]. The 13-item fatigue subscale of the FACIT-F has excellent test-retest reliability (r = .90), internal consistency (α = .93–.95), and has been validated against the Profile of Mood States (POMS) fatigue (r = − .74), POMS vigour (r = .66) and Piper fatigue (r = − .75) [72,73,74].
Sleep quality will be assessed using the Pittsburgh Sleep Quality Index (PSQI), an 18-item questionnaire that assesses sleep quality and disturbances over a 1-month time interval [75]. The PSQI has been used extensively and has good psychometric properties in both clinical (including cancer) and non-clinical samples [76]. In women with breast cancer, the PSQI has been demonstrated to have high internal consistency (α = .80) and good validity when compared against related constructs such as sleep problems (in the Symptom Experience Report; r = .65) and sleep restlessness (in the Center for Epidemiological Studies Depression Scale; r = .69) [77].
Anxiety will be measured with the Generalised Anxiety Disorder Assessment (GAD-7), which has good test-retest reliability (r = .83), excellent internal consistency (α = .92), and has been validated against the Beck Anxiety Inventory (r = .72) [78]. Depression will be measured with the Patient Health Questionnaire (PHQ-9), which shows adequate diagnostic accuracy in cancer patients [79,80,81].
Physical activity self-efficacy will be measured using the Physical Activity Appraisal Inventory (PAAI), which has demonstrated excellent reliability in women with breast cancer (α = .96) [82]. The PAAI also has established validity [82]. Self-efficacy to self-manage cancer will be measured using the Cancer Survivors Self-Efficacy Scale (CS-SES), an 11-item questionnaire with excellent reliability (α = .92) [83].
Habit strength for walking (‘going for a walk’ and ‘walking briskly’) will be measured with the Self-Report Behavioural Automaticity Index (SRBAI), which has established reliability and has shown to be sensitive to hypothesised effects of habit on behaviour [84].
Health and social care service use will be measured using the Client Service Receipt Inventory (CSRI), which has been validated against objective primary care records and is also recommended for usage of hospital and other community health services [85].
App use (T1)
Participants in both groups will also be asked to report their usage of any physical activity app, or any other attempts to change their physical activity, during the study period, and asked what prompted them to do this. Intervention group participants will be asked to complete brief intervention feedback questions (including a self-report question of whether they ever downloaded Active 10) and will be asked to self-report their usage of Active 10 throughout the study period (never, once, less than monthly, monthly, fortnightly (every 2 weeks), weekly, 3–4 times per week, almost every day or every day). Intervention participants will also be asked to complete questions from the Digital Behaviour Change Interventions Engagement Scale [86].
Timing of physical activity (T1)
In a definitive trial, we would conduct an exploratory analysis using the activPAL data alongside habit strength to investigate whether those who walk in the morning have higher habit strength than those who walk in the evening. Previous research suggests that habits form quicker in the morning than the evening [87]. Chronotype (individual differences in sleep timing and in preferences for a given time of day) will be considered a covariate in this analysis and assessed using a sub-scale of the Morningness-Eveningness questionnaire (MEQ) [88, 89]. This sub-scale has been found to be reliable and to correlate well with the full scale [90].
Qualitative interviews (decliners at T0 and participants at T1)
To further understand how a future intervention could be designed to be as inclusive as possible, individuals who decline to participate will be asked to briefly provide reasons if they are willing. They will also have the option to consent to participate in an interview to further explore reasons, and up to 30 interviews will be conducted. This method has been utilised succesfully in an ongoing exercise trial with myeloma patients [63].
Interviews will also be carried out at the end of the trial with any participants who agree to be interviewed. The interviews will take place after a participant has completed all other data collection at T1 and will be conducted to explore experiences of participation, being randomised, and views on providing permission to access NCRAS/HES data. Intervention arm participants will also be interviewed about their experiences of using the app, the intervention materials, and their perceptions of app usage and engagement. Participants will be invited to submit photographs of the places that they walk. This is optional, but if participants consent, they will be able to upload pictures to a secure website along with brief details of where the photograph was taken, why they were walking there, how long they walked and any additional details they wish to share. Photographs will be used to prompt discussion about the environments chosen for brisk walking. This method of photo-elicitation has been used to understand walking environments in previous work and led to a more in-depth understanding of barriers and facilitators to walking [91]. All interviews will be semi-structured and based on a topic guide, take place via telephone, and will be audio-recorded and transcribed verbatim.
Statistical analysis
Baseline comparability of the randomised groups will be assessed using descriptive statistics (e.g. age (continuous), gender (categorical), key outcome measures (e.g. physical activity participation)). The outcomes outlined in Table 1 will be reported descriptively. Any reasons provided for declining not covered in the specific outcomes will also be reported, as well as details of answers provided to the feedback questionnaire, within the intervention group. Mean and standard deviation estimates for the intended primary outcome measure at 3 months (activPAL measured average daily minutes of brisk walking (> 100 steps/min)) will be calculated and reported descriptively. These results will be used to inform a sample size calculation for the future definitive trial.
An initial economic evaluation will be conducted to provide an estimate of the potential cost-effectiveness of the intervention. QALYs will be estimated based upon the EQ-5D-5L combined with standard valuation sources [92, 93]. Costs will include the costs associated with the intervention (promotional materials, time spent delivering the intervention recommendation) and other NHS resource use measured using the CSRI. Costs and QALYs will be combined in an analysis to estimate the incremental cost-effectiveness ratio (ICER) associated with the intervention compared to the control group. Uncertainty in the results will be characterised using cost-effectiveness planes and cost-effectiveness acceptability curves [94]. A value of information analysis will also be conducted at this stage. The expected value of perfect information (EVPI) and expected value of perfect partial information (EVPPI) will be estimated.
Qualitative analysis
Qualitative interviews will be analysed thematically using an approach that is both deductive and inductive, to ensure that the full range of participants' responses are represented, following the steps outlined by Braun and Clarke [95]. These findings will add more in-depth understanding to the feasibility and acceptability analysis described above.
Ethical considerations
The trial protocol has been approved by the Yorkshire & The Humber - South Yorkshire Research Ethics Committee (21/YH/0029) and by the Health Research Authority. The Research and Development Department at the study site has also authorised the study to go ahead following a capacity and capability review. Potential amendments to the protocol will only be implemented if ethical and regulatory approval, including NHS permission where required, is obtained.
Consent
Consent from each participant prior to participation in the trial will be collected online through REDCap hosted within UCL’s Data Safe Haven (details below) [96]. All participants will be informed that they are under no obligation to enter the trial. All participants will also be informed that they can withdraw at any time during the trial without having to give a reason, and that withdrawal will not affect the medical care they receive.
Confidentiality
This study has been registered for Data Protection at UCL Records Office (Reference: Z6364106/2020/10/29). All data will be handled in accordance with the General Data Protection Regulation (2018) and the UK Data Protection Act (2018). Personal data will only be collected if it is deemed essential for the study. Wherever possible, personal data that has been collected will be pseudoanonymised. No identifiable personal data will be used in dissemination of the research.
Adverse event reporting
All adverse events (AEs) and serious AEs (SAEs) that the research team become aware of will be assessed for severity, causality, and seriousness. All AEs and SAEs will be recorded. All SAEs will be reported to the sponsor. Events that are unexpected and thought to be related to the intervention will be reported to the Health Research Authority. In this population, a range of SAEs would be expected that relate to their cancer diagnosis and treatment, including episodes of acute illness, infection, new medical problems, and deterioration of existing medical problems. These could result in hospitalisation, permanent disability or incapacity, or death. These would not however be related to the intervention. There is one potential expected AE related to participation in the study; participants might experience mild skin irritation from the adhesive dressing when wearing the activPAL device as this has been observed previously in older adults [97].
Data monitoring
The sponsor of this trial is UCL. A Trial Management Group (TMG) consisting of the Chief Investigators (AF and PL) and research staff employed on the grant (FK and CM) will be responsible for overseeing the trial. An external Trial Steering Committee, including two independent members, the trial co-investigators, and a lay representative will meet once-twice per year (alongside the TMG) and will provide overall supervision of the trial. There will be no formal data monitoring committee and no criteria have been set for stopping the study early as this is a pilot study and walking is a very low-risk intervention.
Data management
All study data will be stored securely within the UCL Data Safe Haven encrypted platform [91]. The Data Safe Haven is built using a walled garden approach where the data is stored, processed, and managed within a secure environment [91]. Only members of the research team will be able to access the dataset.
Data archiving
All electronic research data will be stored securely within the UCL Data Safe Haven for 12 years after the trial end date, after which point the data will be completely anonymised (by removing the study pseudonym and deleting all contact details including proof of consent) [91]. The anonymised dataset will be entered into the UCL Data Repository and available here for at least 20 years from the trial end date. Other study-related documents will be archived at UCL and each participating site for 20 years from the trial end date and in line with all relevant legal and statutory requirements.
Dissemination
The results of this study will be disseminated through peer-reviewed publications and conference presentations. The results will also be disseminated through social media outlets such as Twitter, and via our PPI representatives.
Discussion
Conducting a pilot and assessing the feasibility of study procedures is an essential part of developing and evaluating complex interventions [98]. This pilot study follows recommendations to use both quantitative and qualitative measures to assess the feasibility and acceptability of the study design in as much detail as possible [98]. The definitive trial that will be run following revision to the current methodology, as required, will address the limited evidence base for theory-driven smartphone app-based interventions designed to promote physical activity in people LWBC. Yorkshire Cancer Research have awarded our group a grant to complete this pilot study and a larger trial, meaning that the definitive trial will be able to proceed quickly once the results of this pilot have been analysed.
The strengths of the intervention include its tailored and theoretically-informed approach to promote brisk walking, an activity that is perceived as safe, achievable, enjoyable, and sustainable by people LWBC [29]. The design of the intervention has been informed based on input from people LWBC. This intervention is accessible, low cost, and scalable, due to the use of an app maintained by Public Health England (or its successor bodies). It is hoped that the intervention could be replicated by a HCP as part of routine care for people LWBC, should it be implemented on a larger scale. A strength of the trial design is the use of objective physical activity measurement.
A limitation of the intervention is that it is only accessible to participants who own a smartphone. However, this intervention would be most appropriate for those who already have a smartphone and as these rates are increasing, this is becoming the majority of the population [23, 24]. We will collect data on how many participants are excluded because they do not own a smartphone so that this can be addressed in a future RCT if required. Another limitation is that the study is taking place in one hospital site in South Yorkshire, and thus it may be that the feasibility results do not generalise to all hospital sites. However, as this hospital is in a deprived area recruitment rates would likely be similar or better in other areas. Also, as the recruitment approach does not involve face-to-face contact with participants it is likely transferable across sites. It is possible that the intervention may be more or less acceptable in this area than others; however, if it is acceptable here, this would imply that it is acceptable to those most in need.
As the Active 10 app is publicly available it is possible that those in the control group could use it during the study. However, the intervention is a package that includes a recommendation to use this app alongside behavioural support to do so. Therefore, even if a control group participant does start using the app this is not equivalent to being in the intervention group. Patients have reported that they are keen for health professionals to recommend an app to them [29] therefore we think it unlikely that many of the control group will seek out the app on their own. We will assess this as a feasibility outcome.
To conclude, this pilot RCT will provide information regarding the feasibility and acceptability of testing this intervention to inform a future definitive RCT. The trial will also provide estimates of the parameters of the intended primary outcome measure for a sample size calculation for a future trial. Should the intervention be feasible, with or without adaptations, a future definitive RCT will aim to investigate the clinical and cost-effectiveness of the intervention among people affected by cancer.
Availability of data and materials
No data is reported in this protocol. The full intervention materials are not supplied with the protocol paper since recruitment for the pilot had not started at the time of paper submission and we do not want our materials to be available in the public domain until the end of the trial.
Abbreviations
- AE:
-
Adverse event
- BCT:
-
Behaviour change technique
- BMI:
-
Body mass index
- CONSORT:
-
Consolidated Standards of Reporting Trials
- CSRI:
-
Client Service Receipt Inventory
- CS-SES:
-
Cancer Survivors Self-Efficacy Scale
- ECOG:
-
Eastern Cooperative Oncology Group
- EQ-5D-5L:
-
Five-level EuroQol-5D
- EVPI:
-
Expected value of perfect information
- EVPPI:
-
Expected value of perfect partial information
- FACIT-F:
-
Functional Assessment of Chronic Illness Therapy-Fatigue
- FACT-F:
-
Functional Assessment of Cancer Therapy-Fatigue
- FACT-G:
-
Functional Assessment of Cancer Therapy-General
- FLIC:
-
Functional Living Index-Cancer
- GAD-7:
-
Generalised Anxiety Disorder Assessment
- GLTEQ:
-
Godin Leisure-Time Exercise Questionnaire
- HCP:
-
Healthcare professional
- HES:
-
Hospital Episode Statistics
- ICER:
-
Incremental cost-effectiveness ratio
- LWBC:
-
Living with and beyond cancer
- MVPA:
-
Moderate-to-vigorous physical activity
- NCRAS:
-
National Cancer Registration and Analysis Service
- PAAI:
-
Physical Activity Appraisal Inventory
- PHQ-9:
-
Patient Health Questionnaire-9
- POMS:
-
Profile of Mood States
- PPI:
-
Patient and Public Involvement
- PSQI:
-
Pittsburgh Sleep Quality Index
- QALY:
-
Quality-adjusted life year
- RCT:
-
Randomised controlled trial
- SAE:
-
Serious adverse event
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
- SRBAI:
-
Self-Report Behavioural Automaticity Index
- TIDieR:
-
Template for intervention description and replication
- TMG:
-
Trial Management Group
- UCL:
-
University College London
References
Maddams J, Utley M, Moller H. Projections of cancer prevalence in the United Kingdom, 2010-2040. Br J Cancer. 2012;107(7):1195–202.
Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;19(1):943.
Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609.
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–90 e9.
Roy S, Vallepu S, Barrios C, Hunter K. Comparison of comorbid conditions between cancer survivors and age-matched patients without cancer. J Clin Med Res. 2018;10(12):911–9.
Demoor-Goldschmidt C, de Vathaire F. Review of risk factors of secondary cancers among cancer survivors. Br J Radiol. 2019;92(1093):20180390.
Annunziata MA, Muzzatti B, Flaiban C, Gipponi K, Carnaghi C, Tralongo P, et al. Long-term quality of life profile in oncology: a comparison between cancer survivors and the general population. Support Care Cancer. 2018;26(2):651–6.
Mosher CE, Sloane R, Morey MC, Snyder DC, Cohen HJ, Miller PE, et al. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer. 2009;115(17):4001–9.
Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–311.
Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the Cancer of the Prostate Strategic Urologic Research Endeavor. Cancer Res. 2011;71(11):3889–95.
Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22(19):4766–75.
Christensen JF, Simonsen C, Hojman P. Exercise training in cancer control and treatment. Compr Physiol. 2018;9(1):165–205.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–49.
World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report. Diet, nutrition, physical activity and breast cancer. 2018.
Garcia DO, Thomson CA. Physical activity and cancer survivorship. Nutr Clin Pract. 2014;29(6):768–79.
Independent Cancer Taskforce. Achieving world-class cancer outcomes - a strategy for England 2015-2020. https://www.cancerresearchuk.org/sites/default/files/achieving_world-class_cancer_outcomes_-_a_strategy_for_england_2015-2020.pdf. Accessed 10 Sept 2021.
Smith L, Croker H, Fisher A, Williams K, Wardle J, Beeken RJ. Cancer survivors’ attitudes towards and knowledge of physical activity, sources of information, and barriers and facilitators of engagement: A qualitative study. Eur J Cancer Care (Engl). 2017;26(4). https://doi.org/10.1111/ecc.12641. Epub 2017 Jan 30.
Koutoukidis DA, Beeken RJ, Lopes S, Knobf MT, Lanceley A. Attitudes, challenges and needs about diet and physical activity in endometrial cancer survivors: a qualitative study. Eur J Cancer Care (Engl). 2017;26(6). https://doi.org/10.1111/ecc.12531. Epub 2016 Jun 21.
Humphreys L, Crank H, Dixey J, Greenfield DM. An integrated model of exercise support for people affected by cancer: consensus through scoping. Disabil Rehabil. 2020:1–10. https://doi.org/10.1080/09638288.2020.1795280. Epub ahead of print.
Koutoukidis DA, Lopes S, Fisher A, Williams K, Croker H, Beeken RJ. Lifestyle advice to cancer survivors: a qualitative study on the perspectives of health professionals. Bmj Open. 2018;8(3):e020313.
Grimmett C, Corbett T, Brunet J, Shepherd J, Pinto BM, May CR, et al. Systematic review and meta-analysis of maintenance of physical activity behaviour change in cancer survivors. Int J Behav Nutr Phys Act. 2019;16(1):37.
Greenwood E, Swanton C. Consequences of COVID-19 for cancer care — a CRUK perspective. Nat Rev Clin Oncol. 2021;18(1):3–4.
OFCOM. OFCOM TECHNOLOGY TRACKER 2021. 14th January to 31st March 2021. https://www.ofcom.org.uk/__data/assets/pdf_file/0015/219102/technology-tracker-2021-data-tables.pdf. Accessed 10 Sept 2021.
OFCOM. OFCOM NATIONS & REGIONS TECHNOLOGY TRACKER - 2020. 9th January to 7th March 2020. https://www.ofcom.org.uk/__data/assets/pdf_file/0037/194878/technology-tracker-2020-uk-data-tables.pdf. Accessed 10 Sept 2021.
Roberts AL, Fisher A, Smith L, Heinrich M, Potts HWW. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2017;11(6):704–19.
Khoo S, Mohbin N, Ansari P, Al-Kitani M, Müller AM. mHealth interventions to address physical activity and sedentary behavior in cancer survivors: a systematic review. Int J Environ Res Public Health. 2021;18(11):5798. https://doi.org/10.3390/ijerph18115798.
Gardner B. A review and analysis of the use of ‘habit’ in understanding, predicting and influencing health-related behaviour. Health Psychol Rev. 2015;9(3):277–95.
Lally P, Gardner B. Promoting habit formation. Health Psychol Rev. 2013;7(sup1):S137–S58.
Roberts AL, Potts HW, Koutoukidis DA, Smith L, Fisher A. Breast, prostate, and colorectal cancer survivors’ experiences of using publicly available physical activity mobile apps: qualitative study. JMIR Mhealth Uhealth. 2019;7(1):e10918.
Roberts AL, Potts HWW, Stevens C, Lally P, Smith L, Fisher A. Cancer specialist nurses’ perspectives of physical activity promotion and the potential role of physical activity apps in cancer care. J Cancer Surviv. 2019;13(5):815–28. https://doi.org/10.1007/s11764-019-00801-w. Epub 2019 Sep 2.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64.
Schulz KF, Altman DG, Moher D, the CG. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. Bmj. 2013;346:e7586.
South Yorkshire’s Community Foundation. South Yorkshire’s Vital Signs Report 2019. 2019.
Public Health England, NHS England, Yorkshire Cancer Research. Cancer in Yorkshire and the Humber. 2016.
Aldiss S, Fern LA, Phillips RS, Callaghan A, Dyker K, Gravestock H, et al. Research priorities for young people with cancer: a UK priority setting partnership with the James Lind Alliance. Bmj Open. 2019;9(8):e028119.
Taylor RM, Solanki A, Aslam N, Whelan JS, Fern LA. A participatory study of teenagers and young adults views on access and participation in cancer research. Eur J Oncol Nurs. 2016;20:156–64.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–12.
Browne RH. On the use of a pilot sample for sample-size determination. Stat Med. 1995;14(17):1933–40.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208. Epub 2019 May 9.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
MinimPy. MinimPy 0.3 Reference Manual. http://minimpy.sourceforge.net. Accessed 10 Sept 2021.
Altman DG, Bland JM. Treatment allocation by minimisation. Br Med J. 2005;330(7495):843.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Bmj. 2014;348:g1687.
Brannan MGT, Foster CE, Timpson CM, Clarke N, Sunyer E, Amlani A, et al. Active 10 - a new approach to increase physical activity in inactive people in England. Prog Cardiovasc Dis. 2019;62(2):135–9. https://doi.org/10.1016/j.pcad.2019.02.001. Epub 2019 Feb 21.
UK Chief Medical Officers’ Physical Activity Guidelines. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf. Accessed 10 Sept 2021.
Stamatakis E, Kelly P, Strain T, Murtagh EM, Ding D, Murphy MH. Self-rated walking pace and all-cause, cardiovascular disease and cancer mortality: individual participant pooled analysis of 50 225 walkers from 11 population British cohorts. Br J Sports Med. 2018;52(12):761–8.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95.
Ormel HL, van der Schoot GGF, Westerink NL, Sluiter WJ, Gietema JA, Walenkamp AME. Self-monitoring physical activity with a smartphone application in cancer patients: a randomized feasibility study (SMART-trial). Support Care Cancer. 2018;26(11):3915–23.
Page EJ, Massey AS, Prado-Romero PN, Albadawi S. The use of self-monitoring and technology to increase physical activity: a review of the literature. Perspect Behav Sci. 2020;43(3):501–14.
Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Taylor SJ, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9:CD010192.
Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, Saxton JM, Taylor SJ. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2013;(9):CD010192. https://doi.org/10.1002/14651858.CD010192.pub2. Update in: Cochrane Database Syst Rev. 2018;9:CD010192. PMID: 24065550.
Gardner B, Rebar AL, Lally P. Habit Interventions. In: Hamilton K, Cameron LD, Hagger MS, Hankonen N, Lintunen T, editors. The Handbook of behavior change. Cambridge Handbooks in Psychology. Cambridge: Cambridge University Press; 2020. p. 599–616.
Howlett N, Trivedi D, Troop NA, Chater AM. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Transl Behav Med. 2019;9(1):147–57.
Rebar AL, Elavsky S, Maher JP, Doerksen SE, Conroy DE. Habits predict physical activity on days when intentions are weak. J Sport Exerc Psychol. 2014;36(2):157–65.
Gardner B, Rebar AL. Habit formation and behavior change. In Psychology (Psychology). Oxford University Press; 2019. https://doi.org/10.1093/obo/9780199828340-0232.
Gardner B, Phillips LA, Judah G. Habitual instigation and habitual execution: Definition, measurement, and effects on behaviour frequency. Br J Health Psychol. 2016;21(3):613–30.
Kaushal N, Rhodes RE, Meldrum JT, Spence JC. The role of habit in different phases of exercise. Br J Health Psychol. 2017;22(3):429–48.
Phillips LA, Gardner B. Habitual exercise instigation (vs. execution) predicts healthy adults’ exercise frequency. Health Psychol. 2016;35(1):69–77.
The English Indices of Deprivation 2019 (IoD2019). Ministry of Housing CLG; 2019.
Edwardson CL, Winkler EAH, Bodicoat DH, Yates T, Davies MJ, Dunstan DW, et al. Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci. 2017;6(2):162–78.
Ryan CG, Grant PM, Tigbe WW, Granat MH. The validity and reliability of a novel activity monitor as a measure of walking. Br J Sport Med. 2006;40(9):779–84.
McCourt O, Fisher A, Ramdharry G, Roberts AL, Land J, Rabin N, et al. PERCEPT myeloma: a protocol for a pilot randomised controlled trial of exercise prehabilitation before and during autologous stem cell transplantation in patients with multiple myeloma. BMJ Open. 2020;10(1):e033176.
Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–6.
Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60.
Ross KM, Wing RR. Concordance of in-home ‘smart’ scale measurement with body weight measured in-person. Obes Sci Pract. 2016;2(2):224–8.
Yorkin M, Spaccarotella K, Martin-Biggers J, Quick V, Byrd-Bredbenner C. Accuracy and consistency of weights provided by home bathroom scales. BMC Public Health. 2013;13:1194. https://doi.org/10.1186/1471-2458-13-1194.
Contardo Ayala AM, Nijpels G, Lakerveld J. Validity of self-measured waist circumference in adults at risk of type 2 diabetes and cardiovascular disease. Bmc Med. 2014;12:170.
Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25(5):365–84.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer-therapy scale - development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9.
Schipper H, Clinch J, McMurray A, Levitt M. Measuring the quality of life of cancer patients: the Functional Living Index-Cancer: development and validation. J Clin Oncol. 1984;2(5):472–83.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74.
Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–84.
McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego: Educational and Industrial Testing Services; 1971.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index - a New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989;28(2):193–213.
Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73.
Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1):5–13.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Esser P, Hartung TJ, Friedrich M, Johansen C, Wittchen HU, Faller H, et al. The Generalized Anxiety Disorder Screener (GAD-7) and the anxiety module of the Hospital and Depression Scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology. 2018;27(6):1509–16.
Hartung TJ, Friedrich M, Johansen C, Wittchen HU, Faller H, Koch U, et al. The Hospital Anxiety and Depression Scale (HADS) and the 9-item Patient Health Questionnaire (PHQ-9) as screening instruments for depression in patients with cancer. Cancer. 2017;123(21):4236–43.
Kroenke K, Spitzer RL, Williams JBW. The PHQ-9 - Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Haas BK, Northam S. Measuring self-efficacy: development of the physical activity assessment inventory. Southern Online J Nurs Res. 2010;10(4). Available: http://www.resourcenter.net/images/snrs/files/sojnr_articles2/vol10num04art03.html.
Foster C, Breckons M, Cotterell P, Barbosa D, Calman L, Corner J, et al. Cancer survivors’ self-efficacy to self-manage in the year following primary treatment. J Cancer Surviv. 2015;9(1):11–9.
Gardner B, Abraham C, Lally P, de Bruijn GJ. Towards parsimony in habit measurement: Testing the convergent and predictive validity of an automaticity subscale of the Self-Report Habit Index. Int J Behav Nutr Phys Act. 2012;9:102. https://doi.org/10.1186/1479-5868-9-102.
Byford S, Leese M, Knapp M, Seivewright H, Cameron S, Jones V, et al. Comparison of alternative methods of collection of service use data for the economic evaluation of health care interventions. Health Economics. 2007;16(5):531–6.
Perski O, Lumsden J, Garnett C, Blandford A, West R, Michie S. Assessing the psychometric properties of the digital behavior change intervention engagement scale in users of an app for reducing alcohol consumption: evaluation study. J Med Internet Res. 2019;21(11):e16197.
Fournier M, d’Arripe-Longueville F, Rovere C, Easthope CS, Schwabe L, El Methni J, et al. Effects of circadian cortisol on the development of a health habit. Health Psychol. 2017;36(11):1059–64.
Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–100.
Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–63. Downloadable at http://www.cet.org/.
Randler C, Schredl M, Göritz AS. Chronotype, sleep behavior, and the big five personality factors. SAGE Open. 2017;7(3):2158244017728321.
Sawyer A, Ucci M, Jones R, Smith L, Fisher A. Supportive environments for physical activity in deprived communities in the United Kingdom: a qualitative study using photo elicitation. Soc Sci Med. 2018;197:49–58.
van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15.
Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22.
Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. New York: Oxford University Press Inc.; 2006.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
UCL. Data Safe Haven (DSH). https://www.ucl.ac.uk/isd/services/file-storage-sharing/data-safe-haven-dsh. Accessed 10 Sept 2021.
Schrack JA, Cooper R, Koster A, Shiroma EJ, Murabito JM, Rejeski WJ, et al. Assessing daily physical activity in older adults: unraveling the complexity of monitors, measures, and methods. J Gerontol A Biol Sci Med Sci. 2016;71(8):1039–48.
Medical Research Council. Developing and evaluating complex interventions. https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/. Accessed 10 Sept 2021.
Acknowledgements
We would like to thank the National Cancer Research Institute Consumer Forum and their members who provided feedback on the intervention materials as they were developed.
The Trial Sponsor is UCL and can be contacted at uclh.randd@nhs.net. The sponsor has, and will have, no involvement in writing this manuscript, the design of the study, or collection, analysis, and interpretation of data. They will have no role in deciding whether to submit results for publication. We would like to thank the ongoing contributions of our trial steering committee.
Funding
This project is funded by Yorkshire Cancer Research (Reference UCL420) (PI: PL and AF). The funding body has, and will have, no involvement in writing this manuscript, the design of the study, or collection, analysis, and interpretation of data. They will have no role in deciding whether to submit results for publication.
Author information
Authors and Affiliations
Contributions
PL, AR, RJB, DMG, HWWP, NC, NL, CT, SL, JG, and AF conceptualised the study. PL, NM, and AF drafted the manuscript. All authors provided critical intellectual input and edits to the study protocol and manuscript. The authors read and approved the final manuscript.
Authors’ information
Diana Greenfield is a National Institute for Health Research (NIHR) Senior Nurse Research Leader. The views expressed in this article are those of the author and not necessarily those of the NIHR, or the Department of Health and Social Care.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial protocol has been approved by the Yorkshire & The Humber - South Yorkshire Research Ethics Committee (21/YH/0029) and by the Health Research Authority on 23rd March 2021. An amendment was approved on 24th December 2021 and the changes incorporated into this paper.
Any further protocol changes will be submitted to the HRA for approval as required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lally, P., Miller, N., Roberts, A. et al. An app with brief behavioural support to promote physical activity after a cancer diagnosis (APPROACH): study protocol for a pilot randomised controlled trial. Pilot Feasibility Stud 8, 74 (2022). https://doi.org/10.1186/s40814-022-01028-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-022-01028-w