Abstract
Background
The human microbiota has a broad range of functions contributing to metabolic processes and the activities of our immune system. Its influence on health, well-being and chronic diseases are discussed in various studies. The intestinal microbiota and the mucosal integrity are influenced by diet, environment and other lifestyle factors, including physical activity. There are correlations between cardiorespiratory fitness and important markers of intestinal health. However, data linking endurance exercise to microbiota composition are sparse. Many endurance athletes take probiotics to reduce gastrointestinal symptoms linked to exercise or immunosuppression, but the longitudinal data is insufficient.
This randomised, controlled cross-over pilot study will examine the impact of specific endurance training and probiotic supplementation on the intestinal microbiota and mucosa in healthy, athletic students.
Objective
The aim of this pilot study is to elucidate the impact of physical activity on the intestinal microbiota and mucosa with regard to the effects of a probiotic supplementation.
Methods
In this pilot study, thirty non-specifically trained student athletes will participate in an intervention consisting of a two-week rest (baseline) period, a four-week exercise programme and a four-week probiotic intervention using SymbioLactComp®. The exercise programme consists of three 60-min running workouts per week at 70–85% of the peak heart rate (HRpeak). Primary endpoint of this pilot study is the feasibility and practicality of the intervention as well as a sample size estimation. Furthermore, anthropometric measurements and information on nutrition and lifestyle will be obtained. The peak oxygen uptake (VO2peak) and peak heart rate (HRpeak) (determined during a shuttle run test) as well as selected blood and saliva parameters (haemogram, cytokines) will be evaluated. Changes to the intestinal microbiota will be analysed by stool diagnostics (KyberKompaktPRO®, KyberPlus®). The potential changes may include microbiota composition, bacterial metabolites and mucosa- and immune markers.
Conclusion
Results will be used for the design of a main randomised controlled trial with a larger collective based on feasibility, validity and sample size estimation as well as the potential effects of endurance exercise on intestinal microbiota and mucosa. Evidence-based information of an exercise-altered microbiota could be of importance for the prevention and therapy of intestinal or immune disorders.
Trial registration
German Clinical Trials Register: DRKS00011108. Retrospectively registered on 28 November 2016.
Similar content being viewed by others
Introduction
The intestinal microbiota composition plays a significant role in metabolism and immunity [1, 2]. Certain species and their associated metabolic products influence physiological homeostasis. For instance, the microbiota is involved in the degradation of food and the synthesis of vitamins, bioactive compounds and hormones such as serotonin. It further plays a role in the training of the immune system, pathogen defence and proliferation of intestinal cells [3, 4]. There is no recognised definition of an intact microbiota. However, the following aspects are believed to play an essential role: an enriched bacterial diversity (alpha diversity), a balanced Firmicutes/Bacteroidetes ratio, an intact mucus layer and an abundance of certain species (e.g. Akkermansia muciniphila and Faecalibacterium prausnitzii) [5,6,7,8,9]. An altered microbiota contributes to the pathogenesis of diseases such as metabolic or (auto)-immune diseases [10,11,12,13]. Recent studies have highlighted that environment and lifestyle factors affect the microbiota composition and its metabolic capacity [14, 15]. There is a growing body of evidence indicating that physical activity is linked to specific markers of intestinal health [16,17,18,19].
Several studies determined a higher species richness (α diversity) in elite athletes and individuals with higher cardiorespiratory fitness (VO2peak) or higher training frequency than those with a sedentary lifestyle or lower fitness level [16, 17, 20].
Furthermore, an increase in number of the two commensal species Akkermansia muciniphila and Faecalibacterium prausnitzii was observed in athletes and highly active individuals [17, 18, 20]. This was related to a higher concentration of the health-promoting bacterial metabolite butyrate [18, 20].
Some professional athletes suffer from immunosuppression or gastrointestinal symptoms, including abdominal pain, diarrhoea or leaky gut syndrome. This is because excessive exercise reduces intestinal blood flow due to increased circulation in the strained muscles and heart. This may lead to microbial imbalances and mucosa disruption [19, 21,22,23,24]. An increased permeability of the intestinal mucosa and epithelium (intestinal barrier) has been associated with bacterial/pathogen translocation to extraintestinal organs manifesting as intestinal or systemic inflammation [25,26,27].
Many athletes use probiotics to enhance barrier integrity and to improve gastrointestinal symptoms. The consumption of a probiotic supplement may induce immune processes, including the synthesis of cytokines and immunoglobulins, thereby contributing to the host’s health [28,29,30]. Previous research with trained athletes revealed the beneficial effects of probiotic intake on zonulin, a marker of mucosal integrity and several cytokines. Interferon-gamma, tumour necrosis factor-alpha and interleukins were all improved [19, 29, 31,32,33].
Regular moderate exercise is well-known for its anti-inflammatory effects, but diet may be a confounding factor in exercise-related impacts on the gastrointestinal system [17, 18]. Data that determines causality between microbiota composition, mucosa permeability and moderate endurance training is sparse. Longitudinal studies are needed to determine how the intestinal microbiota and mucosa as well as immune markers and cytokines vary with exercise as compared to probiotic supplementation. A combination of exercise and probiotics may be a new method for the prevention and therapy of intestinal or immune-related diseases [34,35,36].
Research hypothesis
We hypothesise that a training intervention with regular running exercises may alter microbiota composition in healthy adults. Participants receiving the intervention might further show higher rates of selected markers of intestinal health such as Akkermansia muciniphila, Faecalibacterium prausnitzii, Bifidobacterium spp. and lower rates of intestinal immune markers, e.g. zonulin, alpha-1-antitrypsin or secretory immunoglobulin A (sIgA).
Aims and objectives
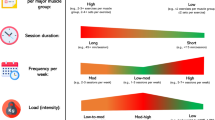
Primary outcomes of this randomised, controlled, cross-over pilot trial are the feasibility and practicality of the intervention, the validity of the study design and the provision of a reliable estimate for sample size calculation. To assess the practicality of the intervention, the duration (60 min), frequency (three times per week) and intensity (70–80% HRpeak) of the exercise programme will be considered as well as the adherence to the daily probiotic supplementation. The validity of the study design will be assessed with regard to the collection and immediate shipping of faecal samples. A further assessment will be made considering the time commitment for participants to complete endurance training, anthropometric testing, faecal samples and food diaries. In order to evaluate the efficacy of the intervention, the influence of moderate endurance training compared to probiotic supplementation (SymbioLactComp®) on the intestinal microbiota composition and barrier function (selected mucosal and immune parameters) as well as on selected blood and saliva biomarkers (haemogram, selected cytokines) in healthy student athletes will be examined.
Methods
Pilot trial design, study setting and ethics
This randomised controlled pilot cross-over trial is conducted at the German Sport University Cologne in cooperation with the MVZ Institute of Microecology GmbH. Data will be collected through bachelor endurance courses at the German Sport University Cologne (Cologne, Germany).
The study was approved by the Ethics Committee of the German Sport University Cologne (No. 136/2016). A schematic of the study design is given in more detail (see Additional file 1). To ensure all necessary aspects were addressed in the study protocol, the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist was adhered [37, 38] (Additional file 2).
Experimental design
Participants will receive a 12-week intervention programme including a 2-week rest period, a 4-week endurance training programme and a 4-week probiotic intervention with SymbioLactComp®. Subjects will not perform endurance sports or take probiotics during the rest period but will participate in the university courses during the programme (e.g. gymnastics, volleyball, tennis).
Exercise intervention
Exercise intensity will be calculated based on the HRpeak determined during a shuttle run test performed at the outset of the study. The training goal is a 10-km run in 50 min (males) or 55 min (female). The intensity of exercises will increase from 70% to 85% of the HRpeak to improve the subject’s basic endurance [39]. The three 60-min running sessions per week will consist of extensive and intensive continuous methods, interval training and stretching [39]. The exercises will be performed in groups and supervised by a sports scientist. Ratings of perceived exertion will be checked using a Borg Scale; the heart rate will be measured using a pulse monitor and belt from Polar® (Polar® Typ FT1). Participants will keep a training diary to log the completion of exercises. A training plan is shown in more detail in the appendix (see Additional file 3).
Probiotic intervention
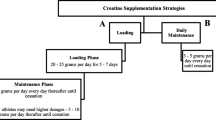
The probiotic period involves supplementation with the probiotic SymbioLactComp® (SymbioPharm GmbH, Herborn, Germany). SymbioLactComp® contains different lactic acid bacteria (Lactobacillus paracasei, Lactobacillus acidophilus, Lactococcus lactis and Bifidobacterium animalis subsp. lactis) as well as 30 μg of biotin. The total bacteria count of one sachet (2 g) is ≥ 1 × 109 KBE/g. One sachet will be mixed with water and taken daily at breakfast.
Pre-experimental procedures
Anthropometry
Anthropometric characteristics, arterial blood pressure and body composition will be measured. Body weight will be determined barefoot and in light clothes using a calibrated digital scale (Seca® Typ 771). Body height will be measured by a wall-mounted measuring tape (Seca® Typ 225). Body mass index will be calculated by the World Health Organization’s specifications (BMI = body weight (kg)/body size (m)2) [40]. Systolic and diastolic blood pressures will be measured three times after 5 min of sitting calmly using a digital blood pressure monitor (OMRON® Typ M5-1). Body fat and muscle mass will be estimated using bioelectric impedance analysis (EGOFIT GmbH, Eggstätt, Germany).
Furthermore, information on the participant’s personal history and lifestyle including physical activity levels will be collected prior to the start of the trial (T0) using a modified questionnaire (International Physical Activity Questionnaire; [41].
Cardiorespiratory fitness
A standardised multistage 20-m shuttle run test will be performed at the outset of the study to calculate HRpeak and the VO2peak as an indicator of the participants’ cardiorespiratory fitness [42].
The test will be executed by running back and forth on a 20-m course. A sound signal emitted from a prerecorded tape will set the pace. The starting speed is 8.5 km/h and will be increased by 0.5 km/h each minute, representing a stage. Participants must touch the 20-m line even after the acoustic signal. If the participant is not able to follow the pace, the test is finished. The last stage number will be used to determine the HRpeak by a pulse monitor and belt (Polar® Typ FT1) as well as the VO2peak. VO2peak will be calculated according to Léger and Lambert [42]: VO2max (mL kg−1 min−1) = − 24.4 + 6.0 × “maximal shuttle run speed”/km h−1. The shuttle run test will be repeated after the 4-week training period in order to define the potential performance improvement.
Data collection
Blood samples
Venous blood samples (of approximately 25 mL) will be collected under medical supervision using the BD Vacutainer® Butterfly Safety Lok, EDTA tubes and SST™ II Advance serum tubes (Becton Dickinson, Heidelberg, Germany). For the evaluation of the chosen parameters, participants are not required to be fasted. A haemogram will be measured from the EDTA blood using a Sysmex KX-21N automated haematology analyser (Sysmex Deutschland GmbH, Bornbach, Germany). Two serum samples will be centrifuged at 4000×g for 10 min and pipetted and stored at − 80 °C in the Center for Molecular Medicine Cologne (University of Cologne, Germany) until it will be analysed. The thawed serum samples will be used to measure the following cytokines by multiplex ELISA (Bio-Plex Pro Human Cytokine 8-plex ELISA Kit, Bio-Plex Pro Human Chemokine IL-1β Singleplex Set; Bio-Rad Laboratories GmbH, Munich, Germany): tumour necrosis factor-alpha, interleukin-6, interleukin-8 and interleukin-1β.
Saliva samples
Saliva samples will be collected after a 30-min fast via Salivettes® from Sarstedt (Sarstedt AG & Co. KG, Nümbrecht, Germany). Samples will be centrifuged at 4000×g for 10 min and pipetted and stored at − 80 °C in the Center for Molecular Medicine Cologne (University of Cologne, Germany). The following selected cytokines will be analysed by a multiplex ELISA (Bio-Plex Pro Human Cytokine 8-plex, Bio-Rad Laboratories GmbH, Munich, Germany): tumour necrosis factor-alpha, interleukin-6, interleukin-8 and interferon-gamma.
Faecal samples
Faecal analyses will be carried out at the Institute of Microoecology, Herborn, Germany. Faecal samples will be collected during the final day of the dietary programme. Subjects will be given a sterile stool sampling kit and detailed sample collection instructions. Subjects will send samples to the laboratory, and if shipping is not possible immediately after collection, the sample will be stored in a refrigerator at 7 °C but will be sent on the same day.
Nutritional data
Each participant will complete four detailed 7-day food diaries based on self-reported intake prior to the next faecal sample collection. A standardised protocol will be developed by a research dietitian supervisor. Subjects will maintain a habitual diet, such that potential gastrointestinal modifications can only be attributed to the intervention of sports or probiotics.
Nutritional quality and quantity of consumed foods and drinks as well as nutritional supplements will be recorded. A prepared protocol with instructions will help to guide the data recorded: breakfast, lunch, dinner, snacks, recipe information, preparation method (raw, steamed, boiled, fried, breaded, baked, with/without skin), fat/fructose content and food amount (gramme/millilitre).
Dietary data and personal data (gender, age, body height, body weight, body mass index) will be entered into the DGExpert software (version 1.8.9.0) to generate each subject’s energy and nutrient intake as well as respective reference values.
The following list of dietary parameters will be analysed each day in grammes/microgrammes/percentage:
-
Energy/caloric intake (kilocalories, kilojoule)
-
Proteins
-
Fats
-
Total fatty acids
-
Polyunsaturated fatty acids
-
Monounsaturated fatty acids
-
Saturated fatty acids
-
Linoleic acid
-
Linolenic acid
-
Quotient of linoleic acid to linolenic acid
-
Total carbohydrates
-
Absorbable carbohydrates
-
Fibres
-
Lactose
-
Sucrose
-
Monosaccharides
-
Disaccharides
-
Total sugar
-
All vitamins and micronutrients
-
Water
-
Alcohol
Laboratory procedures
Identification and enumeration of microorganisms
Bacteria will be enumerated using the KyberKompaktPro® test, which combines the identification of viable bacteria by classical microbial analysis and quantitative polymerase chain reaction (qPCR).
Viable bacteria will be enumerated on the following selective media: Columbia blood agar (total cell count; BioMérieux, Nürtingen, Germany), U3G agar (Enterobacteriacae, enterococci; Heipha, Heidelberg, Germany), Rogosa agar, (Lactobacilli; Heipha), DIC agar (Bifidobacteria; Heipha), Schaedler agar (Bacteroides; Heipha) and SPM agar (Clostridia; Heipha). Faecal samples will be serially diluted in 1 mL of phosphate-buffered saline (PBS, pH 7.2) and subsequently plated on selective agar plates by a fully automated spiral plater (PreviIsola, BioMérieux). The plates will be incubated under either aerobic or anoxic conditions at 37 °C for at least 2 days. Bacteria will be identified by gram staining and colony morphologies. Additionally, identifications will be performed by the API and VITEK systems (BioMérieux). All counts will be recorded by the number of log10 CFU per grammes of sample.
The following bacteria will be routinely analysed: Clostridium spp., Bifidobacterium spp., Bacteroides spp., (E. coli, Enterococcus spp., Lactobacillus spp.) and other bacteria (Pseudomonas spp., Klebsiella spp., Proteus spp., Citrobacter spp., Enterobacter spp., other aerobic bacteria).
DNA extraction from faecal samples for further qPCR analysis
Microbial DNA will be extracted from 200 mg of faecal samples using the QIAsymphony® DSP Virus/Pathogen Mini-Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions on the QIAsymphony® SP (Qiagen). Automated isolation and pipetting of 96-well plates (MicroAmp Optical 96-Well Reaction Plate, Applied Biosystems, Darmstadt, Germany) will be performed by the QIAsymphony® SP/AS instrument (Qiagen) using the QIAsymphony DSP Virus/Pathogen Mini-Kit.
Quantification of target bacteria by quantitative real-time PCR (qPCR)
Primers will be selected to recognise the phyla Firmicutes, Bacteroidetes [43], Verrucomicrobia, the genus Enterobacteriacea [44] and Methanobrevibacter as well as the species Faecalibacterium prausnitzii and Akkermansia muciniphila.
PCR amplification and detection will be performed using an ABI PRISM 7900HT sequence detection system (Applied Biosystems, Darmstadt, Germany) in optical-grade 96-well plates sealed with optical sealing tape. Each reaction mixture (25 μL) will be composed of 12.5 μL of QuantiTect SYBR Green PCR Master Mix (Qiagen), 2 μL primer mix (10 pmol/μL each), 9 μL sterile distilled H2O and 1.5 μL stool DNA (10 ng/μL). For the negative control, 2 μL of sterile distilled H2O will be added to the reaction solution instead of the template DNA solution. A standard curve will be produced using the appropriate reference organism to quantify the qPCR values into the number of bacteria per grammes. The standard curves will be prepared in the same PCR assay as the samples. The fluorescent products will be detected in the last step of each cycle. A melting curve analysis will be carried out following amplification to distinguish the targeted PCR product from the non-targeted PCR product. The melting curves will be obtained by slowly increasing the temperature to 55 and 95 °C at a rate of 0.2 °C/s, with continuous fluorescence collection. The data will be analysed using ABI Prism software. The real-time PCRs will be performed in triplicate, and average values will be used for enumeration.
Laboratory analyses of short-chain fatty acids
Human stool samples analysed for short-chain fatty acid (SCFA) content will be freeze-dried and subsequently analysed using a gas chromatograph. The samples will be weighed (~ 200 mg of dry matter) and a 10-fold dilution with physiological NaCl saline (1.8 mL) will be produced. Following vortexing (2 min, Vortexer MS 3 basic, IKA-Werke GmbH & Co. KG, Staufen, Germany), an aliquot of 200 μL will be mixed with 0.36 M HClO4 (280 μL) and 1 M NaOH (270 μL). The solution will be lyophilised at − 35 °C (alpha 1–4 LSC, CHRIST, Osterode am Harz, Germany). The obtained lyophilisate will be dissolved in 100 μL 5 M HCOOH and 400 μL acetone and centrifuged (5 min at 4000×g, RT; Pico 17, Thermo Electron LED GmbH, Langenselbold, Germany). Concentrations of the SCFA will be determined in the supernatant using a GC-2010 Plus gas chromatograph (Shimadzu Deutschland GmbH, Duisburg, Germany) equipped with flame ionisation detection and a thin-film capillary column Stabilwax®-DA 30 m × 0.25 mm × 0.5 μm (Restek, Bad Homburg, Germany). The samples will be spread out by split injection using the auto-sampler AOC-20s/I (Shimadzu Deutschland GmbH). GCsolution Chromatography Data System (Shimadzu Deutschland GmbH) will be used for data processing. For the determination of the SCFA, an external standard (Supelco™ WSFA-1 Mix, Supelco Sigma-Aldrich Co., Bellefonte PA) will be used.
Laboratory analyses of faecal markers
Faecal calprotectin, eosinophil protein x (EPX), zonulin, beta-defensin 2 (β-defensin 2) and secretory immunoglobulin A (sIgA) concentrations will be measured by an ELISA kit (Immundiagnostik AG, Bensheim, Germany). The tests to analyse calprotectin and EPX use the sandwich ELISA technique. Monoclonal anti-calprotectin-antibodies or anti-EPX-antibodies will be added to the samples, standards and controls. Colour intensity is directly proportional to the concentration of calprotectin and EPX. Samples will be read at 450 nm. Standard curves will be formed by the four-parameter algorithm.
A competitive binding technique will be used to determine zonulin concentrations. Samples, standards and controls will be added with biotinylated zonulin and incubated with polyclonal anti-zonulin antibodies. Colour intensity is inversely proportional to the sample concentration of zonulin. Samples will be read at 450 nm. The four-parameter algorithm will be used to create the standard curve [45].
Quantitative analysis of β-defensin 2, standards and controls will be performed using polyclonal anti-β-defensin 2 antibodies. The amount of bound enzymes is directly proportional to the β-defensin 2 content. Samples will also be measured at 450 nm.
To determine secretory immunoglobulin A (sIgA), polyclonal anti-sIgA antibodies will be added to the samples. The test uses a calibrator and calibration curve to detect sIgA content. Colour development is proportional to the amount of analyte.
Faecal alpha-1-antitrypsin concentrations will be measured using the AAT test (Maier Analytic, Sinsheim, Germany). The assay uses the sandwich technique and polyclonal antibodies will be utilised to analyse samples, standards and controls. Colour intensity is directly proportional to the alpha-1-antytrypsin concentration in the sample. The samples will be read at 450 nm and the standard curve formed via the four-parameter algorithm.
Sample size
It is estimated that a sample size of a minimum of 30 participants is required in order to provide a pooled standard deviation that provides enough precision for the development of a future trial [46, 47].
Participants and recruitment
The study will have a half-year enrollment phase. Recruitment will be carried out by the study director. Healthy students with an age between 18 and 29 and a body mass index of ≤ 25 kg/m2 will be recruited through bachelor endurance courses at the German Sport University Cologne (maximal number of participants around 320 students). Therefore, our feasibility study enrollment rate has to exceed 10%. The recruitment rate will be analysed by the number of eligible individuals recruited within half a year, as presented above. A recruitment rate of > 80% will be considered as feasible to conduct a future randomised control trial.
Randomisation
Participants will be randomised in a 1:1 ratio and allocated to group one (endurance-probiotics) or group two (probiotics-endurance) via block randomisation by the study director [48]. Exclusion criteria will be injuries; diseases (e.g. inflammatory bowel diseases), an antibiotic therapy just before or during the intervention; and continuing gastrointestinal disturbances based on probiotic intake. Subjects who meet the inclusion criteria will obtain detailed information about the trial and will give written informed consent before starting the study.
Statistical analysis
This pilot study intends to examine the confidence interval estimation and feasibility of the presented approach (physical activity and probiotic supplementation) [49, 50]. Attendance and retention rates will be assessed to calculate adherence. Attendance rate will be analysed by dividing the mean value for the number of participants present by the number of sessions offered during the four-week exercise period and the four-week probiotic intervention. The retention rate will be analysed by the percentage of participants completing all sessions of the endurance training (twelve in total), shuttle run test (two in total), nutrition questionnaire (four in total), blood/saliva measurement (four in total) and stool diagnostics (four in total) present in each step of the study. A retention rate of > 80% will be considered as feasible to conduct a future randomised controlled trial. Hypothesis testing and results of regression modelling will be considered entirely exploratory in nature and interpreted with caution. Our trial is mainly conducted in preparation for a future definitive randomised controlled trial to assess the efficacy of the intervention/study design [51]. All data will be entered into the database management software and analysed by the SPSS Version 25.0 (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test will be used for variable normal distribution and the usual descriptive statistics for background information and mean values with a 95% confidence interval. Data will be used for sample size estimation and to determine the most appropriate outcome measure for the main study. Descriptive statistics and confidence intervals will also be calculated to examine the trends in the analysed parameters. This information will help to identify which parameters to focus on in future trials. Depending on the normality of the underlying data, parametric or non-parametric (e.g. unpaired t test/Mann-Whitney U test) tests will be carried out. Furthermore, a paired t test/Wilcoxon test might be computed for the longitudinal comparison (T0–T3). Bivariate correlation analyses might be assessed by the Spearman/Pearson correlation coefficient. Multivariate analyses with linear or logistic regressions might be performed to explore potential effects of the intervention and further potential covariates and cofactors. Moreover, multiple regression models might be used if multiple variables are predictive. The level of statistical significance is p < 0.05.
Discussion
Several cross-sectional studies have determined microbial differences between people living in an active or sedentary lifestyle. However, there is a lack of investigations exploring the effects of sport independent of diet [16,17,18]. By comparing a controlled exercise programme to probiotic supplementation (cross-over design) and including nutritional analyses, this pilot trial intends to examine a causal relationship in this complex system. Progression criteria were described to support the concept and the effectiveness of the intervention in order to analyse the outcome parameters in the future. The use of the progression criteria intends to validate the plausibility and clinical importance of this kind of intervention, which can serve as the basis for a main randomised controlled trial. This approach is of importance because evidence-based information on exercise-altered microbiota is needed for the prevention and therapy of intestinal or immune disorders.
Abbreviations
- EPX:
-
Eosinophil protein x
- HRpeak:
-
Peak heart rate
- IPAQ:
-
International Physical Activity Questionnaire
- PCR:
-
Polymerase chain reaction
- SCFAs:
-
Short-chain fatty acids
- sIgA:
-
Secretory immunoglobulin A
- VO2peak:
-
Peak oxygen uptake
- β-defensin 2:
-
beta-defensin 2
References
Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14(7):676–84.
Balducci S, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20(8):608–17.
Guarner F, Malagelada J-R. Gut flora in health and disease. Lancet (London, England). 2003;361(9356):512–9.
Sekirov I, et al. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904.
Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science (New York, NY). 2005;308(5728):1635–8.
Farhadi A, et al. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18(5):479–97.
Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–48.
Dao MC, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–36.
Miquel S, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16(3):255–61.
Serino M, et al. Intestinal microflora and metabolic diseases. Diabetes Metab. 2009;35(4):262–72.
Forbes JD, van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081.
Kassinen A, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133(1):24–33.
Macfarlane GT, et al. The gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15(13):1528–36.
Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7(1):17–44.
Monda V, et al. Exercise modifies the gut microbiota with positive health effects. Oxidative Med Cell Longev. 2017;2017:3831972.
Estaki M, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016; 4.1:42.
Clarke SF, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–20.
Bressa C, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. 2017;12(2):e0171352.
Lamprecht M, et al. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012;9:45.
McFadzean R. Exercise can help modulate human gut microbiota, 2014.
Lachtermann E, Jung K. Sport und gastrointestinales System - Einfluss und Wechselwirkungen. Deutsches Ärzteblatt. 2006;103:2116–20.
Pedersen BK. Special feature for the Olympics: effects of exercise on the immune system: exercise and cytokines. Immunol Cell Biol. 2000;78(5):532–5.
Petersen AMW, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57(Suppl 10):43–51.
de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med. 2014;44(Suppl 1):S79–85.
Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91(1):151–75.
Alonso C, et al. Intestinal barrier function and the brain-gut axis. Advances in experimental medicine and biology, vol. 817; 2014. p. 73–113.
Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5(6):685–94.
Pyne DB, et al. Probiotics supplementation for athletes - clinical and physiological effects. Eur J Sport Sci. 2015;15(1):63–72.
Cox A, Pyne DB, Saunders PU, Fricker PA. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br J Sports Med. 2010;44:222–6.
Rescigno M. Intestinal microbiota and its effects on the immune system. Cell Microbiol. 2014;16(7):1004–13.
Kekkonen RA, et al. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int J Sport Nutr Exerc Metab. 2007;17(4):352–63.
Gleeson M, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15.
Gleeson M, et al. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int J Sport Nutr Exerc Metab. 2012;22(4):235–42.
McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–22.
West CE. Probiotics for allergy prevention. Benefic Microbes. 2016;7(2):171–9.
Ukena SN, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2(12):e1308.
Chan AW, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. Bmj. 2013;346:e7586.
Chan AW, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Beck H. Das große Buch vom Marathon: Lauftraining mit System: Marathon-, Halbmarathon- und 10-km-Training für Einsteiger, Fortgeschrittene und Leistungssportler: Trainingspläne, Jahrestraining, Krafttraining, Ernährung, Gymnastik. 9., überarbeitete Neuauflage ed. Grünwald: Copress Sport; 2016. p. 368.
Organization WH. Obesity - preventing and managing the global epidemic: report on a WHO consultation. Geneva: World Health Organization; 2000. p. 266.
Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
Léger LA, Lambert J. A maximal multistage 20-m shuttle run test to predict VO2 max. Eur J Appl Physiol Occup Physiol. 1982;49(1):1–12.
Pedersen R, et al. Changes in the gut microbiota of cloned and non-cloned control pigs during development of obesity: gut microbiota during development of obesity in cloned pigs. BMC Microbiol. 2013;13:30.
Bartosch S, et al. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol. 2004;70(6):3575–81.
Ohlsson B, et al. Calprotectin in serum and zonulin in serum and feces are elevated after introduction of a diet with lower carbohydrate content and higher fiber, fat and protein contents. Biomed Rep. 2017;6(4):411–22.
Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol. 2012;65(3):301–8.
Hayman A, et al. How big should a pilot trial be? A case for continuous outcomes in randomised controlled trials. Telford: Annual conference of the Royal Statistical Society; 2012.
Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Human Reprod Sci. 2011;4(1):8–11.
Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–9.
Arain M, et al. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67.
Eldridge SM, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64.
Acknowledgements
We would like to thank the study participants.
Funding
The study is supported by the MVZ Institute of Microecology GmbH, Herborn, Germany. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The dataset that will be used and analysed during the current study will be available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
CG, LS, NF, AS, UW and KR contributed to the study concept. LS and CG contributed to the data collection, data analyses, interpretation of data and preparation of manuscript. LS, CG, AS, KR and EM contributed the data handling. LS, AS, KR and EM contributed to the laboratory analyses. NF, AS, EM and CG contributed to the critical manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee of the German Sport University Cologne
Competing interests
The authors declared that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Study design (PDF 1470 kb)
Additional file 2:
SPIRIT checklist (PDF 243 kb)
Additional file 3:
Training plan (PDF 185 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Schmitz, L., Ferrari, N., Schwiertz, A. et al. Impact of endurance exercise and probiotic supplementation on the intestinal microbiota: a cross-over pilot study. Pilot Feasibility Stud 5, 76 (2019). https://doi.org/10.1186/s40814-019-0459-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-019-0459-9