Abstract
Background
Patients with a low serum blood hemoglobin concentration suffer from a pathologic state that contributes significantly to morbidity and mortality figures worldwide. Oral iron supplementation, the most common method of treatment, is reported to have poor patient adherence, due to its unwanted side effects. Lactoferrin is a globular glycoprotein of the transferrin family that has shown promising results in patients with a low hemoglobin profile. This systematic review and meta-analysis of randomized clinical trials explore its effect on blood hemoglobin compared to conventional iron preparations.
Methods
We followed the PRISMA Guidelines for reporting systematic reviews and meta-analyses. A systematic search was conducted in electronic databases (PubMed, CINAHL, Scopus, and Cochrane) from inception to June 2022. Meta-analysis was performed on studies where the primary outcome was the mean Hb concentration, comparing lactoferrin to ferrous sulfate subgroups. We assessed the methodological quality of the trials using the Jadad scoring scale.
Results
Nineteen trials published between 2006 and 2022 met the eligibility criteria. It has been found that the levels of Hb concentration in different populations with varying health conditions undergo a moderate to significant change after treatment with all types of trialed interventions, including both iron and lactoferrin treatment, in both the intervention group and the comparison group. Most of the studies report that LF showed a statistically significant increase in Hb concentration levels, compared to those in the iron group. The meta-analysis included seven trials comparing the effectiveness of lactoferrin to ferrous sulfate for patients with low Hb concentration. The analysis showed a statistically significant increase in Hb levels in the oral bovine lactoferrin group compared to ferrous sulfate (SMD -0.81, 95% CI: -1.21, -0.42, p < 0.0001, I2 = 95.8%, P heterogeneity < 0.001).
Conclusions
Lactoferrin is an effective intervention at doses of 100–250 ng/day, for patients with a low Hb concentration. As a safer option and with high compliance evidence, lactoferrin can serve as an iron replacement treatment for patients who may be experiencing adverse side effects due to iron intake.
Similar content being viewed by others
Background
When the number of red blood cells is insufficient to support the body’s metabolic needs, it leads to anemia, a pathologic state defined by a low hemoglobin concentration, which contributes significantly to morbidity and mortality figures worldwide [1, 2]. In 2019, the estimated global prevalence of anemia in women and children, based on the distribution of blood hemoglobin concentration values in different populations globally, was strikingly reported as 39.8% in children up to five years of age, 29.9% in women of reproductive age (non-pregnant), and 36.5% in pregnant women [3]. Oral iron supplementation is the most common conventional method of treatment. However, it usually causes unwanted gastrointestinal side effects in patients in 70% of cases, which may consequently affect the patients’ adherence to treatment [4, 5].
Lactoferrin (LF) is a globular glycoprotein of the transferrin family, possessing a high iron binding affinity, and it is structurally and chemically similar to serum transferrin [6]. Bovine LF (bLF) shares a high degree of similarity (up to 70%), with human lactoferrin, which is found in body exocrine secretions (human milk, tears, and saliva) and in the secondary granules of neutrophils [7, 8]. It has shown promising results in many studies evaluating its effect on blood hemoglobin levels in women, children, and patients with chronic disease [9].
A comprehensive analysis of the randomized trials conducted to date, examining the effects of oral bLF as a supplement on hemoglobin, in different populations and health states, is needed to evaluate and clarify the effect and safety of oral LF supplementation. Therefore, the scope of this systematic review and meta-analysis is to describe its effect on blood hemoglobin and to quantify the effect of oral bLF on hemoglobin in different populations and health states for both genders.
Methods
Information sources and search strategy
For conducting this systematic review and meta-analysis, the PRISMA Guidelines for reporting systematic reviews and meta-analyses (PRISMA) were followed [10]. The protocol of this study was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022348383).
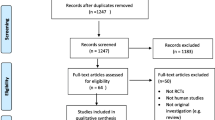
The following electronic databases were independently searched by two researchers (MC, AC) from inception to June 2022: PubMed, CINAHL, Scopus, Cochrane and Embase. Studies were retrieved by using the following ‘Medical Subject Heading’ (MesH) and text words combined using Boolean operators as a search strategy for each electronic database: (Lactoferrin OR ‘‘bovine lactoferrin’’ OR lactotransferrin) AND (‘‘anemia treatment’’ OR ‘‘anaemia trea tment’’ OR ‘‘iron deficiency anemia’’ OR ‘‘iron deficiency anaemia’’ OR anaemia OR anemia OR anemic OR anaemic OR ‘‘iron deficiency’’ OR hemoglobin OR haemoglobin). The search was limited to title and abstract and clinical trial articles, and articles published in English. Following the completion of the search, the title and abstract of all the retrieved articles were screened for their relevancy to the subject being studied, and articles were eliminated if irrelevant. Finally, the full text of the potentially eligible articles was examined for potential inclusion in the analysis. Disagreements were settled through mediation and discussion with a third author (KG). The citation lists of the articles retrieved were manually checked to identify similar studies. The database search strategy is presented in Fig. 1.
Eligibility criteria
We used the Participants, Intervention, Comparison, Outcomes, and Study design (PICOS) approach to identify included studies [11]. Eligible studies included male and female adult participants and children ≥ 2 years of age. The clinical trials selected for inclusion used oral bLF as the main intervention, with co-interventions allowed. The trials needed to clearly state the daily dose given in both the intervention group and the control/comparison group. Trials that compared the intervention to a control or comparison group were eligible for inclusion, including those using oral iron preparations or any other form of conventional treatment for anemia. The change in mean Hb concentration values was considered the primary outcome measure. Randomized trials were the only type of study design that was eligible for inclusion.
The trials concerned participants with a mean Hb group value of ≤ 11.5 g/dL as a baseline characteristic. Only studies involving interventions on human subjects were considered, while those focused solely on the protective or potential effect of LF on healthy subjects were excluded. The publication year of the articles was not considered a restrictive factor in any case. Duplicate publications of the same trial were excluded. Only studies published in English were considered. Narrative reviews, dissertations or theses, conference papers, case studies, editorials, and letters were excluded.
Study selection and data extraction
After the removal of irrelevant studies and duplicates, the full text of the retrieved articles was assessed to determine whether the content of the article and methodology pertained to the inclusion and exclusion criteria. Data was extracted independently by two authors (MC and AC) and tabulated using a table designed by the authors based on the ‘Cochrane Checklist of Items’ [12]. For the purpose of the systematic review, extracted information from the included articles referred to the authors and date of publication of the article, the participants’ characteristics that were included in the trial, their health condition, age, and mean baseline values for each group of participants included in the trial, the type of interventions used, the dose of the applied intervention in each group, the time frame of the intervention, and the main outcome of the trial shown as the mean value of Hb concentration for each group that completed the given intervention in the trial and its calculated p value. Disagreements were settled through mediation and discussion with a third author (KG).
Quality assessment
The methodological quality of the included trials was assessed using the Jadad scoring scale. The Jadad scale is a validated tool for evaluating the methodological quality and risk of bias of randomized clinical trials [13]. It is based on the assessment of specific methodological qualities of clinical trials: randomization, blinding, and accountability of withdrawals and dropouts. Each trial may be scored within the range of 0–5 [14]. For the purposes of this meta-analysis, studies with a Jadad score of 3 or above were considered to have low methodological bias, while those with a Jadad score of 2 or less were considered to have high methodological bias.
Data synthesis and statistical analysis
The inverse-variance weighted approach was used for continuous data meta-analysis with a Standardized Mean Difference (SMD) and 95% Confidence Interval (CI) to estimate the size of the effect calculated by pooling the SMD of each individual study based on the mean, standard deviations (SD), and sample size. SMD were chosen due to the variability in Hb concentrations between assays used by different laboratories and between measurements of Hb in different components of blood. Due to a lack of data, we only calculated a pooled effect size for the association between the lactoferrin group and ferrous sulfate on Hb concentration. We assessed heterogeneity among individual effect estimates and reported the P-value of the χ2-based Cochran Q test. The variation in estimates due to heterogeneity was quantified using the I2 metric for inconsistency and the I2 index (> 50% indicating significant heterogeneity) [15]. A random effects model was used to pool the results if I2 was greater than 50%; otherwise, a fixed-effect model was used [16]. To further explore sources of heterogeneity, we carried out subgroup analyses considering treatment duration. The presence of publication bias was examined by visual inspection of funnel plots and evaluated formally with Egger's regression asymmetry test [17, 18]. All statistical analyses were performed by STATA 14.0 software (STATA Corp. College Station, TX). A two-tailed P value < 0.05 was considered statistically significant.
Results
Study selection
The process of the search strategy and article selection for the systematic review and the meta-analysis is summarized in Fig. 1, following the PRISMA flow diagram 2020 [10]. A total of 158 articles were identified through database searches, with 25 from PubMed, 33 from CINAHL, 44 from Scopus, 13 from Cochrane and 43 from Embase. After the removal of duplicates, 111 article titles and abstracts were screened for relevance to the study’s field. Among these, 46 article titles were identified as potentially relevant. Among these, 25 article titles were excluded because only the abstract was published. The full manuscript of the remaining 21 article titles were assessed further for eligibility. Ultimately, only 10 articles met the selection criteria and were included in the systematic review. Additionally, 13 more articles were discovered by screening the citation lists of these included articles. Out of these, 8 met the inclusion criteria for this systematic review and were analysed.
One of the articles [19] included for review reported two separate trials—one involving non-pregnant women and the other involving pregnant women. We counted these as two separate trials. Therefore, a total of 19 randomized trials from 18 articles, yielded through database search and citation lists, were analyzed in this systematic review.
Study characteristics
The 19 trials included in this systematic review were published between 2006 and 2022. Out of these, 5 of the trials reported were conducted in Italy [19,20,21,22] and 14 in Egypt [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The total number of participants who completed these trials was 2992 patients, with a mean Hb concentration level of ≤ 11.5 g/dL. Among them, 637 were children and adolescents, and 2355 were adults. There were 1353 participants in the LF intervention groups, and 1639 participants in the comparison/control groups. In terms of gender distribution, there were significantly more females, with 2516 (including 2266 women > 18 years and 250 children and adolescents < 18 years), as opposed to 326 males (including 89 men > 18 years and 237 children and adolescents < 18 years).
Among the 19 analyzed trials, the majority (53%—10 trials) focused on pregnant women with iron deficiency anemia (IDA) at or after 12 weeks of gestation, comprising a 2041 out of 2992 participants (939 in the intervention, 1102 in the control). This represented 68% of all participants involved in the trials [19, 21,22,23, 25, 26, 28, 29, 33,34,35]. Seven trials (37%) involved children aged over 2 [24, 27, 30,31,32, 36, 37], and one trial targeted cancer patients undergoing chemotherapy [20]. Additionally, four trials included 334 individuals with chronic health conditions (cancer, cerebral palsy, inflammatory bowel disease, obesity), all of whom had anemia (Hb < 11.5 g/dL) [20, 27, 32, 37].
Among the 1353 participants in the intervention groups, all received oral bLF. They were compared to the following groups: 1160 received ferrous sulfate [21, 22, 24,25,26,27,28,29, 33, 34, 38], 73 received ferric gluconate [20], 73 received ferrous fumarate [35], 30 received ferrous bisglycinate [30], 70 received ferric (III) hydroxide polymaltose [31, 37], 62 received iron polymaltose complex [30, 32], 33 received iron (III) hydroxide dextran complex [23], and 112 received a combination of LF and iron [24, 30, 31]. The bLF dosage ranged from 100 to 250 mg per day, and the treatment duration varied between 30 and 90 days. The study characteristics of the trials included in the systematic review are presented in Table 1.
Methodological quality
According to the Jadad scoring scale, there was a variation in the methodological bias of the studies included in the meta-analysis, as detailed in Table 2. Studies with a Jaded score of 3 or above were considered to have low methodological bias, while those with a Jadad score of 2 or less were considered to have high methodological bias. Out of the included studies, five received 1/5 rating [29,30,31, 36, 37], six received a 2/5 rating [19, 20, 22, 25, 34, 35], six received a 3/5 rating [23, 24, 26,27,28, 32, 33], and 1 received a 4/5 rating [21]. Overall, 8 out of 18 studies were considered to have low methodological bias, while 10 out of 18 studies were considered to have high methodological bias. It is evident in the scoring process (Table 2.) that the problem originates from the lack of description of the randomisation process by most authors. The major issue, however, is the non-blinding of both patients and researchers/healthcare practitioners, which can give rise to treatment bias and may eventually influence the results. Only one study (Nappi et al., 2009) out of 18 studies described the application of an appropriate blinding process during the trial.
Meta-analysis results of Hb concentration outcomes
Out of the 19 included trials, seven were eligible for meta-analysis focusing on the effectiveness of lactoferrin versus iron supplementation in patients with a low Hb concentration profile. These trials included a total of 1397 participants: 698 supplemented with ferrous sulfate, and 699 with oral bLF. The participants were pregnant women with IDA, at a gestational age of at least 12 weeks but not more than 36 weeks.
The overall pooled SMD, using a random-effects model, revealed a statistically significant increase in Hb concentration levels in the oral bLF group in comparison with the ferrous sulfate group (SMD 0.81, 95% CI: 0.42, 1.21, p < 0.0001, I2 = 95.8%, P heterogeneity < 0.001). Based on the random-effects model, the pooled SMD, after one month of treatment was 0.59 (95% CI: 0.18, 0.99, p = 0.004; I2 = 92.1%, P heterogeneity < 0.001). For those women who received the intervention for the longest duration, the pooled SMD, was 1.04 (95% CI: 0.34, 1.74, p = 0.004; I2 = 97.1%, P heterogeneity < 0.001). The results of the meta-analysis regarding the effectiveness of bLF compared to ferrous sulfate supplementation in pregnant women with low Hb concentration are presented in Fig. 2.
Publication bias
Funnel plots (Fig. 3 and Fig. 4) were used for the visual inspection of asymmetry or outliers for the purpose of determining publication bias. The shape of the funnel plots indicated data asymmetry. The Egger asymmetry test revealed no evidence of publication bias (P Egger’s test P > 0.05).
Discussion
The present systematic review and meta-analysis evaluated the effectiveness of oral bLF versus iron supplementation for patients with a low hemoglobin. It has been found that the levels of Hb concentration in different populations with varying health conditions undergo a moderate to significant change after treatment with all types of trialed interventions, including both iron and lactoferrin treatment, in both the intervention group and the comparison group. The trend of the results appears to favor bLF treatment. In all 19 randomized trials included in this study, bLF has been suggested as an effective and safe alternative to iron supplementation by the authors. Our meta-analysis demonstrated a statistically significant increase in Hb concentration favoring the oral bLF group over the ferrous sulfate group, with an overall pooled SMD of 0.81 (95% CI: 0.42, 1.21, p < 0.0001, I2 = 95.8%, P heterogeneity < 0.001). While several studies conducted, mainly on pregnant women [19, 22, 25, 26, 33, 35] and children [24, 27, 32, 36, 37], support this result, stating that LF showed a statistically significant increase in Hb concentration levels, compared to those in the iron group, some state that there were no statistically significant differences in the mean Hb increase between both treatments and that both treatments can be considered equally effective in improving Hb concentration levels [21, 29, 20].
For those who received the intervention for the longest duration, the pooled SMD was 1.04 (95% CI: 0.34, 1.74, p = 0.004; I2 = 97.1%, P heterogeneity < 0.001). Based on these results, subgroup analysis at the longest treatment duration suggests a slightly more significant effect compared to the one-month intervention, favoring bLF treatment. This finding warrants further investigation to determinate the optimal treatment duration. It is possible that daily bLF treatment for more than one month may lead to a more substantial increase in Hb concentration in the blood. This, in turn, may suggest that patients might need multiple cycles of LF treatment to significantly elevate their Hb concentration.
Several studies, included in this systematic review, propose that the Hb concentration enhancing effect of LF is attributable to its anti-inflammatory properties. More precisely, Paesano et al., 2010 [38], investigated this hypothesis by measuring the fluctuations in pro-inflammatory and inflammatory markers, IL-6, CRP, ESR, in both the LF and iron populations recruited in their trials. It was observed that serum IL-6 levels were significantly decreased in gravidas with IDA at third trimester, who were treated with bLF, from a mean of 34.0 ± 8.0 pg/ml to 12.0 ± 10.0 pg/ml (p < 0.0001). The mean value of IL-6 increased in patients who were treated with ferrous sulfate, from a mean of 33.0 ± 13.0 pg/ml to 52.0 ± 13.0 pg/ml. This could possibly suggest that increased levels of the pro-inflammatory cytokine IL-6 in serum may be associated with lower levels of serum Hb mean value. This same effect was observed in two other included studies, involving children suffering from obesity and inflammatory bowel disease [27, 37]. This metanalysis analyzed the mean Hb values of pregnant women. Pregnancy is characterized by inflammatory processes which are normal and condition the course of physiological processes and body changes that take place during pregnancy till childbirth [39]. Therefore, it could be that the anti-inflammatory activity of LF plays a role in the modulation of Hb in pregnancy as demonstrated by Paesano et al. (2014) in anemic pregnant women affected from hereditary thrombophilia [40].
It is well established that inflammation exerts negative effects on iron homeostasis. Under inflammatory conditions ferroportin is downregulated thus impairing iron release into blood plasma. Hepcidin synthesis is controlled by IL-6, among other factors. Elevated levels of the pro-inflammatory cytokine IL-6, elevates hepcidin which in turn interacts with ferroportin at the cell surface, impairing iron release into plasma, thus causing hypoferremia [41].
Perhaps, a deeper inside into the mechanism of effect of LF can be concluded from previously published data taken from healthy individuals. In a randomized double blind study, Koikawa et al., 2008 [42], recruited sixteen female long distance runners, with a healthy mean Hb value before treatment, divided into two groups (control and intervention). It was observed that there was no rise or significant difference in Hb mean values, (from 13.1 ± 0.8 g/dl to 13.0 ± 0.6 g/dl), after treatment with LF for 8 weeks, whilst the daily intake of oral iron supplementation was a constant in both groups. Furthermore, the rest of the hematology parameters examined (ferritin, serum iron, red blood cell count) didn’t show any significant change either. This evidence suggests that the therapeutic potentiality of LF with regard to Hb seems to be attributable to its capacity to modulate iron homeostasis. The failure of LF to increase blood parameters in healthy subjects is supporting evidence to this statement. Data demonstrate that lactoferrin may be a modulating agent in iron homeostasis by targeting the IL-6, Hepcidin, Ferroportin interplay [8].
A high patient compliance was recorded in participants receiving the LF treatment. Bayoumy, Ragab and Elghareeb, 2021 [26] and Hemeda et al., 2018 [35], highlighted its statistical significance (p = 0.009 and p < 0.05 respectively). As compliance to treatment is always a prerequisite for optimum results, a higher adherence to LF treatment over iron supplementation may further explain the more favorable results of LF supplementation over oral iron supplementation. These results may be credited to the fact that fewer side effects to no side effects were reported with LF intervention than conventional iron supplementation, with significant difference (p < 0.001 and p < 0.05) [24, 33].
Strengths and limitations
To our knowledge, this systematic review and meta-analysis represents the first evaluation of data from randomized clinical trials regarding the impact of LF on Hb concentrations in non-healthy patients, of both genders and different population groups. However, there are some limitations to this study. It only considered articles published in English, potentially excluding similar eligible studies in other languages. Although the study's overall quality of evidence suggests a potential risk of bias, it's important to consider that the study also had a high level of heterogeneity at 95.8%, which indicates a significant level of variability. This level of variability indicates inconsistent treatment results. This heterogeneity may stem from variations in treatment types, product formulation, timeframes, and population characteristics, as well as variations in the methods used by the researchers or even bias. Furthermore, all included studies were conducted in Italy and primarily in Egypt, limiting the demographic representation of the results. While the results of this systematic review and meta-analysis were not intended to support the introduction of a guideline, treatment protocol or treatment recommendation, nor to be used as a basis for accurate decision-making by healthcare providers, readers should be aware that these limitations may impact the reliability of results, and therefore, it is advisable to interpret these results with caution.
Conclusion
This systematic review and meta-analysis provides representative data on the effectiveness of oral LF at doses of 100–250 mg/day, compared to conventional iron preparations in patients with low hemoglobin levels. The evidence suggests that bLF is an effective intervention for patients with low Hb concentration profiles, particularly in patients with inflammatory conditions. As a safer option and with high compliance evidence, lactoferrin can serve as an iron replacement treatment for patients who may be experiencing adverse side effects due to iron intake. Lactoferrin's ability to reduce pro-inflammatory cytokines seems crucial in regulating and restoring Hb levels. Reducing inflammation could potentially serve as the the first step toward normalizing Hb concentration levels in patients with low hemoglobin and inflammation-related conditions.
Future studies need to focus on reducing the risk of bias in their clinical trials to improve the methodological quality of the studies. This will help ensure the validity of the results and maintain the credibility and integrity of the study. Furthermore, it is important to examine whether product formulation, i.e. different types of lactoferrin preparations, affect Hb concentration differently. This will determine if different types of lactoferrin preparations are a confounding variable in such studies and may explain the high heterogeneity of results observed so far.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- blf:
-
Bovine Lactoferrin
- Hb:
-
Hemoglobin
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- LF:
-
Lactoferrin
- IDA:
-
Iron Deficiency anemia
- IL-6:
-
Interleukin 6
- SMD:
-
Standardized mean difference
- SD:
-
Standard deviation
- CI:
-
Confidence interval
References
Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PHM, Newman AB, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: The cardiovascular health study. Arch Intern Med. 2005;165(19):2214–20.
Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116:3–10.
WHO. Anaemia in women and children. https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children, assessed on 10th June 2021. p. 1–6. Available from: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children. Cited 2022 Mar 23
Ziauddin Hyder SM, Persson LÅ, Chowdhury AMR, Ekström EC. Do side-effects reduce compliance to iron supplementation? A study of daily- and weekly-dose regimens in pregnancy. J Heal Popul Nutr. 2002;20(2):175–9. Available from: https://www.jstor.org/stable/23498939. Cited 2022 Mar 23
DeLoughery TG. Safety of Oral and Intravenous Iron. 142, Acta Haematologica. Karger Publishers; 2019. p. 8–12. Available from: https://www.karger.com/Article/FullText/496966. Cited 2022 Mar 23
Jegasothy H, Weerakkody R, Selby-Pham S, Bennett LE. In vitro heme and non-heme iron capture from hemoglobin, myoglobin and ferritin by bovine lactoferrin and implications for suppression of reactive oxygen species in vivo. BioMetals. 2014;27(6):1371–82. Available from: https://link.springer.com/article/https://doi.org/10.1007/s10534-014-9798-4. Cited 2022 Mar 24
Sienkiewicz M, Jaśkiewicz A, Tarasiuk A, Fichna J. Lactoferrin: an overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. 101080/1040839820211895063. 2021; Available from: https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/10408398.2021.1895063. Cited 2022 Mar 24
Rosa L, Cutone A, Lepanto MS, Paesano R, Valenti P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int J Mol Sci 2017, Vol 18, Page 1985. 2017;18(9):1985. Available from: https://www.mdpi.com/1422-0067/18/9/1985/htm. Cited 2022 Mar 24
Rosa L, Lepanto MS, Cutone A, Siciliano RA, Paesano R, Costi R, et al. Influence of oral administration mode on the efficacy of commercial bovine Lactoferrin against iron and inflammatory homeostasis disorders. BioMetals. 2020;33(2–3):159–68. Available from: https://pubmed.ncbi.nlm.nih.gov/32274616/. Cited 2022 Mar 23
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. Available from: https://www.bmj.com/content/372/bmj.n71. Cited 2022 Mar 26
Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Pract. 2001;1(2):136–41.
Higgens J, Green S. Table 7.3.a: Checklist of items to consider in data collection. Cochrane Handbook’s checklist of items for inclusion. 2011. Available from: https://handbook-5-1.cochrane.org/chapter_7/table_7_3_a_checklist_of_items_to_consider_in_data_collection.htm. Cited 2022 Mar 26
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Contr Clin Trials. 1996;17(1):1–12.
Berger V, Alperson S. A General Framework for the Evaluation of Clinical Trial Quality. Rev Recent Clin Trials. 2009;4(2):79–88. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1574-8871&volume=4&issue=2&spage=79
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. Available from: https://onlinelibrary.wiley.com/doi/https://doi.org/10.1002/sim.1186
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Available from: https://www.bmj.com/content/327/7414/557. Cited 2022 Oct 27
Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5. Available from: https://www.bmj.com/content/323/7304/101. Cited 2022 Oct 27
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Available from: https://pubmed.ncbi.nlm.nih.gov/9310563/. Cited 2022 Oct 27
Paesano R, Berlutti F, Pietropaoli M, Goolsbee W, Pacifici E, Valenti P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int J Immunopathol Pharmacol. 2010;23(2):577–87.
Macciò A, Madeddu C, Gramignano G, Mulas C, Sanna E, Mantovani G. Efficacy and safety of oral Lactoferrin supplementation in combination with rHuEPO-β for the treatment of anemia in advanced cancer patients undergoing chemotherapy: open-label, randomized controlled study. Oncologist. 2010;15(8):894.
Nappi C, Tommaselli GA, Morra I, Massaro M, Formisano C, Di Carlo C. Efficacy and tolerability of oral bovine lactoferrin compared to ferrous sulfate in pregnant women with iron deficiency anemia: a prospective controlled randomized study. Acta Obstet Gynecol Scand. 2009;88(9):1031–5.
Paesano R, Torcia F, Berlutti F, Pacifici E, Ebano V, Moscarini M, et al. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. In: Biochemistry and Cell Biology. Biochem Cell Biol; 2006;377–80. Available from: https://pubmed.ncbi.nlm.nih.gov/16936810/. Cited 2022 Feb 28
Darwish AM, Fouly HA, Saied WH, Farah E. Lactoferrin plus health education versus total dose infusion (TDI) of low-molecular weight (LMW) iron dextran for treating iron deficiency anemia (IDA) in pregnancy: a randomized controlled trial. 101080/1476705820181429396. 2018;32(13):2214–20. Available from: https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/14767058.2018.1429396. Cited 2021 Oct 4
El-Asheer OM, Ahmed AG, Hafez ZAA, Dahpy MA, Soliman AA. Lactoferrin efficacy versus ferrous sulfate in treatment of children with iron deficiency anemia. J Child Sci. 2021;11(1):E199-204.
Fawzy Mohamed M, Soliman Taha W, Farrag Ismaeil Farag M. Lactoferrin versus Ferrous Sulphate for the Treatment of Iron Deficiency Anemia during Pregnancy (A Randomized Clinical Trial). Al-Azhar Med J. 2020;49(1):271–82. Available from: https://amj.journals.ekb.eg/article_67107.html. Cited 2022 Jul 17
Bayoumy KM, Ragab SG, Elghareeb NAM. Compliance and efficacy of oral lactoferrin versus ferrous sulfate in treatment of nutritional iron deficiency anemia during second trimester among Egyptian ladies. Egypt J Hosp Med. 2021;83(1):903–9. Available from: https://ejhm.journals.ekb.eg/article_158077.html. Cited 2022 Jul 17
El Amrousy D, El-Afify D, Elsawy A, Elsheikh M, Donia A, Nassar M. Lactoferrin for iron-deficiency anemia in children with inflammatory bowel disease: a clinical trial. Pediatr Res. 2022;92:762. https://doi.org/10.1038/s41390-022-02136-2.Cited2022Jul18.
Rezk M, Kandil M, Dawood R, Shaheen A-E, Allam A. Oral lactoferrin versus ferrous sulphate and ferrous fumerate for the treatment of iron deficiency anemia during pregnancy. J Adv Nutr Hum Metab. 2015;2:740. Available from: https://smartscitech.com/index.php/JANHM/article/view/550. Cited 2022 Jul 18
Ali A, Nasr M, Rasheedy MI El, Gaber M, Aaty A El, Hashim A. Oral Iron Preparations for Prevention of Iron Deficiency Anaemia During Pregnancy a Prospective Randomized Comparative Clinical Trial. AamjEgNet. 2015;13(2):244–50. Available from: http://www.aamj.eg.net/journals/pdf/2839.pdf. Cited 2022 Sep 14
El-Hawy MA, Abd Al-Salam SA, Bahbah WA. Comparing oral iron bisglycinate chelate, lactoferrin, lactoferrin with iron and iron polymaltose complex in the treatment of children with iron deficiency anemia. Clin Nutr ESPEN. 2021;46:367–71.
Kamal MY, Rezk MM, Hafez MH. A comparative study for the efficacy of lactoferrin-100 versus lactoferrin-100 and ferrous gluconate versus ferric hydroxide on iron deficiency anemia. Curr Pediatr Res. 2021;25(3):444–9. Available from: www.currentpediatrics.com. Cited 2022 Feb 28
Omar OM, Assem H, Ahmed D, Abd Elmaksoud MS. Lactoferrin versus iron hydroxide polymaltose complex for the treatment of iron deficiency anemia in children with cerebral palsy: a randomized controlled trial. 2021;180(8):2609–18. Available from: https://link.springer.com/article/https://doi.org/10.1007/s00431-021-04125-9. Cited 2021 Oct 4
Rezk M, Dawood R, Abo-Elnasr M, Al Halaby A, Marawan H. Lactoferrin versus ferrous sulphate for the treatment of iron deficiency anemia during pregnancy: a randomized clinical trial. J Matern Neonatal Med. 2016;29(9):1387–90.
El-Nasr IAS, Mahmoud SA, Elnaddar EM, Ammar HA. Ferrous Sulphate Alone Versus Combination of Ferrous Sulphate and Lactoferrin for The Treatment of Iron Deficiency Anemia during Pregnancy and Their Effect on Neonatal Iron Store: A Randomized Clinical Trial. Egypt J Hosp Med. 2021;84(1):1955–60. Available from: https://ejhm.journals.ekb.eg/article_178616.html. Cited 2022 Feb 28
Hemeda HM, Abd El Kader Mohamed A, Aly Islam B, Hasan Eweis AA. Effectiveness of Bovine Lactoferrin versus Ferrous Fumarate in the Management of Iron Defi ciency Anemia in Pregnancy: Randomized Clinical Trial-International Journal of Reproductive Medicine & Gynecology SCIRES Literature-Volume 4 Issue 1-www.sciresl. 4, Int J Reprod Med Gynecol. 2018. Available from: http://ua-pharm.com/wp-content/uploads/docs/laktoferrin-i-zhelezo-protiv-anemii-beremennyh-issledovanie-original.pdf. Cited 2022 Jul 15
El-Khawaga A, Abdelmaksoud H. Effect of Lactoferrin Supplementation on Iron Deficiency Anemia in Primary School Children. Int J Med Arts. 2019;0–0. Available from: https://ijma.journals.ekb.eg/article_34024_5278.html. Cited 2022 Jul 15
Atia MM, Gama RM, Saad MA, Hamam MA. Comparative Study of the Effects of Lactoferrin versus Oral Iron Therapy in Obese Children and Adolescents with Iron Deficiency Anemia. J Adv Med Med Res. 2021;33(21):104–14. Available from: http://apsciencelibrary.com/handle/123456789/6364. Cited 2022 Jul 16
Paesano R, Berlutti F, Pietropaoli M, Pantanella F, Pacifici E, Goolsbee W, et al. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. Biometals. 2010;23(3):411–7.
Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221(1):80. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3078586/. Cited 2022 Sep 22
Paesano R, Pacifici E, Benedetti S, Berlutti F, Frioni A, Polimeni A, et al. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: an interventional study. BioMetals. 2014;27(5):999–1006. Available from: http://link.springer.com/https://doi.org/10.1007/s10534-014-9723-x
Diederich M, Morceau F, Dicato M. Pro-inflammatory cytokine-mediated anemia: Regarding molecular mechanisms of erythropoiesis. 2009, Mediators of Inflammation. 2009. Available from: https://www.hindawi.com/journals/mi/2009/405016/. Cited 2022 Mar 23
Koikawa N, Nagaoka I, Yamaguchi M, Hamano H, Yamauchi K, Sawaki K. Preventive effect of lactoferrin intake on anemia in female long distance runners. Biosci Biotechnol Biochem. 2008;72(4):0803040802–0803040802.
Acknowledgements
The authors would like to thank Panagiota Neofytou and Styliana Eleftheriadou for their help with the literature search and retrieval, in the production of this report.
Funding
Open access funding provided by the Cyprus Libraries Consortium (CLC). This study was not funded.
Author information
Authors and Affiliations
Contributions
M.D.C. conceptualized the theme, drafted the manuscript, collected the data, appraised the articles, summarized the results, analyzed the data, prepared the tables, and interpreted the results. K.G. analyzed the data, performed the meta-analysis, prepared the statistical figures, interpreted the results, and revised the manuscript critically. M.M. interpreted the results and revised the manuscript critically. A.M. interpreted the results and revised the manuscript critically. M.V.S. interpreted the results and revised the manuscript critically. A.C. conceptualized the theme, supervised the process, interpreted the results, and revised the manuscript critically. All authors contributed to the article and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Articles were included in this systematic review and meta-analysis only if they reported that the authors had obtained informed consent from the participants of the clinical trial.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Christofi, MD., Giannakou, K., Mpouzika, M. et al. The effectiveness of oral bovine lactoferrin compared to iron supplementation in patients with a low hemoglobin profile: A systematic review and meta-analysis of randomized clinical trials. BMC Nutr 10, 20 (2024). https://doi.org/10.1186/s40795-023-00818-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-023-00818-6