Abstract
An extremely halophilic archaeon, Haladaptatus cibarius D43T, was isolated from traditional Korean salt-rich fermented seafood. Strain D43T shows the highest 16S rRNA gene sequence similarity (98.7 %) with Haladaptatus litoreus RO1-28T, is Gram-negative staining, motile, and extremely halophilic. Despite potential industrial applications of extremely halophilic archaea, their genome characteristics remain obscure. Here, we describe the whole genome sequence and annotated features of strain D43T. The 3,926,724 bp genome includes 4,092 protein-coding and 57 RNA genes (including 6 rRNA and 49 tRNA genes) with an average G + C content of 57.76 %.
Similar content being viewed by others
Introduction
The extremely halophilic archaea, called haloarchaea, possess the small retinal protein halorhodopsin [1–3] and currently consists of more than 47 genera that live in hypersaline environments [4, 5]. Three members of the genus Haladaptatus — H. paucihalophilus [6], H. litoreus [7], and H. cibarius [8]—were isolated from a low-salt, sulfide-rich spring; marine solar saltern; and salt-fermented seafood, respectively. Haladaptatus comprises Gram-negative staining, non-motile haloarchaea that have polar lipids including phosphatidylglycerol, phosphatidylglycerol phosphate methyl ester, and phosphatidylglycerol sulfate [6]. The genomic analysis revealed that H. paucihalophilus survives in low salinity conditions because of trehalose synthesis with OtsAB pathway and trehalose glycosyl-transferring synthase pathway, and glycine betaine uptake [9]. However, other members in the genus Haladaptatus have not been analyzed at the genome level.
H. cibarius was isolated from the traditional Korean salt-fermented seafood, which is made with shellfish [8]. D43T (= DSM 19505T = JCM 15962T ) is a representative strain and designated as the type strain of the species. It can grow in 10%–30% (w/v) NaCl (optimum, 15%), with Mg2+ required for growth. In addition, cells are not lysed in distilled water. The genome sequences of this genus are expected to provide fundamental information for the halotolerant features and biotechnological applications of the haloarchaea. Here, we describe the first whole genome sequence of H. cibarius along with its annotated features, and summarize the taxonomic classification.
Organism information
Classification and features
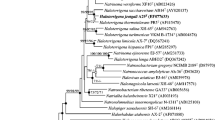
The taxonomic position for H. cibarius D43T was identified with type strains obtained from the EzTaxon-e server [10]. The 16S rRNA sequences of D43T and closely related strains were aligned using the ClustalW multiple sequence alignment program [11] and were subsequently used for the phylogenetic analysis. Phylogenetic trees were constructed using the neighbor-joining [12], maximum-parsimony [13], and maximum likelihood [14] algorithms with bootstrap values of 1,000 using MEGA version 5 molecular evolutionary genetics analysis program [15]. Strain D43T clustered with type strains of Haladaptatus species (Fig. 1), exhibiting 16S rRNA gene sequence similarities of 98.7% and 95.1% between strain D43T (EF660747) and the type strain of H. litoreus and H. paucihalophilus , respectively. Classification and general features of H. cibarius D43T are shown in Table 1.
Phylogenetic tree constructed using the neighbor-joining method based on 16S rRNA gene sequences, showing the taxonomic position of strain D43T in genus Haladaptatus. Bootstrap values (>70%) at nodes are shown as percentages calculated using the neighbor-joining/maximum parsimony/maximum likelihood probabilities based on 1000 replicates. Filled circles indicate identical branches generated using three algorithms. Methanosarcina semesiae MD1T was used as an outgroup. Bar, 0.05 substitutions per nucleotide position
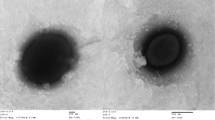
Strain D43T is a Gram-negative staining, coccus or coccobacillus, motile archaeon approximately 1.0 μm in diameter (Fig. 2). Catalase and oxidase tests yielded positive results, but reduction of nitrate to nitrite under aerobic conditions was negative. Cells contained the polar lipids phosphatidylglycerol, phosphatidylglycerol phosphate methyl ester, and two unidentified glycolipids. Strain D43T hydrolyzed gelatin and Tween 80, utilized formate and acetate as carbon sources, and produced acid from sucrose and d-glucose. The strain was sensitive to anisomycin, aphidicolin, chloramphenicol, and rifampicin, and was resistant to ampicillin, erythromycin, kanamycin, streptomycin, and polymycin B.
Genome sequencing and annotation
Genome project history
The genome project and sequence of the H. cibarius D43T genome were deposited in the Genomes OnLine Database [16] (project ID: Gp0086819) and GenBank (accession number: JDTH00000000), respectively. The BioProject number was PRJNA236630. Sequencing and annotation were performed by Chun Lab Inc. (Seoul, Korea) and Integrated Microbial Genomes Expert Review (IMG-ER) [17].
Growth conditions and genomic DNA preparation
H. cibarius D43T grew optimally on halophilic medium [6] supplemented with 15% (w/v) NaCl and 20 mM Mg2+ adjusted to pH 7.0, producing colonies with a pink color after incubation at 37°C as previously described [8]. Genomic DNA was extracted and purified using a G-spin DNA extraction kit (iNtRON Biotechnology Inc., Sungnam, Korea), according to the manufacturer’s instructions.
Genome sequencing and assembly
Genomic sequences of H. cibarius D43T were generated from a total of 9,237,360 quality-filtered reads (710.3-fold coverage) by combining 5,074,634 reads (374.9-fold coverage) obtained from Mi-Seq 300 bp paired-end library (Illumina, San Diego, CA, USA), 4,112,798 reads (292.1-fold coverage) obtained from an Ion Torrent Personal Genome Machine 318v2 chip (Life Technologies, Carlsbad, CA, USA), and 49,928 reads (43.3-fold coverage) obtained from PacBio RS 10 kb library (Pacific Biosciences, Menlo Park, USA). Illumina and PGM data were assembled de novo with CLC Genomics Workbench 6.5.1 (CLC bio, Boston, MA, USA) and PacBio data were assembled using the HGAP2 algorithm in SMRT Analysis 2.1 (Pacific Biosciences). Resultant contigs were assembled with CodonCode Aligner 3.7 (CodonCode Corporation, Centerville, MA, USA). Sequences were assembled to 13 scaffolds with an N50 contig size of 985,075 bp; the genome sequencing project information and its associated MIGS version 2.0 compliance levels [18] are shown in Table 2.
Genome annotation
The open reading frames of the assembled genome were predicted and annotated using IMG-ER [17], NCBI COG [19], Pfam [20], and EzTaxon-e [10] databases. The rRNA and tRNA genes were identified using RNAmmer 1.2 [21] and tRNA scan-SE 1.23 [22], respectively.
Genome properties
The draft genome sequence for H. cibarius D43T contained 3,926,724 bp, with 13 scaffolds. The G + C content was 57.76 % (Fig. 3 and Table 3), and 4,092 protein-coding genes were predicted along with 57 RNA genes, including six rRNA (two 5S, three 16S, and one 23S rRNA), 49 tRNA, and two additional RNA genes. There were 2,676 protein-coding genes with predicted functions: 773 were enzymes, 98 encoded signal peptides, and 1,049 encoded transmembrane proteins. The distribution of genes in the COG functional categories is shown in Table 4. A large number of genes were associated with the COG functional categories of cell wall biogenesis (79, 3.3 %); transcription (100, 4.1 %); and transport and metabolism of amino acids (299, 12.3 %), carbohydrates (121, 5.0 %), and lipids (80, 3.3 %). Further analysis with dbCAN [23], a database for annotation of carbohydrate-active enzymes, showed that the genome contains genes encoding various enzymes for the breakdown and biosynthesis of carbohydrates such as chitinase (GH18), chitosanase (GH5), pullulanase (GH13), trehalose synthase (GT4 and 20), cellulose synthase (GT2), and alginate lyase (PL6).
Graphical map of the H. cibarius D43T pseudochromosome. From outside to center: RNA genes (red, tRNA and blue, rRNA) and genes on the antisense and sense strands (colored according to COG categories). Inner circle shows the GC skew, with yellow and blue indicating positive and negative values, respectively. GC content is indicated in red and green. The genome map was visualized using CLgenomics 1.06 (Chun Lab Inc.)
Insights from the genome sequence
The genome analysis of H. cibarius D43T revealed genes involved in glycine betaine synthesis—including betaine aldehyde dehydrogenase, glycine betaine demethylase, and choline-glycine betaine transporter gene—that allow H. cibarius to maintain osmotic balance in hypersaline environments. In addtion, trehalose-related genes of trehalose-6-phosphate synthase, trehalose-6-phosphatase, trehalose-6-phosphate synthase and trehalose-6-phosphate hydrolase, and trehalose-utilization protein genes were analyzed in the genome sequences of H. cibarius D43T. The genes related with trehalose synthesis in the genome show the possibility of trehalose production that is important in food industry.
Conclusions
The draft genome sequences of the extremely halophilic archaeon isolated from the salt-fermented seafood were analyzed. Genes related with glycine betaine and trehalose for the survival in extreme environments were identified. The extremely halophilic archaeon could be a valuable resource for biotechnological applications because hypersaline conditions minimize the risk of contamination by other microorganisms. Further characterization of halophilic enzymes of the haloarchaea based on the genomic analyses can provide more detailed information on enzyme structures and potential industrial applications.
Abbreviations
- PGM:
-
Personal genome machine
- IMG-ER:
-
Integrated microbial genomes expert review
- ORF:
-
Open reading frame
References
Lanyi JK. Halorhodopsin: a light-driven chloride ion pump. Annu Rev Biophys Biophys Chem. 1986;15:11–28.
Lanyi JK. Halorhodopsin, a light-driven electrogenic chloride-transport system. Physiol Rev. 1990;70:319–30.
Oesterhelt D, Tittor J, Bamberg E. A unifying concept for ion translocation by retinal proteins. J Bioenerg Biomembr. 1992;24:181–91.
Oren A. The order Halobacteriales. In: Dworkin M, Falkow S, Rosenberg E, Shleifer KH, Stakebrandt E, editors. The prokaryotes. Volume 3. 3rd ed. New York: Springer; 2006. p. 113–64.
Oren A, Ventosa A. International Committee on Systematics of Prokaryotes Subcommittee on the taxonomy of Halobacteriaceae and Subcommittee on the taxonomy of Halomonadaceae: minutes of the joint open meeting, 31 July 2014, Montreal, Canada. Int J Syst Evol Microbiol. 2014;64:3915–8.
Savage KN, Krumholz LR, Oren A, Elshahed MS. Haladaptatus paucihalophilus gen. nov., sp. nov., a halophilic archaeon isolated from a low-salt, sulfide-rich spring. Int J Syst Evol Microbiol. 2007;57:19–24.
Cui HL, Sun FF, Gao X, Dong Y, Xu XW, Zhou YG, et al. Haladaptatus litoreus sp. nov., an extremely halophilic archaeon from a marine solar saltern, and emended description of the genus Haladaptatus. Int J Syst Evol Microbiol. 2010;60:1085–9.
Roh SW, Lee ML, Bae JW. Haladaptatus cibarius sp. nov., an extremely halophilic archaeon from seafood, and emended description of the genus Haladaptatus. Int J Syst Evol Microbiol. 2010;60:1187–90.
Youssef NH, Savage-Ashlock KN, McCully AL, Luedtke B, Shaw EI, Hoff WD, et al. Trehalose/2-sulfotrehalose biosynthesis and glycine-betaine uptake are widely spread mechanisms for osmoadaptation in the Halobacteriales. ISME J. 2014;8:636–49.
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, et al. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–21.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25.
Kluge AG, Farris JS. Quantitative phyletics and the evolution of anurans. Syst Biol. 1969;18:1–32.
Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–76.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9.
Liolios K, Chen IM, Mavromatis K, Tavernarakis N, Hugenholtz P, Markowitz VM, et al. The Genomes On Line Database (GOLD) in 2009: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res. 2010;38:D346–354.
Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics. 2009;25:2271–8.
Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol. 2008;26:541–7.
Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–6.
Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–230.
Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–8.
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64.
Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:W445–451.
Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–9.
Garrity GM, Holt JG. Phylum AII. Euryarchaeota phy. nov. In: Boone DR, Castenholz RW, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. Volume 2. 2nd ed. New York: Springer; 2001. p. 211–355.
Grant WD, Kamekura M, McGenity TJ, Ventosa A. Class III. Halobacteria class nov. In: Boone DR, Castenholz RW, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. Volume 2. 2nd ed. New York: Springer; 2001. p. 294.
Grant WD, Kamekura M, McGenity TJ, Ventosa A. Order I. Halobacteriales. In: Boone DR, Castenholz RW, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. Volume 2. 2nd ed. New York: Springer; 2001. p. 299–301.
Gupta RS, Naushad S, Baker S. Phylogenomic analyses and molecular signatures for the class Halobacteria and its two major clades: a proposal for division of the class Halobacteria into an emended order Halobacteriales and two new orders, Haloferacales ord. nov. and Natrialbales ord. nov. Int J Syst Evol Microbiol. 2015;65:1050–69.
Grant WD, Kamekura M, McGenity TJ, Ventosa A. Family I. Halobacteriaceae. In: Boone DR, Castenholz RW, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. Volume 2. 2nd ed. New York: Springer; 2001. p. 299–301.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (2012R1A1A2040922) and a Korea Basic Science Institute NAP grant (T34780).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KJY and HSS carried out the microbial cultivation and DNA isolation. HWL, DWK, SWR, BYK, YJC and KJY participated in the sequence analyses. HWL, DWK and SWR drafted the manuscript. MJS, JKR, DGL and CY helped to draft the manuscript. SWR and YDN conceived of the study and participated in its design. HJC and JSC participated in its design and coordination. All authors read and approved the final manuscript.
Hae-Won Lee and Dae-Won Kim contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, HW., Kim, DW., Lee, MH. et al. Draft genome sequence of the extremely halophilic archaeon Haladaptatus cibarius type strain D43T isolated from fermented seafood. Stand in Genomic Sci 10, 53 (2015). https://doi.org/10.1186/s40793-015-0051-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-015-0051-8